Back to Journals » Infection and Drug Resistance » Volume 14
Magnitude of Surgical Site Infections, Bacterial Etiologies, Associated Factors and Antimicrobial Susceptibility Patterns of Isolates Among Post-Operative Patients in Harari Region Public Hospitals, Harar, Eastern Ethiopia
Authors Shakir A, Abate D, Tebeje F, Weledegebreal F
Received 20 July 2021
Accepted for publication 23 September 2021
Published 5 November 2021 Volume 2021:14 Pages 4629—4639
DOI https://doi.org/10.2147/IDR.S329721
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Anwar Shakir, 1 Degu Abate, 2 Fikru Tebeje, 2 Fitsum Weledegebreal 2
1Department of Medical Laboratory Sciences, Jugal Regional Hospital, Harar, Ethiopia; 2Department of Medical Laboratory Sciences, College of Health and Medical Science, Haramaya University, Harar, Ethiopia
Correspondence: Fitsum Weledegebreal Email [email protected]
Background: Surgical site infections (SSIs) are infections that occur one month after a surgical operation or one year after implant surgery and a surgical procedure, either at the injury site or near the injury site. Surgical site infections are still a major global problem, especially in developing countries, where they cause increased morbidity and mortality. There is a dearth of information regarding SSIs in the eastern Ethiopia, particularly in this study area.
Objective: This study aimed to assess the prevalence of SSIs, bacterial etiologies, associated factors, and antimicrobial susceptibility patterns of isolates among post-operated patients admitted to public hospitals in the Harari Region, eastern Ethiopia.
Methods: A cross-sectional study was conducted among 306 patients who had undergone surgery. A pre-tested structured questionnaire was used for assessing the sociodemographic and clinical factors. Following standard microbiological techniques, wound swabs and pus specimens were collected and transported to Harar Health Research and Regional Laboratory for isolation, identification of bacteria, and antibiotic susceptibility test. Data were double entered onto Epi Data version 3.5.1 software and transferred to Statistical Package for the Social Sciences version 20.0 for analysis. P-value < 0.05 was declared as statistical significant.
Results: In this study, the overall prevalence of surgical site infection was 11.8% (95% CI: 8.3– 15.4%) and Staphylococcus aureus (30.3%) was the most frequent isolate. Both S. aureus and coagulase negative Staphylococci were 100% resistant to penicillin. Wound with drain (AOR = 24.538; 95% CI: 10.053– 59.898), being diabetic patient (AOR = 7.457, 95% CI 2.893– 19.221), age > 60 years (AOR = 4.139, 95% CI 1.278– 13.40), surgical procedure duration of more than 2 hours (AOR = 0.159, 95% CI 0.040, 0.630), being alcohol drinker (AOR = 2.58, 95% CI 1.091– 6.102) and having dirty surgical wound (AOR = 9.026; 95% CI: 3.503– 23.255) were factors significantly associated with SSIs.
Conclusion: In this study, single and multiple drug resistance to the commonly used antibiotics was high. Therefore, intensifying the implementation of infection prevention and patient safety measures and identifying an etiological cause may minimize the burden.
