Back to Journals » Journal of Pain Research » Volume 14
Long-Term Outcomes of Single versus Multiple Courses of Viscosupplementation for Osteoarthritic Knee Pain: Real-World, Multi-Practice Experience Over a Six-Year Period
Authors Johnston J, Brown K , Muir J, Sloniewsky MJ
Received 23 March 2021
Accepted for publication 27 July 2021
Published 10 August 2021 Volume 2021:14 Pages 2413—2421
DOI https://doi.org/10.2147/JPR.S312418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Janice Johnston,1 Karen Brown,2 Jeffrey Muir,3 Michael J Sloniewsky4
1Arrowhead Health Centers, Glendale, AZ, USA; 2KLEO Research, Missoula, MT, USA; 3Motion Research, Ancaster, ON, Canada; 4RMG Holding, Inc., Florence, SC, USA
Correspondence: Jeffrey Muir
Motion Research, 3-35 Stone Church Road, Ste. 215, Ancaster, ON, L9K 1S4, Canada
Email [email protected]
Introduction: Viscosupplementation (VS) is a safe and effective local treatment for osteoarthritis (OA) of the knee. While much research has been completed evaluating its efficacy, comparatively little research has been completed examining the effects of multiple, repeat courses of treatment versus a single course of treatment.
Methods: We retrospectively reviewed real-world data from a large cohort of patients receiving treatment for OA of the knee at 16 rehabilitation clinics. Patients were grouped based on whether they received a single course of treatment or multiple courses. Outcomes for this study included pain (measured via the visual analog scale, VAS) and functional ability (measured via the Western Ontario and McMaster Universities Arthritis Index (WOMAC)). Pain and function scores were collected at baseline (prior to treatment administration) and one week following each course of treatment.
Results: Patients receiving multiple courses of treatment saw greater improvements than those receiving a single course. For VAS, maximal improvement occurred after the fourth course (66% improvement: 1.7± 1.2 vs 5.0± 2.4 at baseline, p< 0.0001). WOMAC scores saw maximal improvement up to the fourth course for all domains (pain: 74%: 2.5± 3.3 vs 9.5± 5.3, p< 0.0001; stiffness: 61%: 1.3± 1.0 vs 3.3± 2.0, p< 0.0001; function: 66%: 9.5± 7.2 vs 28.3± 14.1, p< 0.0001). When scores from multiple courses were averaged, improvements were maintained through the fourth course for VAS (3.4± 2.8) and all WOMAC domains (pain: 6.1± 5.0; stiffness: 3.0± 2.2; function: 23.4± 17.3).
Discussion: Our results indicate that multiple courses of treatment are associated with greater improvements than a single course of VS, and that these improvements continue through four courses of treatment.
Keywords: viscosupplementation, hyaluronic acid, knee osteoarthritis, repeat treatment, longitudinal study
Introduction
Osteoarthritis (OA) of the knee remains a significant and prevalent condition worldwide, with over 650 million individuals over 40 years of age affected in 2020.1 With an estimated 86 million patients newly affected each year,1 the clinical and economic burden on worldwide healthcare is daunting. Treatment of symptoms in early and mid-stage OA is often multimodal, taking a stepwise approach that combines non-pharmacologic and/or pharmacological (over the counter or prescription) treatments. In cases where these treatments fail but where surgical intervention is not indicated – due to co-morbidities or patient preference – viscosupplementation (VS) offers a viable treatment alternative.
The ability to deliver treatment locally and without any known drug interactions makes VS particularly useful in the treatment of typical knee OA symptoms. Over the last three decades, various formulations of VS have been evaluated in clinical studies,2–6 as well as systematic reviews and meta-analyses,7–11 all of which have affirmed its safety and efficacy in addressing symptoms of knee OA. The 2015 Consensus Statement12 further noted that VS is a “positive” therapy and should not be restricted to use only in patients who have failed to respond adequately to analgesic or non-steroidal anti-inflammatory (NSAID) medication. In the United States, 16 different VS formulations have been reviewed and approved by the Food and Drug Administration since 1997, the majority of which are labelled as safe for use in repeat treatments. However, the body of evidence evaluating the long-term benefits of repeated treatment, while growing, remains somewhat limited. The 2015 update of the EUROVISCO recommendations13 supported the retreatment of symptomatic patients following an initial course of VS and there is clinical evidence demonstrating a benefit to repeated treatment, although this evidence generally represents either highly regimented, randomized, saline-controlled studies or small-to-medium sized cohort studies of retreatment.6,14 Less work has been completed examining larger, real-world populations and thus, the evidence in this important setting is more limited.
