Back to Journals » Journal of Pain Research » Volume 15
The Lighter Side of Pain: Do Positive Affective States Predict Memory of Pain Induced by Running a Marathon?
Authors Anunciação L, Portugal AC , Landeira-Fernandez J, Bajcar EA, Bąbel P
Received 18 May 2021
Accepted for publication 16 September 2021
Published 11 January 2022 Volume 2022:15 Pages 105—113
DOI https://doi.org/10.2147/JPR.S319847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jonathan Greenberg
Luis Anunciação,1 Anna Carolina Portugal,2 J Landeira-Fernandez,1 Elżbieta A Bajcar,3 Przemysław Bąbel3
1Pontifical Catholic University of Rio de Janeiro – Brazil, Rio de Janeiro, 22451-900, Brazil; 2Federal University of Rio de Janeiro, Rio de Janeiro, 22290-902, Brazil; 3Pain Research Group, Institute of Psychology, Jagiellonian University, Kraków, Poland
Correspondence: Luis Anunciação
Pontifical Catholic University of Rio de Janeiro – Brazil, Marques de São Vicente, 225/L201, Gavea, Rio de Janeiro, 22451-900, Brazil
Email [email protected]
Background: Memory and in turn, memory of pain is a reconstructive process. This study considers the relationship between time, memory, affective states, and pain induced by running a marathon by investigating the influence of these factors on a participant’s memory of pain experienced after a marathon. The following two hypotheses were formulated: 1) participants’ recalled-pain of marathon experience is underestimated; and 2) the underestimation of recalled pain would be greater for participants experiencing higher positive affect.
Methods: A longitudinal design was employed to check pain intensities of marathon participants a) at the finish line and b) 6 months following its completion. The sample size was based on a power analysis, and 108 marathonists rated their pain intensities and positive and negative affects at the finish line. From this sample, 58 participants recalled their pain experience of running the marathon 6 months later. Linear models, including computer-based data-mining algorithms, were used.
Results: The experienced pain was higher than their recalled pain (t(55) = 3.412, p < 0.01, d = 0.45), supporting the first hypothesis. The memory of pain faded similarly in all participants, which did not directly support the second hypothesis. Further exploratory analysis suggested that negative and positive affective states were related to participants’ pain memory; positive affective states appeared to be inversely related to the recall (β = − 0.289, p = 0.039).
Discussion: This study shows that time has a significant effect on memory recall and that emotions may also influence the memory of pain. This is the first study that preliminarily showcased the effect of positive affective states on the memory of pain induced by physical exercise.
Keywords: marathon, pain memory, pain, positive affect, negative affect
Introduction
There is ample evidence showcasing that memory retrieval is reconstructive and therefore not always accurate.1 This also applies to pain recollection. Previous studies show that both acute2–9 and chronic pain10–12 might be misremembered. Moreover, the current literature also shows that the way in which pain is remembered influences subsequent pain experiences,13–15 as well as future decisions to engage in activities that may be accompanied by pain.16,17
The majority of studies have analyzed clinical pain associated with an injury, ailments, or medical procedures. Nevertheless, pain can also be experienced daily and caused by activities that people voluntarily engage in and value such as sports like running marathons. People who practice this sport can experience pain, which is accompanied by both negative and positive affective states of varying intensity. Yet, relatively little research has been done on recalling this type of pain.18–21 These previous findings have shown that pain experienced during sports activity was remembered as less intensive18,20,21 or accurately.19 These results are also in line with other studies’ findings on the memory of pain induced by positively valued experiences like giving birth.3,22 However, factors influencing the distortion of pain memory induced by physical exercise have not been thoroughly investigated.
Preceding studies have concluded that the emotional or affective states accompanying pain experiences are significant predictors of pain memory.3,5,8,11,14,15,20,23–29 These studies mostly emphasize the role of negative affect,5,11,14,15,20,23–26,28 whereas some recent studies suggest that positive affect may also be of importance, especially in terms of pain associated with positively valued experiences.3,30 One previous study shows that negative affect experienced after reaching the marathon finish line enabled pain memory predictions. The influence of positive affect on pain memory was statistically non-significant, but a trend was revealed, suggesting the need for further research.20
As there is still uncertainty regarding these factors, this study aims to investigate the influence of time and affective states on participants’ memory of pain induced by running a marathon. Based on previous findings, it was hypothesized that: (1) recalled pain would be underestimated; and (2) the underestimation of recalled pain would be greater for participants experiencing higher positive affect at the finish line. This study and its hypotheses were previously preregistered at https://osf.io/bftqm and http://osf.io/rwuz9/.