Keywords: surgical site infections, bacterial etiologies, associated factors, eastern Ethiopia
Corrigendum for this paper has been published
Introduction
Surgical site infections (SSIs) are infections that occur one month after a surgical operation or one year after implant surgery and a surgical procedure, either at the injury site or near the injury site.1 The Centers for Disease Control and Prevention (CDC) classifies SSIs into three types: superficial infections, deep incisional infections, and infections involving organs or body spaces. The degree of surgical site contamination at the time of surgery affects the likelihood of SSI. Wounds are classified as clean, clean-contaminated, contaminated, or dirty or infected based on the presence and degree of contamination.2 Studies of the epidemiology of SSIs are problematic due to the diverse nature of these surgical infections. The prevalence differs widely between surgical procedures, hospitals, patients, and surgeons.3
Surgical site infections are likely caused by endogenous or exogenous microorganisms. Most SSIs are caused by endogenous microorganisms that appear on the patient’s skin when the surgical incision is made. Gram-positive bacteria, for instance, Staphylococcus aureus are the most predominant causative skin-dwelling microorganisms. Surgical site infections are possibly caused by organisms within the patient’s body that are exposed during surgery. Causative pathogens depend on the surgical site; for example, the risk of developing SSI from enteric gram-negative microorganisms increases with surgery on the gastrointestinal tract.4
Several associated factors have been identified in the literature but the studies are not reproducible. Despite this fact, various authors have repeatedly identified, such as male gender, advanced age and significant blood loss5–8 were highly associated with SSIs. Other risk factors for SSI are typically separated into patient-related (pre-operative), procedure-related (peri-operative), and post-operative categories. Generally, patient-related risk factors for the development of SSI can be categorized as either unmodifiable or modifiable. Changeable patient-related risk factors include poorly controlled diabetes mellitus, obesity, tobacco use, use of immunosuppressive medications, and length of preoperative hospitalization. Procedure-related risk factors include wound class, length of surgery, and shaving of hair, hypoxia, and hypothermia. Unmodifiable or modifiable, like age and sex.9 Although few types of research have been conducted on surgical site infections in Northern, Southern, and Western parts of Ethiopia, there is limited information regarding SSIs in the Eastern part of the country. Therefore, this study was aimed to determine the magnitude, distribution of bacterial pathogens isolated from post-operative wounds associated factors and their antimicrobial susceptibility profiles in public hospitals in Harar town, Ethiopia.
Methods and Materials
Study Area and Period
The study was conducted in two public hospitals: Jugal General Hospital and Hiwot Fana Specialized University Hospital, found in Harar town. Harar town is located 526 km away from Addis Ababa, the capital city of Ethiopia. In the region, there are two public hospitals, one Police hospital, two private general hospitals, and eight health centers, and 26 health posts. Jugal Hospital was founded in 1904 G.C. It is a general hospital with 125 beds, and Hiwot Fana Specialized University Hospital (HFSUH), the referral teaching hospital, was established during the occupation of Ethiopia by Italian soldiers (1928–1933) with 250 beds. Currently, both hospitals provide health-care services to more than 5 million people around Harar and neighboring regions like Oromiya Regional State, Dire Dawa Administrative Council, and Ethiopia Somali Regional State and are delivering different health services to the community.10
The hospitals were selected based on provision of major surgical service in the town. The data were collected from March 16 to April 25, 2020.
Study Design and Population
An institutional-based cross-sectional study design was conducted. Three hundred six patients who were admitted and undertaken surgery either as elective or emergency surgical procedures in both hospitals during the study period were included. Patients who had another operation before a month and developed infection prior to the study period, very ill or weak patients and patients who developed SSIs and were taking antibiotics following the operation were excluded from the study.
Sample Size Determination and Sampling Technique
The sample size of the study was determined using the single population proportion formula by considering the prevalence (p) of SSI (75%) from a study carried out in Ayder Referral and Teaching Hospital, Mekelle,11 at 95% confidence interval (CI), 5% margin of error (d) and 10% non-response rate. Therefore, the final sample size was found to be 317. Study participants were enrolled conveniently from both hospitals until the required sample size was fulfilled.
