Back to Journals » Clinical Interventions in Aging » Volume 17
A Feasibility Study of Individuals Living at Home with Alzheimer’s Disease and Related Dementias: Utilization of Visual Mapping Assistive Technology to Enhance Quality of Life and Reduce Caregiver Burden
Authors Han SS, White K, Cisek E
Received 1 September 2022
Accepted for publication 13 December 2022
Published 23 December 2022 Volume 2022:17 Pages 1885—1892
DOI https://doi.org/10.2147/CIA.S387255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Samuel S Han,1,* Kaylin White,1,* Edward Cisek2
1Research and Development, MapHabit, Inc., Atlanta, GA, USA; 2Program Evaluation, CaringKind, New York, NY, USA
*These authors contributed equally to this work
Correspondence: Samuel S Han, Research and Development, MapHabit, inc, 75 5th Street Ste 2240, Atlanta, GA, 30308, USA, Tel +1 207 991 1955, Email [email protected]
Abstract: Efficacy of assistive technology continues to evolve as a means of helping individuals with cognitive and intellectual disabilities, asserting the importance of its research. We report outcomes of a six-week randomized control feasibility study in a small cohort of 16 family caregivers of individuals living with Alzheimer’s disease and related dementias. An experimental group of seven family caregivers used visual mapping software on smart devices (step-by-step pictures, audio, and videos instructing how to complete a task) to support carrying out activities of daily living with their care recipients. In comparison, control group of nine family caregivers used smart devices to access and view educational videos focused on dementia care. After a six-week study, compared to caregivers using educational videos, caregivers using visual maps assistive technology reported higher satisfaction of use and stronger recommendation of use to others. Caregivers using visual maps technology also exhibited more improved quality of life scores and improved completion of activities of daily living for their dementia care recipients, as well as reduced caregiver burden scores compared to the caregivers in the control group. These promising findings show that the use of assistive technology is feasible in the home setting and suggest time is ripe for undertaking systematic studies of assistive technology’s potential to advance effective behavioral interventions in dementia home and family settings.
Keywords: visual mapping, dementia caregiving, activities of daily living, randomized controlled trial
Plain Language Summary
Efficacy of assistive technology continues to evolve as a means of helping individuals with cognitive and intellectual disabilities. The current study addresses the feasibility of using assistive technology to improve the lives of families living with Alzheimer’s disease and other related dementias. After 6 weeks of using an assistive technology app that helps the user engage in activities of daily living with highly personalized step-by-step visual guides, caregivers reported improved quality of life and completion of activities of daily living for their individual with dementia, all the while reducing burden and stress for themselves. These promising findings underscore the feasibility of using assistive technology in home settings and set the stage for systematic studies of assistive technology’s potential to advance effective behavioral interventions for a wide range of neuro-compromised individuals within home and family settings.
Introduction
Assistive technology software built into consumer grade mobile devices—eg smartphones and tablets—has been used effectively with adult individuals living with Alzheimer’s disease and related dementias (ADRD) to enhance their ability to complete activities of daily living (ADLs) and to improve overall quality of life.1–3 The technology can involve the use of visual maps, which are step-by-step sequenced guides incorporating photos, videos, and audio components, to assist caregivers and individuals living with dementia in engaging daily tasks. Much of this work has been conducted in hospital and senior living facilities where patients were managed by professional caregivers and used experimental designs where subjects served as their own controls.3 The present study, to our knowledge, is the first to systematically assess the feasibility and effectiveness of assistive technology using a randomized controlled trial and to focus on family settings of people living at home with ADRD. For the six-week study, family caregivers used visual maps assistive technology (the Experimental group) or watched educational dementia-care videos (the Control group). A primary goal of the study was assessing the feasibility and efficacy of using assistive technology in the home setting. All caregivers were administered net-promoter questions assessing user-satisfaction and willingness to promote their use to others. Additionally, caregivers in both groups were administered standardized behavioral assessments focused on outcomes in the caregiver recipients and the levels of burden caregivers feel in supporting them.
Materials and Methods
Assistive Technology: The MapHabit System
The MapHabit System (MHS) has been described in detail in previous reports and is a commercially available visual mapping software application, resident on encrypted smart tablets, that utilizes visual, audio, and text media to create step-by-step visual guides to assist participants and their caregivers in structuring and accomplishing ADLs (see Figure 1).1 MHS is the assistive technology used in the Experimental group. Control group used encrypted smart tablets to view a pre-loaded series of educational videos focused on all aspects of dementia, including recognizing symptoms and dementia care.