Method
Participants
A power analysis was initially conducted using the GPower 3.1 software program to determine the necessary sample to carry out the proposed analyses.31 A total of 45 participants were suggested after defining an a priori effect size of 0.5,20 a one-tailed alpha level of 0.05, and β of 0.95 within a dependent or longitudinal analyses. After considering a sample loss of 20%, a minimum of 54 participants were recommended to keep the study well powered.
A total of 108 marathon runners composed the sample of the first phase of the study. This phase was conducted at the finish line. The second phase was conducted 6 months after the marathon, and 56 participants were present (drop-out rate of 48%).
Overall, 58.3% (n = 63) participants were males and the mean age was 40.4 (SD = 8.9). Contextual variables were also assessed: most runners considered themselves athletes (80%) and were running the marathon alone (65.6%). Table 1 provides descriptive characteristics of the sample presented in both phases.
 |
Table 1 Descriptive Characteristics of Participants |
Instruments and Materials
The 11-point Numeric Rating Scale (NRS) ranging from 0 = “no pain” to 10 = “the worst pain imaginable” was used to measure the intensity of current and remembered pain. Previous evidence of its psychometric properties suggests adequate reliability and validity properties. Moderate-to-high correlations were found between the NRS and other self-report scales, such as the Faces Pain Scale-Revised (FPS-R; r range across studies=0.75 to 0.93), Verbal Rating Scale (VRS) (r range, 0.48 to 0.0.79), and the Color Analogue Scale (CAS) (r range across studies=0.58 to 0.84).32,33 In this study, the test-retest reliability had a correlation coefficient of 0.52 (95% CI 0.30 to 0.69, p < 0.001) and an Intraclass Correlation of 0.68 (95% CI 0.50 to 0.80, p < 0.001).
The Positive and Negative Affect Schedule (PANAS),34 a 20-item self-report scale, was used to measure positive and negative affect. The scale consists of 10 items measuring positive affect and 10 items measuring negative affect. Respondents are asked to rate the extent to which they have experienced each particular emotion within a specified period with reference to a 5-point scale. The scale points are the following: 1 “very slightly or not at all”, 2 “a little”, 3 “moderately”, 4 “quite a bit”, and 5 “very much”. Total scores for each affect range between 0 and 50, and greater values suggest greater emotional experience. Several different timeframes have been used together with the PANAS, but this current study adopted the timeframe “now, right after having completed the marathon”. The reliability of the data was investigated with Cronbach’s alpha for Positive affect of 0.88 (95% CI 0.84 to 0.91) and average inter-item correlation of 0.43. The Cronbach’s alpha for the Negative affect was 0.76 (95% CI 0.7 to 0.83), with an average inter-item correlation of 0.26. The entire scale had a Cronbach’s Alpha of 0.87 (95% CI: 0.83 to 0.9) and average inter-item correlation of 0.2.
Sociodemographic questions were asked and answered by each respondent, including information about age and gender, and if participants considered themselves athletes and were running alone. We hinted at further contact at the end of the questionnaire, and asked if the participants would be willing to share their contact information (email and phone numbers) for the sake of the study’s second stage.
This study was performed in accordance with the 1964 Helsinki Declaration and its later amendments. Ethical approval was obtained from the ethics committee of the Pontifical Catholic University of Rio de Janeiro. Written informed consent was obtained from all participants.
Procedures
Based on previous procedures, the current study was composed of two phases.18–21 The first phase was carried out after the marathonists had finished the marathon, and the second 6 months after completing the run. Marathonists were invited to participate in the study once reaching the finish line of the international marathon of Rio de Janeiro in June 2018; the first and second authors explained the study, its objective, and ethical committee approval. Those who agreed to participate were asked to: (1) rate the intensity of their current pain using the NRS; (2) assess their affective state according to the PANAS, considering what they were feeling at that current moment; and (3) reply to demographic questions, in which phone contacts and e-mail addresses were requested. The e-mail information was used to harmonize the two data sets (T1 and T2) and age and gender variables were used to double-check the computational process.