Data Collection Methods
Physical Examination, Specimen Collection, and Transportation
Physical examination of the patients were assessed by a surgeon and confirmed of developing SSIs based on the presence of at least one of the next signs or symptoms of infection within 30 days of surgery: (a) pain, tenderness, localized swelling, redness, heat or purulent discharge (b) evidence of abscess or fever of >38°C in infections of the deep incision; (c) localized pain or tenderness with an organism isolated from an organ/space infection,2 were considered for bacteriological analysis. From the patients who confirmed for SSIs pus swabs and aspirates were aseptically collected from surgical sites before cleansing the wound with antiseptic solutions and were placed in sterile up to 5 mL capacity test tubes and transferred to the microbiology laboratory within 30 minutes after collection. The following reception in the laboratory and the specimens were registered. All specimens were also processed for culturing and Gram-staining.12
Bacterial Isolation and Identification
Aseptically collected sample was inoculated into blood agar, MacConkey agar, and Mannitol salt agar (Oxoid, LTD, UK) by using the standard streak plate technique.12 The plates were incubated in an anaerobic atmosphere at 37°C for 24–48 hours. Growth of bacteria on media confirmed based on their colony morphology (like; mucoid, raised, white, small, large), pigment production (pyocyanine, pyoverdin), hemolysis (beta-hemolysis, alpha-hemolysis, gamma-hemolysis), biochemical tests (fermenting lactose, mannitol, glucose, sucrose) and motility characteristics.12 Organisms that grew on both blood agar and Mannitol salt agar were suspected to be Gram-positive bacteria as Mannitol salt agar is selective media for Staphylococci. Catalase test was performed to differentiate staphylococci from streptococci in which catalase-negative results exclude Streptococci species. Further, a coagulase test was performed to differentiate S. aureus from other species of genus Staphylococci, which are coagulase-negative.12
Bacteria that grew on blood agar and MacConkey agar were suspected to be Gram-negative as MacConkey agar is selective to gram-negative bacteria. Colonies on MacConkey agar were differentiated based on their characteristics to ferment lactose. Pink color characterizes lactose fermenters whereas colorless colonies were non-lactose fermenters. Gram-negative bacteria were further tested for their motility and characterized using arrays of biochemical tests including indole, urea, Triple Sugar Iron agar (TSI), Simmon’s Citrate agar, and Lysine Decarboxylase (LDC). Colonies that produced pigment on blood agar and non-lactose fermenter on MacConkey agar were tested using oxidase to confirm P. aeruginosa, which is oxidase-positive bacterium.12
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility test of the isolates was conducted according to the Clinical Laboratory Standards Institute guidelines,13 using the Kirby-Bauer disk diffusion method on Muller–Hinton agar (OXOID, Basingstoke, United Kingdom).
In brief, four to five bacterial colonies of the same morphological type were selected and suspended into 5mL of sterile normal saline (0.85% NaCl) or nutrient broth (for direct inoculation method) and the turbidity was adjusted to match that of 0.5 McFarland standards to obtain approximately a colony count of 107 or 108 colony-forming units (CFU) per mL. A sterile swab was dipped into the suspension and the excess of inoculums was removed by pressing it against the sides of the tube. Then, the swab was applied to the center of the Mueller–Hinton agar plat and evenly spread onto the medium to obtain confluent growth using the same swab. For antimicrobial susceptibility testing of Streptococci, 5% defibrinated sterile sheep blood was aseptically added to Mueller–Hinton medium.12
Antimicrobial discs (Oxoid, Ltd, UK) including Amoxicillin-clavulanic acid (30µg), Ampicillin (10ʋg) Penicillin (30µg), Ceftriaxone (30µg), Gentamicin (10µg), Ciprofloxacin (5µg), Norfloxacin (10µg), and Trimethoprim sulphamethoxazole (25µg) were placed on the inoculating plates and incubated at 37°C for 18–24 hours.13 In this regard, these antibiotic disks represent commonly prescribed antibiotics for the treatment of SSIs in the study area.