Recruitment, Enrollment, and Consent
The study was carried out in partnership between a leading New York City Alzheimer’s and dementia caregiving organization (CaringKind, New York City Chapter) and a health-care assistive technology company (MapHabit, Inc, Atlanta, GA). Through their social media outreach programs, CaringKind recruited interested ADRD families to the study. Interested families were enrolled by clinical coordinators, who worked with caregivers and their ADRD family members for the duration of the study. Advarra Institutional Review Board (IRB) served as the IRB of record (Pro00039611). The study was registered as a clinical trial with ClinicalTrials.gov (NCT05352529), a registry that meets the requirements of the International Committee of Medical Journal Editors (ICMJE). The study complies with the Declaration of Helsinki regarding the ethical principles of research involving human participants. Electronic informed consent was obtained from the primary caregiver (the legally authorized representative of the ADRD individual) prior to study commencement. Individuals living with ADRD hereafter will be referred to as participants. Each participant was enrolled together with their primary caregiver, who completed the assessment forms from which all the data were derived. Participants used MHS under the supervision of their caregiver.
Study Design and Inclusion and Exclusion Criteria
Participants were individuals diagnosed with mild-to-moderate ADRD. Caregivers and participants were required to be proficient in English and have access to the internet. Participating caregivers needed to be the participant’s primary support partner. A primary support partner was defined as the person who cares for the individual with ADRD who requires assistance with daily activities. Caregivers had to have four or more caregiving-related interactions per day. Examples of such interactions include, but are not limited to, assisting with bathing, dressing, preparing meals, health monitoring, travel, and household chores.
Caregiver-participant dyads (the caregiver and the care recipient) were sequentially assigned to an Experimental group (E) or to a Control group (C), with age and sex balanced (Table 1). A total of 16 dyads completed the study (E=7 dyads; C=9 dyads; Table 2). Caregivers in both groups received encrypted smart tablets, and financial compensation was provided to families for completing the study.
 |
Table 1 Participant Demographics |
 |
Table 2 Participant Enrollment |
Baseline Assessments
Caregivers in both groups completed pre-study behavioral assessments of their participants using the Alzheimer’s Disease Cooperative Study – Activities of Daily Living Inventory (ADCS-ADL), which assesses an individual’s ability to carry out ADLs.4 Additionally, caregivers completed the Margaret Blenker Research Center (MBRC) Caregiver Strain instrument,5 a 14-item questionnaire assessing the various stresses caregivers experienced related to caring for an individual with cognitive impairment. MBRC Caregiver Strain assessment has been an important factor in evaluating the efficacy of interventions for dementia support and caregiving.6,7
Tablet Training
Group E caregivers were trained on how to access a library of visual ADL maps located on their devices and how to customize them for their care recipients. Group C caregivers were trained on how to access a library of educational videos available on their devices, all of which focused on Alzheimer’s disease, dementia care, and caregiver support. Clinical coordinators also facilitated periodic discussion sessions with caregivers in both groups on ADLs, behaviors, or educational videos. This study was carried out amid the COVID pandemic. The study-duration of 6 weeks was based on our unpublished feasibility data collected during the pandemic showing potential dropout of participants for durations longer than 6 weeks.
Post-Study Assessments
At the end of the 6-week period, the ADCS-ADL and MBRC caregiver strain assessments were re-administered to caregivers, along with two additional post-intervention assessments: (1) A net-promoter-type survey consisting of two questions (using a Likert-type scale of 1–5, where a score of 5 was most positive, a score of 3 was neutral, and a score of 1 was most negative) that asked caregivers to rate their overall satisfaction with the MHS, and how strongly they would recommend MHS to others1,2 (2) An 18-item quality-of-life questionnaire (QoL-18) completed by the caregivers to evaluate the status of their care recipient on a range of behaviors (including mood, emotion, independence, and memory) compared to 6 weeks earlier.1,2
Statistical Analyses
For this small-n study, paired group comparisons used the nonparametric Wilcoxon matched-pairs signed ranks test, and unpaired comparisons used the nonparametric Mann–Whitney test. Individual group analyses used the one-sample Wilcoxon signed rank test. All comparisons used 2-tail tests, unless noted otherwise.
Results
Demographic characteristics are provided descriptively in Table 1. Results are reported for the 16 dyads (E=7, C=9) that completed the study. The enrollment data for the study are described in Table 2.