After six months of this first phase, participants were contacted through their e-mail addresses and invited to reply to an online form. This form was available for a week and composed of demographic questions (such as age and gender) and pain intensity rates. Participants were expected to rate their recalled level of pain, namely the pain they felt immediately after they reached the finish line six months ago. At this time, it was emphasized that they were being asked to recall and describe how they remembered the pain they felt during the first phase of the study, rather than to recall how they had rated the pain in the first study phase.
Statistical Analysis
Descriptive statistics were used to summarize the data. Categorical variables were reported as absolute values (counts) and proportions (%), and continuous variables were reported as means (M) and standard deviations (SD). A summary score was computed and included the 10 positive items of PANAS and its counterpart negatives. Following some literature recommendations, a balanced affective state was scored by subtracting the negative score from the positive one35–38 to use as descriptive indicator of the overall affective status of the marathonists.
A paired t-test was carried out to test the first hypothesis and the second hypothesis was formally tested using a Linear-Mixed Effect Model (LMM). In this later model, the recalled memory (dependent variable) was regressed on the baseline results, considering time, positive affects, and their interaction (Time x Positive affect). Participants were defined as random effect. Therefore, a different intercept value was included for each participant, with values based on each participant’s baseline level.39
A computational-based analysis was subsequently conducted using a genetic algorithm, i.e., a data mining procedure, to further explore the predictors of the recalled memory. All possible models were fitted and ranked through the results of the corrected Akaike Information Criterion (AICc). The model with the lower AICc was defined as the final model and its coefficients were estimated.40
The significance level was set at 0.05 and multiple comparisons were not performed. All analyses were conducted in the R 4.0 software program41 using the following packages: tidyverse,42 lme4,43 lmerTest, and glmulti.40 All data, notebooks, and codes are freely available on an online open repository. The hypothesis and the statistical procedure to check them were also preregistered at https://osf.io/rwuz9/.
Results
The second phase dropout rate was 48.1%. We compared sociodemographic characteristics of participants who participated in phase one and dropped out during phase two to rule out the assumption that the achieved difference was related to participant demographic features. Despite the dropout rate, the results revealed that experienced pain was not different between both groups (t(106) = 0.979, p = 0.329). Positive affect (t(106) = 1.287, p = 0.201), negative affect (t(106) = −0.925, p = 0.357), age (t(74) = 0.408, p = 0.684), and sex (X2 (1) = 0.067, p = 0.794) were also not statistically different.
The first hypothesis tested the difference between the experienced and recalled pain and its relationship with time. The experienced pain (M = 6.64, SD = 2.84) was significantly higher than the recalled pain with a small to medium effect size (M = 5.79, SD = 2.57; t(55) = 3.412, p < 0.001, d = 0.45). This result suggests that the participants underestimated their experienced pain in a small to moderate effect size (Table 2). The marathonists also finished the marathon with a higher positive affect, as their positive affect was significantly higher than negative affect (t(107) = 25.868, p < 0.01, d = 3.3).
 |
Table 2 Differences Between Experienced and Recalled Pain and Affective States |
The second hypothesis assumed that the underestimation of recalled pain would be greater for participants with higher positive affect at the finish line. If this hypothesis were to hold, the interaction term between the positive affect and the experienced pain score would be statistically significant. A linear mixed model was performed by specifying a separate intercept for each participant and further concluded that the relationship was not significant (b = −0.001, p = 0.974). Table 3 presents the results.
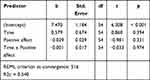 |
Table 3 Linear Mixed Model Results |
After testing the above preregistered hypothesis, an exploratory data analysis was performed to check whether variables could predict the recalled pain. The variable selection was based on a data mining algorithm. About 60% of the variance was accounted for by six variables in model (F(6,28) = 6.66, p < 0.001), presented in Table 4.
 |
Table 4 Exploratory Results |
In this model, the pain intensity experienced after completing the marathon was the most influential predictor of recalled memory (β = 0.620, p < 0.001). Negative and positive affective states were particularly related to the outcome, with negative affect proportionally related to the recalled memory. Thus, the higher the negative affect, the more intense the recalled pain was (β = 0.316, p = 0.029). Conversely, positive affect was inversely related to memory recall (β = −0.289, p = 0.039). Moreover, participants running a marathon for their first time recalled more intense levels of pain in comparison to a veterans’ recalled pain (β = 0.307, p = 0.039). Running alone or self-reporting as an athlete did not reach significance. Figure 1 shows the main results.
 |
Figure 1 Significant predictors of the memory of pain. |
Discussion
This study sought to investigate the influence of time and affect on the participants’ pain memory induced by running a marathon. One of the findings is that pain induced by running a marathon is misremembered. After a six-month delay, the pain was remembered as less intense than it had initially been, which is convergent to the first hypothesis. In turn, the pain memory faded similarly in all participants, regardless of whether they experienced a high or low level of positive affect after reaching the marathon line. This latter data did not directly support the second hypothesis. Further explanatory secondary analyses, to a lesser degree, suggested that both positive and negative affect experienced upon completing the marathon influenced the memory of pain induced by running on certain occasions.