The plates were allowed to dry for 3–5 minutes before putting the antimicrobial disks. Antibiotic discs were placed equidistantly at least 24 millimeters away from each other and 15 millimeters from the edge, to avoid the overlapping zone of inhibition, on to 150mm Petri dish Muller–Hinton agar seeded with each isolate and gently pressed onto the medium with the help of sterile forceps to ensure complete contact with the agar surface and was incubated for 24 hours at 35–37°C. Using a digital or manual caliper, the isolates diameter of the zone of inhibition around the disc was measured to the nearest millimeter and then was classified as sensitive, intermediate, and resistant according to Clinical Laboratory Standard Institute guidelines.12,13
Quality Control
The English language questionnaire was first translated into the local languages (Amharic and Afan Oromo) by Language experts and was then back translated to English to maintain consistency. And, it was pretested on patients (5% of the sample size) who were admitted at Harar General Hospital before commencing the actual data collection. During pretesting, additional information was gathered and vague terms, phrases, and questions were modified. The principal investigator gave training to the data collectors about the procedures of interview and wound swab/pus aspirate sample collection i and on how to clean, and sterilize reusable laboratory materials for laboratory attendants for two days. A manufacturer’s instruction was followed during culture media preparation and sterilization. Sterility of culture media was checked by incubating 3–5% of the batch at 37°C overnight and observed for growth. Culture media that showed any growth was discarded and replaced by a new sterile batch. In addition, laboratory analysis was carried out using standard operating procedures (SOPs) and under the close supervision of experienced microbiologists. First the actual work, reagents were checked for proper functioning. Reference strains of E. coli (ATCC-25922), S. aureus (ATCC-25923), and P. aeruginosa (ATCC-27853) were used as quality control throughout the study for antimicrobial susceptibility testing. All the strains were obtained from the Ethiopian Public Health Institute (EPHI). The inoculums density for bacterial suspension for the antimicrobial susceptibility testing was standardized to 0.5McFarland standard for bacteriological examination. Besides, supervision was undertaken during the whole phase of the study period by the investigator and; thereby, any doubt was cleared on the spot.
Data Analysis
Data were coded and double entered into EPI Info version 3.5.1 and exported to SPSS version 20.0 for analysis. Descriptive statistics was used to assess the frequency distribution of variables. Binary logistic regression has been employed to assess the association of dependent variable with independent variables and to determine predictors of SSI using odds ratios with 95% confidence intervals (CIs). Independent factors with P-value less than 0.25 in the bivariate binary logistic regression analysis were further tested via multivariable binary logistic regression to account for potential confounding. Finally, every variable with P-values less than 0.05 at a 95% confidence interval in the multivariable logistic model was considered statistically significant.
Ethical Consideration
Ethical approval was secured from Haramaya University College of Health and Medical Sciences Institutional Health Research Ethics Review Committee (IHRERC). This study was conducted in accordance with the Declaration of Helsinki. A formal support letter was submitted to Jugal and Hiwot Fana Specialized University hospitals, and the purpose of the study was explained to the hospital head and each participant including objectives, procedures, potential risks, and benefits of the study. Informed, voluntary, written and signed consent was obtained from the head of the hospital, all adult respondents and a parent or legal guardian of participants for under 18 years of age throughout the study. The study participants were informed of their rights to refuse or withdraw from the study at any time and refusing to participate in the study did not affect each study participant. Confidentiality of participants’ information was assured, in such away that the data collection format was prepared anonymous. Any information acquired from participants during the study was kept confidential. Result of positive patients was communicated for their health-care professionals for getting the required treatment.
Result
Socio-Demographic Characteristics
In this study, a total of 306 study participants were included with a response rate of 96.5%, the age of the study participants ranged from 4 to 80 years with mean age (±SD) of 29.9 (±10.61) years and 59.2% were females. About 51.9%, 65.7%, 67%, and 66.7% of the participants were from urban areas, had got formal education, married, and employed, respectively (Table 1).
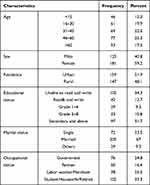 |
Table 1 Socio-Demographic Characteristics of Study Participants Attending Jugal and Hiwot Fana Specialized University Hospitals, Harar, Ethiopia, 2020 (n = 306) |
Behavioral and Clinical Related Factors
Among the study participants 51 (16.67%), 161 (52.61%), and 48 (15.69%) were smokers, chat chewers, and alcohol drinkers, respectively (Table 2). One hundred ninety-three (63.1%) of the study subjects were admitted in the surgical ward and 113 (36.9%) were related to Obstetric/gynecological cases. Two hundred seventy-five (89.87%) wounds were located on the abdomen, 21 (6.86%) around upper and lower extremities, and 10 (3.27%) wounds were in the thigh area. There were 199 (65%) emergency and 107 (35%) elective operations. High proportions of participants 170 (58.8%) were found in the clean wound, 59 (19.3%) in clean-contaminated, 40 (13.1%) in dirty, and low proportions of participants 27 (8.8%) were found in contaminated wound class. One hundred (32.68%) study participants had taken antimicrobial prophylaxis. Besides, in 68 (22.2%) participants, their wound had drain/discharge (Table 2).