To address this lack of evidence, we sought to evaluate the comparative effectiveness of multiple versus single courses of VS in improving pain and function scores in knee OA patients in a real-world setting. We further sought to examine the comparative effect of repeated courses of treatment on patient symptomatology in this cohort.
Methods
Study Design
This study was a retrospective, observational, multi-site study of patients who underwent VS treatment for knee OA. We collected data from 16 rehabilitation clinics affiliated with OsteoArthritis Centers of America. The study was conducted in accordance with the Declaration of Helsinki,15 ethics approval was received from Advarra prior to data collection and all patients provided informed consent for collection of study data. Study participants were grouped based on whether they received a single course of treatment (SC) or multiple courses of treatment (MC), based upon product prescribing information. In cases where patients received treatment for bilateral knee OA, data was collected for each knee separately.
Study Population and Eligibility Criteria
We included data from patients who received VS for primary knee OA between January 2014 and June 2020. Patients were eligible for inclusion in the analysis if:
- they had a confirmed diagnosis of OA, based on criteria set out in the Medicare Local Coverage Determination rules,16 which at a minimum include patient-reported pain in the affected knee which interferes with basic function/activities of daily living (ADL), a physical examination, standing radiographs and a detailed medical history including previous treatment;
- clinical outcomes data was recorded prior to the first VS injection (baseline); and,
- one-week post-treatment outcomes data was available, regardless of the number of treatment courses administered.
Patients presenting for second or subsequent courses of treatment additionally were required to have reported a recurrence of pain in the affected joint and to have objective documentation of previous success with VS treatment. Patients for whom no baseline or post-treatment data were available were excluded from the analysis.
Treatment Regimen
Each course of VS was administered as a series of 5 injections administered at weekly intervals. We sought to replicate real-world conditions for treatment and, as such, patients presented for treatment based on symptomatology and discomfort. No recruitment of patients was undertaken and patients returning for repeat courses of treatment did so of their own volition and were not prompted to return. No restrictions were placed on the time between courses of treatment. The number of courses administered in each case was determined by the treating physician in consultation with the patient and based on patient presentation. During treatment, no restrictions were placed on patients regarding physiotherapy, rehabilitation or medications. The most commonly administered viscosupplements were Genvisc850 (OrthogenRx, Inc., Doylestown, PA, USA), Supartz (Bioventus LLC, Durham, NC, USA), Orthovisc (Pendopharm, Montreal, PQ, Canada) and Hyalgan (Fidia Farmaceutici, Abano Terme, Italy) (Table 1).
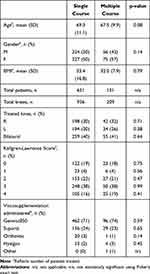 |
Table 1 Demographics and Descriptive Statistics |
Outcomes
The outcomes for this study were pain and function, assessed via the visual analog scale (VAS, 0–10), and the Western Ontario and McMaster Universities Arthritis Index (WOMAC)17 score, respectively. Data for each knee treated was recorded at baseline (prior to initiation of first course of treatment) and one week following completion of each course of treatment. Demographic data including patient age, gender, Kellgren-Lawrence (KL) score at baseline, treatment date, body mass index (BMI) and treated knee (ie, right, left or bilateral) was recorded for all patients. Patients categorized as “bilateral” received treatment for both knees at different time points during the study period. In these cases, data was collected for each knee separately and patients were categorized as “bilateral” for demographic purposes only. Data regarding the occurrence of adverse events (AEs) was also collected.
Statistics Methods and Data Analysis Plan
Alpha was set a priori at 0.05 for all statistical comparisons. Continuous variables are presented as mean or mean ± standard deviation. Mean values were compared using Student’s t-test or analysis of variance (ANOVA), as appropriate. Categorical variables are presented as proportions and compared using chi-squared or Fisher’s exact test (as appropriate). To determine the rate of treatment responders, the minimal clinically important difference (MCID) for each of VAS and WOMAC scores was calculated by comparing the post-treatment score with the baseline score. Thresholds for MCID were set as an improvement of 30% over baseline for VAS18 and an improvement of 20% over baseline for WOMAC.19
Patients were stratified based on the number of courses of treatment completed and comparisons made within groups and between groups. The within-group analysis was a comparison of change from baseline for each group (SC and MC), with mean baseline scores in each group compared with post-treatment scores. The between-groups analysis compared the final, post-treatment scores from the SC group with the MC group. The MC group was stratified based on the number of courses of treatment administered, such that results from a single course could be compared with results from two, three, etc. courses of treatment. Finally, post-treatment scores within the MC group were compared to determine the relative effect of repeated courses of treatment on outcomes.