Previous studies have shown that pain memory could be accurate,26,27,44–46 but is often overestimated.6,10,14,24 Although possible, underestimating past pain is rare, and seems to apply to acute but not chronic pain.8,28,47–49 It has been suggested that the positive context of a painful experience may be an essential factor contributing to the underestimation of the memory of pain.3 Indeed, an underestimation of the memory of pain was found for labor pain3,50 and pain induced by physical exercise.18,20,21 The current study is in line with these precedent findings, that pain induced by running a marathon was underestimated over time.
In the present study, a distortion of pain memory was observed six months after completing a marathon. This result is in line with the previous study.20 However, other findings show that the memory of pain induced by physical exercise may be distorted much earlier, after three months20 or even one month.21 One study suggests that changes in the memory of pain induced by physical effort can start about a week after the pain.21 Thus, it seems that the memory of pain induced by physical exercise is underestimated, regardless of the length of delay between the pain experience and its recall.
The role of experienced pain and negative affect as predictors of acute pain recall is emphasized in the model proposed by Gedney and Logan.24 According to this model, the intensity of the experienced pain predicts the memory of pain when the period of recall delay is relatively short. Over time the importance of the affective factor increases – affect associated with pain experience provides cues for the reconstruction of pain memories. Our data partially agreed with these authors, as they showed that the pain felt after reaching the marathon line, as well as positive and negative affect experienced at that moment predicted the memory of pain induced by running a marathon.
Previous studies investigating the memory of pain induced by running a marathon confirmed the experienced pain’s predictive role.20,21 One of these studies also showed that the relationship between experienced and recalled pain was mediated by pain present at the moment of recall.21 Another study also corroborates the impact of negative affectivity on the memory of pain induced by physical exercise, wherein negative affect and pain resulting from running predicted the memory of pain three and six months after the marathon.20 However, the current study suggests that the third factor, ie positive affect, unaddressed in the model of acute pain recall,24 should preliminarily be considered a predictor of the memory of this type of pain.
In the current study, the forgetting trend was not particularly salient for those participants experiencing higher positive emotional states after the marathon. Therefore, in our study, the second hypothesis was not supported by the data. To a lesser extent, a data mining process was conducted and revealed that positive and negative affective state had significant results after statistical controls were implemented. Although this is a secondary outcome, data demonstrated that positive affect was found to influence the recall. The obtained data showed that the negative affect was proportionally related, while the positive affect was inversely related to pain memory. As standardized coefficients are measures of effect size, we suggest that negative affect has a stronger influence than its positive counterpart on memory recall.51 We stress that this result does not alter or challenge the lack of support found for this hypothesis in the primary analyses, however, the exploratory analysis suggested that positive affect might contribute to an underestimation in the memory of pain induced by physical exercise.
The effect of positive affect on the memory of pain was previously shown for pain resulting from experimental stimulation,8 labor,3 dental procedures,9,27 migraine,46 and surgery.29 Accordingly, positive affect influences the memory of pain experienced due to positive events, such as physical exercise or giving birth, and negative and uncontrolled ones, such as migraine.52 However, it may be of particular importance in memory of pain associated with positive events.
Running a marathon, like giving birth, might lead to a significant increase in beta-endorphin and oxytocin concentration, which can modify the encoding of negative aspects in these experiences. It is hypothesized that oxytocin can inhibit the action of the central nucleus of the amygdala (CeA), which is involved in memory consolidation.30 It has also been found that post-training administration of sub-analgesic doses of beta-endorphin causes retrograde amnesia.53
In the light of these data, the question arises of whether participants’ memory of pain induced by physical exercise was distorted or rather participants did not have enough opportunity to encode and consolidate their pain-related experiences. This study demonstrates the need for further research on the memory of pain which accompanies positive events.