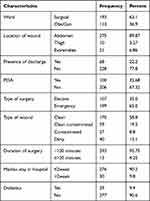 |
Table 2 Clinical and Procedural Characteristics of Study Participants Admitted Jugal and Hiwot Fana Specialized University Hospitals, Harar, Ethiopia, 2020 (n = 306) |
Bacterial Etiologies Isolated from SSI
In the present study, 36 specimens were cultured. Of them, 72.2% (95% CI: 57.9–86.8) showed bacterial growth within 48 hours of incubation. Out of 26 culture-positive specimens, a total of 33 different bacteria were isolated, of these, 19 (57.58%) of the isolates were gram-negative organisms. Besides, single microbial organism isolates were recovered from 73.1% (19/26) patients’ specimens whereas 26.9% (7/26) were double-microbial infections. Staphylococcus aureus was the most frequently isolated 10 (30.3%) followed by E. coli 9 (27.3%) (Table 3).
The Magnitude of Surgical Site Infection
Among 306 study participants; a total of 36 (11.8%) (95% CI: 8.3–15.4%) patients met the definition of SSI. Out of these, about 38.8% (14/36) of patients with SSI belonged to >60 years age group, 52.8% (19/36) were male, 58.3% (21/36) were from urban areas, and 75% (27/36) were non-smokers. Regarding comorbidity conditions, 33.3% (12/36) were diabetic patients with SSI. The majority of the patients with SSI received antimicrobial prophylaxis (91%) and 80.6% had a drain at the surgical site.
Factors Associated with Surgical Site Infection
In bivariate logistic regression analysis variables including age >60years, grades 1–4 and 5–8 in educational level, drinking alcohol, use of pre-operative antibiotics (POA), duration of surgery, location of wound around abdomen and thigh area, presence of discharge or drain at the operated site, dirty, contaminated and clean-contaminated wounds type, and diabetic patients were associated with SSIs at P value less than 0.25 and considered for multivariable analysis. In multivariable analysis variables like age >60years, drinking alcohol, presence of discharge or drain, duration of surgery, dirty and contaminated wound classification, and diabetic patients were remaining statistically significant at a P value less than 0.05 (Table 4).
 |
Table 4 Bivariate and Multivariable Analysis for Factors Associated with SSI of Study Participants Admitted at Jugal and Hiwot Fana Specialized University Hospitals, Harar, Ethiopia 2020 |
Study participants whose ages >60 years were 4.1 times (AOR = 4.139, 95% CI 1.278–13.406) more likely to develop the infection as compared to those <15 years old patients. Alcohol consumers were 2.5 times (AOR = 2.58, 95% CI 1.091–6.102) more likely to develop the infection as compared to their counterparts (Table 4).
In the present study, diabetic patients were 7.5 times (AOR = 7.457, 95% CI 2.893–19.221) more likely to develop SSI as compared to those non-diabetic patients. Participants who undergone the surgical procedure for less than 2 hours were 84.1% (AOR = 0.159, 95% CI 0.040, 0.630) less likely to develop the infection compared with those prolonged for greater than 2 hours procedural time. Patients who had drain or discharge on their operated site were 24.5 times (AOR = 24.514, 95% CI 10.053, 38.867) more likely to develop SSI compared to their counterparts. Furthermore, participants who had dirty on their wound were 9 times (AOR = 9.026 95% CI 3.511–23.205) and those with contaminated wounds were 3.6 times (AOR=3.668,95% CI 1.140–11.720) more likely to develop the infection as compared to those patients with the clean wound (Table 4).
Prevalence of Multiple Drug Resistance (MDR) Among Isolated Strains
The overall antibiotic resistance patterns for multiple drug resistance (MDR ≥ 2 different drugs) causing postoperative wound infection were recorded in 78.8% (26/33) of bacterial isolates. The cumulative resistant pattern of gram-negative organisms was 63.2% (12/19). And greater than 63% (12/19) of them were resistant to ceftriaxone, but Pseudomonas spp were the only species that showed 100% resistance to both amoxicillin-clavulanic acid and ceftriaxone.