Net Promoter-Type Survey
For each of the caregivers, response scores to each of the two questions were averaged together, and group means were compared to a null score of 3.0 (neutral) in the 5-point scale. Group E’s mean score (3.92) was significantly higher than the null score (p < 0.003). Group C’s mean score (3.38) was not significantly different from the null score (p = 0.088). The difference between the mean scores of the two groups did not reach statistical significance (p = 0.082). However, caregivers in group E endorsed positive scores of “4” and “5” 79% of the time, compared to only 39% of the time for caregivers in the group C.
Activities of Daily Living Inventory
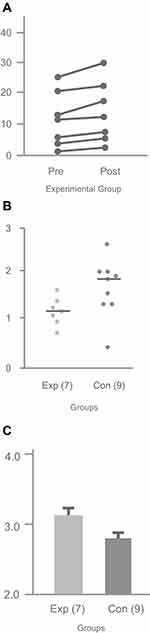
For the ADCS-ADL test, total score can range from 0 to 53, with the higher score indicating stronger performance in independently completing ADLs. We analyzed the mean total scores caregivers reported for their participants in groups E and C, both at baseline and at the end of the 6-week study. The baseline mean score of group E (11.0) did not differ significantly from the baseline mean score of group C (16.7; p = 0.31). At the end of the 6-week study, mean scores between group E (13.6) and group C (16.8) also did not differ statistically. However, it is notable that all scores in group E improved (Figure 2A), while only 6 of the 9 scores in the group C improved. Moreover, for group E, scores were significantly higher at the end of the six-week study (13.6) compared to scores at baseline (11.0; p < 0.015). In contrast, scores for group C did not differ significantly between the two time points: mean score at baseline was 16.7, while mean score at the end of the six-week study was 16.8 (p = 0.78).
MBRC Caregiver Strain
The MBRC Caregiver Strain instrument scoring ranges from 0 to 3 for each item, with the higher score representing larger stress and burden felt by the caregiver. The baseline median scores across the 14 items for groups E and C were not statistically different (group E = 1.21; group C = 1.64; p = 0.19). At the end of the six-week study, caregivers in group E reported significantly lower median scores (1.14), indicating lower reported stress and burden, across the 14 items than caregivers in group C (1.78; p = 0.03; Figure 2B).
Quality of Life
The QOL-18 uses a 5-point Likert scale format, where a higher score indicates a positive change in quality of life (5. Much better, 4. Better, 3. Not much change, 2. Worse, 1. Much worse).8 Figure 1C shows the median scores of the two caregiver groups averaged across the 18 QoL items. Caregivers in group E reported an overall score of 3.21 for their care recipients, significantly higher than the overall score reported by caregivers in group C (2.94, p < 0.01). Additionally, the group E score of 3.21 reported for their care recipients was significant above the null (no change) value of 3 (p = 0.03). Several individual items reached statistical significance in terms of positive change in Group E care recipients: coping, better moments, cooperation (all p < 0.03). By contrast, the overall score reported by group C caregivers of 2.94 was significant below the null (no change) value (p < 0.02), and no individual items reached significance in terms of positive change.
Discussion
With regard to the question of feasibility of using assistive technology in the home setting, the findings were evident: Group E caregivers reported high levels of satisfaction using the visual maps program, and strongly endorsed promoting it to friends and colleagues. By contrast, group C caregivers, who used a series of educational and instructional videos about dementia and dementia care on smart devices, reported substantially lowers satisfaction and endorsement scores.
For group E caregivers, quality of life and ADL scores were improved for their family member care recipients, and the group reported diminished caregiver burden scores. By contrast, for the group C caregivers, quality of life and ADL scores were significantly lower than group E’s. Moreover, the group C’s caregiver strain scores did not exhibit the reduction in burden and stress of caregiving observed in the E group. The finding that the group C’s quality of life scores at the end of the study were reported to be significantly below the null point suggests the possibility that some interventions, although anticipated by researchers or clinicians to be somewhat helpful, may instead be interpreted by participants to be otherwise.
Conclusions
The findings that the use of assistive technology—in this case, the MapHabit visual maps system—was not only embraced by family users but also provided important and effective intervention to help enhance overall quality of life both for individuals living at home with dementia and their family caregivers are encouraging. Nevertheless, the present study did have methodological limitations. Notably, this randomized control trial was conducted during the COVD-19 pandemic. Many of our recruited families were coping with unexpected challenges in managing the care of their family members, and likely impacted the ability of some caregivers to successfully participate and/or complete assessments (Table 2). Accordingly, this study had relatively small number of subjects in groups E and C, a condition possibly contributing to the numerically small although statistically significant differences reported between the groups. The finite number of enrolled dyads additionally limited our ability to meaningfully explore the influence of variables such as age, gender, or additional intellectual disabilities. These points, together with the positive findings reported here, lay the groundwork for more detailed systematic studies of how assistive technology can benefit individuals with cognitive and other intellectual impairments and their family caregivers within a family home setting.