Interestingly, participants who run a marathon for the first time recalled pain induced by running as more intense than veterans did. It seems this could also be related to the affect accompanying this experience. Those who have run a marathon several times might have found this experience predictable and more controllable than first-time runners, which might positively influence their mood. In turn, positive affect could impact processing the pain-related information. This result indicates that both expectancies and emotions related to the potentially painful situation might affect the memory of pain, and future research should consider the contribution of both cognitive and affective factors to the distortion of pain memory. No differences between male and females were found, which may be explained by the distance and length of a marathon. Previous evidence has shown that the longer the distance, the less of a gap between men and women.54
Some limitations of the study should be addressed. First, the observational design does not allow commenting on causality. Second, this study’s results should be generalized with caution to pain induced by other modes of physical exercise. Third, neither affect nor pain experienced at the moment of recall was controlled for. Previous studies showed that these factors might affect pain memory.3,21 Therefore, we cannot rule out that participants underestimated the experienced pain because they presented positive affect when replying to the survey. We also highlight that the positive and negative emotional valence relied on exploratory analyses, and future studies on the effect of mood on the memory of pain is required to check the stability of this finding.
In sum, this study appears to be the first showing that positive affect predicts the memory of pain induced by physical exercise. This study and previous studies on other types of pain show that positive and negative emotions accompany pain, and both of them influence the memory of pain. Thereby, this study’s results provide further evidence to extend the model of acute pain recall.24 The obtained result also confirms previous findings showing that pain induced by physical effort is remembered as less intense. However, more research is needed to investigate whether the memory of pain induced by physical exercise becomes distorted over time, or whether the negative aspects of this painful experience are not encoded.
Conclusion
Memory in general, and memory of pain specifically, is a reconstructive process in which biases, distortions, and errors are present. In this study, we explored the influence of time and affective states on participant’s memory of pain induced by running a marathon. The results mainly strengthen previous findings delimitating that pain induced by physical effort is remembered as less intense. The results of this study suggest that positive affect along with negative affect may be considered a predictor of pain memory distortion.
Acknowledgments
Elżbieta A. Bajcar and Przemysław Bąbel are supported by the grant no. 2016/23/B/HS6/03890 from the National Science Centre, Poland. The open access license of the publication was funded by the Priority Research Area Society of the Future under the programme “Excellence Initiative – Research University” at the Jagiellonian University in Krakow.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Gazzaniga MS, Halpern DF. Psychological Science (Fifth Edition). W. W. Norton & Company; 2015.
2. Aksoy H, Yücel B, Aksoy U, Acmaz G, Aydin T, Babayigit MA. The relationship between expectation, experience and perception of labour pain: an observational study. Springerplus. 2016;5(1):1766. doi:10.1186/s40064-016-3366-z
3. Bąbel P, Pieniążek L, Zarotyński D. The effect of the type of pain on the accuracy of memory of pain and affect. Eur J Pain. 2015;19(3):358–368. doi:10.1002/ejp.554
4. Daoust R, Sirois M-J, Lee JS, et al. Painful memories: reliability of pain intensity recall at 3 months in senior patients. Pain Res Manag. 2017;2017:1–7. doi:10.1155/2017/5983721
5. Kyle BN, McNeil DW, Weaver B, Wilson T. Recall of dental pain and anxiety in a cohort of oral surgery patients. J Dent Res. 2016;95(6):629–634. doi:10.1177/0022034516631977