Discussion
The study addressed the prevalence of SSI, bacterial etiologies, associated factors, and antimicrobial susceptibility patterns of the bacterial isolates among post-operative patients in Harar town.
In this study, the overall prevalence of SSI was 11.8% which is consistent with the study carried out in Rwanda (10.2%)14 and Bahir Dar, Ethiopia (10.2%),7 but relatively lower when compared with studies conducted in Uganda (16.4%),15 Egypt (67.6%),8 Hawassa, Ethiopia (24.6%),16 and Mekelle, Ethiopia (29.8%).11 Besides, this finding is higher compared with studies conducted in Peru (2.5%)17 and Saudi Arabia (2.2%).18 The difference might be due to the presence of modern surgical techniques, surgery rooms, and sufficiently trained professionals in middle- and high-income countries. Such variation could also be ascribed to lack of adequate postoperative wound care, shortage of trained manpower, failure to preserve sterility during surgical procedures, insufficient infection control due to deprived hygiene, and water shortage.
In this study, being elderly (>60 years of age) was significantly associated with SSIs. It is line with the study done in Hawassa where the prevalence of SSI was found higher in older age groups.16 On the contrary, a study done by Asres et al in Addis Ababa contradicts this finding since infection was predominantly observed in under 10 years age group.5 In this regard, the prevalence of SSIs was varied among the age group in the previous studies, and it might be related to the participants' immunity status as well.
This study indicated that the prevalence of SSI among alcohol consumers was higher than non-users, which is in line with the study conducted in Egypt,8 and Hawassa, Ethiopia.16 The association might be due to the fact that chronic alcohol consumption weakens the immune system and hence delays the healing of wounds.15
In this study, the prevalence of SSI was higher in patients who had a drain or discharge on their operated site than those who did not. This finding is in trajectory with a study reported from Egypt.8 One possible explanation is that it is one of the criteria for physically diagnosing SSIs without the need of laboratory testing.
This study found that the prevalence of SSI was higher in patients with dirty and contaminated wound types than those patients with clean wounds. This finding is consistent with reports from Uganda,15 Hawassa, Ethiopia,16 and Mekelle, Ethiopia.11 The significant influence of endogenous contamination during the operation or exogenous contamination during the wound care procedure might be a scientifically justifiable reason.
In this study, the prevalence of SSI was higher in patients who had a surgical procedure of more than 2 hours. The findings are consistent with the previous reports from Rwanda14 and Hawassa, Ethiopia.16 The higher odds of SSI in prolonged surgical procedures is attributed to a decrease in the use of aseptic measures during surgery due to fatigue, tissue hypoxia due to increased blood loss.
In this study, the overall rate of bacterial isolates from post-surgical infected wounds was 72.2%, which is comparable with a study carried out in Addis Ababa, Ethiopia (75.6%),5 and Jimma, Ethiopia (61.6%).20 On the other hand, it is lower than a report from Gondar, Ethiopia (88.1%).21 The difference could partly be the use of prophylactic antimicrobials resulting early resolution of infection, type of antiseptics used during wound cleaning, the nature of bacteria (unable to grow due to their fastidious nature), samples containing already dead bacteria or wound infections of unidentifiable pathogens.
This study revealed that 57.6% of the isolates were gram-negative organisms, which is consistent with the previous research from Hawassa22 and Jimma, Ethiopia,20 whereas the study conducted in Egypt8 contradicts this finding. Such variation might be ascribed to bacterial etiology habitat and infection prevention practices in various health-care settings.
This research indicates that the most frequently isolated species was S. aureus (30.3%). The finding is in line with studies done in Addis Ababa, Ethiopia (33.3%)5 and Bahir Dar, Ethiopia (26.2%),7 whereas the study conducted inUganda (50%) revealed that of the most prevalent isolate was K. pneumoniae.15 This difference in the distribution of bacterial species might be due to variation in common hospital-acquired pathogens, and infection prevention and control policies and guidelines across countries.