Data Sharing Statement
Data supporting the results reported in the manuscript will be made readily available in a data repository to researchers or publishers who provide a methodologically sound proposal. Proposals should be directed to [email protected]. To gain access, data requestors will need to sign a data access agreement. Data that involves individual participant data will be deidentified. All data will be shared when requests are accepted. Additionally, study protocol, statistical analysis plan, and informed consent form will be made available. The data will be eligible to be accessed immediately following publication, with no end date unless stated otherwise when the proposal is accepted.
Consent for Publication
The authors consent for the journal to publish the details of all materials and content included in the manuscript. Authors are prepared to provide copies of signed consent forms to the journal editorial office if requested.
Acknowledgments
This manuscript and supporting research were made possible in part by the support and participation of families within the ADRD community. We thank Yanan Wang, Emory School of Public Health, Department of Biostatistics and Bioinformatics, for assistance and validation of statistical analyses, Paolo Aguila, and Brittany Montgomery, MapHabit, Inc., for assistance with figure illustrations, and for assistance with all aspects of manuscript preparation, respectively. The recruitment of subjects participating in the study was done independently of MapHabit, Inc., and carried out initially by CaringKind organization, a national support entity for caregivers and individuals with ADRD. Statistical analyses of all data were carried out independently of MapHabit, Inc. by a biostatistical resource department of an academic health center. Other than sharing the costs necessary to carry out the study, neither MapHabit, Inc., nor any MapHabit Inc. employees have any financial link to CaringKind, or to the independent biostatistical resource used in the study.
Funding
This manuscript and supporting research were made possible in part by the National Institute of Aging of the National Institutes of Health under award number 5R43AG065081-02. This includes 48% ($24,000) funded with federal money and 52% ($26,000) in non-government sources. MapHabit, Inc. contributed the 52% in funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
Samuel S. Han, BA, was the Clinical Lead of MapHabit, Inc., the company that developed the assistive technology (the MapHabit System) that is used in this feasibility study. Mr Han received salary from MapHabit, Inc. Mr Han is now enrolled in a master’s program in Social Work at Boston University. Kaylin White, MS, was the Lead Clinical Researcher at MapHabit, Inc., the company that developed the assistive technology (the MapHabit System) that is used in this feasibility study. Ms White received salary from MapHabit, Inc. Ms White is now enrolled in a PhD program in Public Health at Emory University. The authors report no other conflicts of interest in this work.
References
1. Boatman F, Golden M, Jin J, et al. Assistive technology: visual mapping combined with mobile software can enhance quality of life and ability to carry out activities of daily living in individuals with impaired memory. Technol Health Care. 2020;28(2):121–128. doi:10.3233/thc-191980
2. Kelleher J, Zola S, Cui X, et al. Personalized visual mapping assistive technology to improve functional ability in persons with dementia: feasibility cohort study. JMIR Aging. 2021;4(4). doi:10.2196/28165
3. Parker MW, Davis C, White K, Johnson D, Golden M, Zola S. Reduced care burden and improved quality of life in African American family caregivers: positive impact of personalized assistive technology. Technol Health Care. 2022;30(2):379–387. doi:10.3233/thc-213049
4. Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1997;11:33–39. doi:10.1097/00002093-199700112-00005
5. Bass DM, McClendon MJ, Deimling GT, Mukherjee S. The influence of a diagnosed mental impairment on family caregiver strain. J Gerontol. 1994;49(3):S146–S155. doi:10.1093/geronj/49.3.s146
6. Horvath KJ, Trudeau SA, Rudolph JL, Trudeau PA, Duffy ME, Berlowitz D. Clinical trial of a home safety toolkit for Alzheimer’s disease. Int J Alzheimers Dis. 2013;2013. doi:10.1155/2013/913606
7. Bass DM, Noelker LS, Rechlin LR. The moderating influence of service use on negative caregiving consequences. J Gerontol B Psychol Sci Soc Sci. 1996;51(3):S121–S131. doi:10.1093/geronb/51B.3.S121
8. McLeod S. Likert scale definition. Examples and analysis. Simply Psychol. 2019;2019:1.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.