6. McNeil DW, Helfer AJ, Weaver BD, Graves RW, Kyle BN, Davis AM. Memory of pain and anxiety associated with tooth extraction. J Dent Res. 2011;90(2):220–224. doi:10.1177/0022034510385689
7. Mentula M, Kalso E, Heikinheimo O. Same-day and delayed reports of pain intensity in second-trimester medical termination of pregnancy: a brief report. Contraception. 2014;90(6):609–611. doi:10.1016/j.contraception.2014.06.031
8. Bąbel P. The effect of positive affect on the memory of pain. Pain Manag Nurs. 2017;18(3):129–136. doi:10.1016/j.pmn.2017.02.198
9. Bąbel P, Krzemień M. Memory of dental pain induced by tooth restoration. Stud Psychol. 2015;53(1):5–17. doi:10.2478/V10167-010-0116-9
10. Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. Pain. 2008;139(1):146–157. doi:10.1016/j.pain.2008.03.024
11. Bryant RA. Memory for pain and affect in chronic pain patients. Pain. 1993;54(3):347–351. doi:10.1016/0304-3959(93)90036-O
12. Lefebvre JC, Keefe FJ. The effect of neuroticism on the recall of persistent low-back pain and perceived activity interference. J Pain. 2013;14(9):948–956. doi:10.1016/j.jpain.2013.03.006
13. Chen E, Zeltzer LK, Craske MG, Katz ER. Children’s memories for painful cancer treatment procedures: implications for distress. Child Dev. 2000;71(4):933–947. doi:10.1111/1467-8624.00200
14. Gedney JJ, Logan H. Pain related recall predicts future pain report. Pain. 2006;121(1):69–76. doi:10.1016/j.pain.2005.12.005
15. Noel M, Chambers CT, McGrath PJ, Klein RM, Stewart SH. The influence of children’s pain memories on subsequent pain experience. Pain. 2012;153(8):1563–1572. doi:10.1016/j.pain.2012.02.020
16. Kahneman D, Fredrickson BL, Schreiber CA, Redelmeier DA. When more pain is preferred to less: adding a better end. Psychol Sci. 1993;4(6):401–405. doi:10.1111/j.1467-9280.1993.tb00589.x
17. Redelmeier DA, Katz J, Kahneman D. Memories of colonoscopy: a randomized trial. Pain. 2003;104(1–2):187–194. doi:10.1016/S0304-3959(03)00003-4
18. Anunciação L, Landeira-Fernandez J. Porcelanas inquebráveis: dor, afeto e memória em corredores [Unbreakable porcelain: pain, affection, and memory in runners]. Rev Bras Prescrição e Fisiol Do Exerc. 2017;11(64):63–73. Portuguese.
19. Anunciação L, Portugal AC, Landeira-Fernandez J. A elegia da lembrança impossível: dor, memória e afeto em corredores de longa distância [The elegy of impossible remembering: pain, memory and affection in long distance runners]. Sci Cognit. 2018;23(2):227–236. Portuguese. doi:10.5281/zenodo.2543016
20. Bąbel P. Memory of pain induced by physical exercise. Memory. 2016;24(4):548–559. doi:10.1080/09658211.2015.1023809
21. Bąbel P, Bajcar EA, Śmieja M, et al. Pain begets pain. When marathon runners are not in pain anymore, they underestimate their memory of marathon pain–A mediation analysis. Eur J Pain. 2018;22(4):800–809. doi:10.1002/ejp.1166
22. Niven CA, Murphy‐Black T. Memory for labor pain: a review of the literature. Birth. 2000;27(4):244–253. doi:10.1046/j.1523-536x.2000.00244.x
23. Gedney JJ, Logan H, Baron RS. Predictors of short-term and long-term memory of sensory and affective dimensions of pain. J Pain. 2003;4(2):47–55. doi:10.1054/jpai.2003.3
24. Gedney JJ, Logan H. Memory for stress-associated acute pain. J Pain. 2004;5(2):83–91. doi:10.1016/j.jpain.2003.11.005
25. Rocha EM, Marche TA, Von Baeyer CL. Anxiety influences children’s memory for procedural pain. Pain Res Manag. 2009;14(3):233–237. doi:10.1155/2009/535941
26. Bąbel P. The influence of state and trait anxiety on the memory of pain. Pain Med. 2017;18(12):2340–2349. doi:10.1093/pm/pnw354
27. Bąbel P. The effect of affect on memory of pain induced by tooth restoration. Int Dent J. 2014;64(5):246–251. doi:10.1111/idj.12115
28. Eli I, Baht R, Kozlovsky A, Simon H. Effect of gender on acute pain prediction and memory in periodontal surgery. Eur J Oral Sci. 2000;108(2):99–103. doi:10.1034/j.1600-0722.2000.00777.x
29. Halicka M, Bąbel P. Factors contributing to memory of acute pain in older adults undergoing planned and unplanned hip surgery. Clin J Pain. 2018;34(6):543–551. doi:10.1097/AJP.0000000000000568
30. Farley D, Piszczek Ł, Bąbel P. Why is running a marathon like giving birth? The possible role of oxytocin in the underestimation of the memory of pain induced by labor and intense exercise. Med Hypotheses. 2019;128:86–90. doi:10.1016/j.mehy.2019.05.003