In the present study, chloramphenicol was a relatively effective drug for the treatment of SSIs caused by gram-negative bacteria which is consistent with a study reported from Gondar, Ethiopia.21 On the contrary, the study conducted in Addis Ababa, Ethiopia reported lower efficacy of this drug 52.2%.5 Probably it might be due to irrational use of anti-infective medicines together with inadequate measures to control the spread of infections, variation in common hospital-acquired pathogens inhabitant, acquiring anti-microbial resistant organism is then related to host risk factors as well as to the amount of time that is spent in a setting where they are exposed to these microorganisms.
This study also indicated that clindamycin was an effective drug for SSIs caused by gram-positive organisms in 78.6% isolates and is lower than a study carried out in Addis Ababa, Ethiopia (90.7%).5 This might be partly due to these drugs oral route of administration might affect their rate of absorption into the bloodstream, frequent use of it as empiric treatment and/or prophylaxis options in the study area might contribute to dropping its efficacy.
The current study emphasizes the alarming rate of multiple antimicrobial resistance to be 78.8% which is higher compared to the previous studies conducted in Egypt (37.2%)8 and Addis Ababa, Ethiopia (65.5%)5 whereas lower than the one reported from Hawassa, Ethiopia (93.2%).22 This might be due to the fact that empiric treatment of the isolates and ̸or indiscriminate and frequent use of antibiotics by unskilled practitioners along with unavailability of guidelines for the use of antibiotics play a pivotal role in the emergence and spread of resistance.
According to this study, being diabetic patients was associated with a higher risk of developing SSI compared to non-diabetes. This might be due to the fact that a deficiency of vasoactive neuropeptides in patients with neuropathy may impair normal soft tissue leading to delay wound healing in diabetic patients. In the current study, socio-demographic factors like being male gender and urban residence showed higher odds of SSI with no significant association and is similar with studies carried out in Addis Ababa,5 and Gondar19 in Ethiopia.
Limitation of the Study
This study did not address anaerobic bacterial pathogens due to limited laboratory facilities. Being a cross-sectional study made it difficult to determine the causal relationship (temporal sequence of cause and effects could not be explored).
Conclusion
In this study, the prevalence of SSI was consistent with most of the studies carried out in Ethiopia. Age >60 years, drinking alcohol, dirty wound, wound drain/discharge and being diabetic patients were factors associated with the occurrence of such infection. The most frequently isolated species was S. aureus. The drug resistance to the commonly used antibiotics forced the clinicians with very few choices of drugs for the treatment of SSIs. Therefore, to alleviate this problem, the concerned body should focus on WHO surgical safety protocol and appropriately manage comorbidity. In addition apply infection prevention and control practices tailored to the specified health-care settings is recommended. Further study should be conducted on the prevalence, associated factors, bacterial etiologies and antibiotic susceptibility patterns to address the emerging and gradually evolving nature of antimicrobial resistance.
Acknowledgment
We acknowledge the Haramaya University College of Health and Medical Sciences Institutional Health Research Ethical Review Committee for giving the ethical clearance. We also thank study participants and all individuals who have in one way or another contributed to the completion of this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, and revising or critically reviewing the article. And gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
Fund for data collection for this research was covered by Haramaya university postgraduate directorate.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization (WHO). Global guidelines for the prevention of surgical site infection; 2016. Available from: https://pubmed.ncbi.nlm.nih.gov/28139389/.
2. Centers for Disease Control and Prevention (CDC). Procedure-Associated Module: Surgical Site Infection (SSI) Event. Atlanta, GA: Centers for Disease Control and Prevention; 2017.
3. Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection. JAMA Surg. 2017;152(8):784–791. doi:10.1001/jamasurg.2017.0904
4. Naderi HR, Ebrahim ZM. Evaluation of postoperative infections in patients undergoing abdominal surgery: a systematic review. Patient Saf Qual Improv. 2015;3(4):300–303.