31. Mayr S, Erdfelder E, Buchner A. G Power 3.1 manual. Tutor Quant Methods Psychol. 2017;3:51–59.
32. Thong ISK, Jensen MP, Miró J, Tan G. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain. 2018;18(1):99–107. doi:10.1515/sjpain-2018-0012
33. Castarlenas E, Jensen MP, von Baeyer CL, Miró J. Psychometric properties of the numerical rating scale to assess self-reported pain intensity in children and adolescents. Clin J Pain. 2017;33(4):376–383. doi:10.1097/AJP.0000000000000406
34. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi:10.1037/0022-3514.54.6.1063
35. Kormi-Nouri R, Farahani M-N, Trost K. The role of positive and negative affect on well-being amongst Swedish and Iranian university students. J Posit Psychol. 2013;8(5):435–443. doi:10.1080/17439760.2013.823511
36. Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–265. doi:10.1348/0144665031752934
37. Liu Y, Wang Z, Lü W. Resilience and affect balance as mediators between trait emotional intelligence and life satisfaction. Pers Individ Dif. 2013;54(7):850–855. doi:10.1016/j.paid.2012.12.010
38. Kanjo E, Kuss DJ, Ang CS. NotiMind: utilizing responses to smart phone notifications as affective sensors. IEEE Access. 2017;5:22023–22035. doi:10.1109/ACCESS.2017.2755661
39. Stroup WW. Generalized Linear Mixed Models - Modern Concepts, Methods and Applications. Boca Raton: CRC Press; 2012. doi:10.1201/b13151
40. Calcagno V, de Mazancourt C. glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Softw. 2010;34(12). doi:10.18637/jss.v034.i12
41. R Development Core Team. R Development Core Team, R: A Language and Environment for Statistical Computing; 2020.
42. Wickham H. Tidyverse: easily install and load “Tidyverse” packages; 2016. Available from: https://cran.r-project.org/package=tidyverse.
43. Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1). doi:10.18637/jss.v067.i01
44. Lefebvre JC, Keefe FJ. Memory for Pain: the relationship of pain catastrophizing to the recall of daily rheumatoid arthritis pain. Clin J Pain. 2002;18(1):56–63. doi:10.1097/00002508-200201000-00009
45. Hovasapian A, Levine LJ. Reappraisal mitigates overestimation of remembered pain in anxious individuals. Cogn Emot. 2016;30(6):1222–1231. doi:10.1080/02699931.2015.1049937
46. Bąbel P. Memory of pain and affect associated with migraine and non-migraine headaches. Memory. 2015;23(6):864–875. doi:10.1080/09658211.2014.931975
47. Fors EA, Götestam KG. The accuracy of memory for acute pain induction: an experimental study. Int J Rehabil Health. 1996;2(4):253–263.
48. Rode S, Salkovskis PM, Jack T. An experimental study of attention, labelling and memory in people suffering from chronic pain. Pain. 2001;94(2):193–203. doi:10.1016/S0304-3959(01)00356-6
49. De Pascalis V, Cacace I, Massicolle F. Focused analgesia in waking and hypnosis: effects on pain, memory, and somatosensory event-related potentials. Pain. 2008;134(1–2):197–208. doi:10.1016/j.pain.2007.09.005
50. Lowe NK, Roberts JE. The convergence between in-labor report and postpartum recall of parturition pain. Res Nurs Health. 1988;11(1):11–21. doi:10.1002/nur.4770110104
51. Bisby JA, Burgess N. Negative affect impairs associative memory but not item memory. Learn Mem. 2013;21(1):760–766. doi:10.1101/lm.032409.113
52. Arruda MA, Arruda R, Landeira-Fernandez J, Anunciação L, Bigal ME. Resilience and vulnerability in adolescents with primary headaches: a cross-sectional population-based study. Headache. 2021;61(3):546–557. doi:10.1111/head.14078
53. Izquierdo I, Dias RD, Souza DO, Carrasco MA, Elisabetsky E, Perry ML. The role of opioid peptides in memory and learning. Behav Brain Res. 1980;1(6):451–468. doi:10.1016/0166-4328(80)90001-7
54. Temesi J, Arnal PJ, Rupp T, et al. Are females more resistant to extreme neuromuscular fatigue? Med Sci Sport Exerc. 2015;47(7):1372–1382. doi:10.1249/MSS.0000000000000540
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