5. Asres GS, Legese MH, Woldearegay GM. Prevalence of multidrug resistant bacteria in postoperative wound infections at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Arch Med. 2017;9(4):12.
6. Moghadamyeghaneh Z, Hwang GS, Hanna MH. Risk factors for prolonged ileus following colon surgery. Surg Endosc. 2016;30:603–609. doi:10.1007/s00464-015-4247-1
7. Mulu W, Kibru G, Beyene G, Damtie H. Associated risk factors for postoperative nosocomial infections among patients admitted at Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. Clin Med Res. 2013;2(6):140–147. doi:10.11648/j.cmr.20130206.15
8. Zahran WA, Zein-Eldeen AA, Hamam SS, Sabal MSE. Surgical site infections: problem of multidrug-resistant bacteria. Menoufia Med J. 2017;30(4):1005–1013. doi:10.4103/mmj.mmj_119_17
9. Anderson DJ, Moehring RW, Sloane R, et al. Bloodstream infections in community hospitals in the 21st century: a Multicenter Cohort Study. PLoS One. 2014;9:e91713. doi:10.1371/journal.pone.0091713
10. Harari region health office annual report of 2019 (Unpublished).
11. Mengesha RE, Kasa BG-S, Saravanan M, Berhe DF, Wasihun AB. Aerobic bacteria in post-surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Ethiopia. BMC Res Notes. 2014;7(1):575. doi:10.1186/1756-0500-7-575
12. Cheesbrough M. District Laboratory Practice in Tropical Countries.
13. Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests.
14. Mukagendaneza MJ, Munyaneza E, Muhawenayo E, et al. Incidence, root causes, and outcomes of surgical site infections in a tertiary care hospital in Rwanda: a prospective observational cohort study. Patient Saf Surg. 2019;13(1):1–8. doi:10.1186/s13037-019-0190-8
15. Lubega A, Joel B, Justina Lucy N. Incidence and etiology of surgical site infections among emergency postoperative patients in mbarara regional referral hospital, South Western Uganda. Surg Res Pract. 2017;2017:1–6. doi:10.1155/2017/6365172
16. Deribe B, Jemebere W, Bekele G Surgical site infection prevalence and associated factors in Hawassa University comprehensive specialized hospital, southern Ethiopia; 2019. doi:10.21203/rs.2.15549/v1.
17. Ramirez-Wong FM, Atencio-Espinoza T, Rosenthal VD, et al. Surgical site infections rates in more than 13,000 surgical procedures in three cities in Peru: findings of the international nosocomial infection control consortium. Surg Infect. 2015;16(5):572–576. doi:10.1089/sur.2014.201
18. Al-Mulhim FA, Baragbah MA, Sadat-Ali M, Alomran AS, Azam MQ. Prevalence of surgical site infection in orthopedic surgery: a 5-year analysis. Int Surg. 2014;99(3):264–268. doi:10.9738/INTSURG-D-13-00251.1
19. Amare B, Abdurrahman Z, Moges B, et al. Postoperative surgical site bacterial infections and drug susceptibility patterns at Gondar University Teaching Hospital, Northwest Ethiopia. J Bacteriol Parasitol. 2011;2:126. doi:10.4172/2155-9597.1000126
20. Tesfaye S, Esseye S, Beyene G, Ali S. 2016 Patterns of bacteria isolated from admitted patients with signs of infection at Jimma University specialized hospital, Jimma, Ethiopia. Int J Trop Dis Health. 2011;17(4):1–12.
21. Gelaw A, Gebre-Selassie S, Tiruneh M, Mathios E, Yifru S. Isolation of bacterial pathogens from patients with postoperative surgical site infections and possible sources of infections at the University of Gondar Hospital, Northwest Ethiopia. J Environ Occup Health. 2014;3(2):103–108.
22. Dessalegn L, Shimelis T, Tadesse E, Gebre-selassie S. Aerobic bacterial isolates from post-surgical wound and their antimicrobial susceptibility pattern: a hospital based cross-sectional study. J Med Res. 2014;3(2):18–23.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