Results
Descriptive Statistics
A total of 782 patients (1145 knees) were included in the analysis. Descriptive statistics are summarized in Table 1. The entire study patient cohort, comprised of 52% females (409/782), had a mean BMI of 32.1 (SD: 9.5) and a mean age of 68.9 years (SD: 10.9). There were no statistically significant differences in demographic variables between study groups (SC vs MC). GenVisc850 and Supartz were the two most prescribed VS in both study groups: Supartz was prescribed slightly more often to patients in the SC group while GenVisc850 was prescribed slightly more often in the MC group.
Within-Groups Analysis: Change from Baseline
Statistically significant improvements from baseline for both VAS and WOMAC scores were noted in all treatment groups, regardless of the number of courses of treatment received (Figure 1). For the entire cohort, an average 36% improvement over baseline was noted in VAS scores following the final course of treatment, regardless of the number of courses administered (3.4±2.9 post-treatment vs 5.3±2.9 at baseline, p<0.0001). Similar observations were noted for WOMAC Pain (37% improvement: 6.4±4.6 vs 10.1±4.6 at baseline, p<0.0001), Stiffness (33% improvement: 3.0±2.1 vs 4.5±2.1 at baseline, p<0.0001) and Function (33% improvement: 23.7±16.1 vs 35.6±15.6 at baseline, p<0.0001) scores.
The changes from baseline for patients in the MC group were greater than improvements in patients in the SC group, with improvements generally increasing with each subsequent course of treatment (Figure 1). For VAS, maximal improvements were noted following the fourth course of treatment (66% improvement: 1.7±1.2 vs 5.0±2.4 at baseline, p<0.0001). Significant improvements were noted up to the fourth course for all WOMAC domains. The greatest improvements in WOMAC Pain scores were noted after four (74%: 2.5±3.3 vs 9.5±5.3, p<0.0001) courses, as were the greatest improvements in WOMAC Stiffness scores (61%: 1.3±1.0 vs 3.3±2.0, p<0.0001) and WOMAC Function scores (66%: 9.5±7.2 vs 28.3±14.1, p<0.0001) courses.
Between-Groups Analysis: Final Post-Treatment Scores
Post-treatment, VAS and WOMAC scores in the MC group improved gradually over those in the SC group, peaking following the fourth course. The mean final VAS score for patients having received a single course of treatment was 3.3±2.9. Scores in patients who received two (3.1±2.7, p=0.61 vs single course) and three (3.7±2.6, p=0.56) courses were improved, with four courses associated with a significant improvement (1.7±1.2, p<0.01) (Table 2). Similar observations held true for all domains of WOMAC scores. For WOMAC Pain, the largest improvements noted following the fourth course (2.5±3.3, p=0.04 vs single course). WOMAC Stiffness (1.3±1.0, p=0.05) and Function (9.5±7.2, p=0.03) scores were significantly improved over a single course (Stiffness: 2.9±2.1; Function: 23.5±15.9) following the fourth course of treatment.
 |
Table 2 Between Groups Comparison of Post-Treatment Scores Following the Final Course of Treatment |
Between-Groups Analysis: Averaged Post-Treatment Scores
To further examine the effect of repeated courses of treatment on the primary outcome, a second between-groups analysis was completed in the MC group using the average score over all courses of treatment. In this comparison, scores for two courses of treatment were compared with those from three or greater courses of treatment. For VAS, improvements following multiple courses of treatment were maintained but were not significantly different. A similar response was noted for all WOMAC domains, with improvements maintained through multiple courses of treatment but no significant differences noted between various courses of treatment (Table 3).
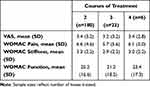 |
Table 3 Between Groups Comparison of Average of All Post-Treatment Scores Following Each Patient’s Final Course of Treatment for Patients Receiving Multiple Courses of Treatment |
Responder Rates
Multiple courses of treatment resulted in a greater proportion of patients achieving a minimum clinically important difference in both VAS and WOMAC scores when compared with a single course of treatment (Table 4). For VAS scores, two (112/180, 62%, p=0.42), three (14/23, 61%, p=0.86) and four (4/6, 67%, p=0.70) courses of treatment resulted in more patients achieving a clinically important improvement than a single course (552/936, 59%), although these improvements were not significant.
 |
Table 4 Summary of Responder Rates |
Responder rates for WOMAC scores were higher than for VAS scores. For WOMAC Pain, maximal improvements were noted following two (135/180, 75%, p=0.15) and four (5/6, 83%, p=0.48) courses of treatment when compared with a single course (652/936, 70%). For both WOMAC Stiffness and Function, three courses (Stiffness: 20/23, 87%, p=0.03) and two courses (122/180, 68%, p=0.47), respectively, saw important improvements over a single course (Stiffness: 607/936, 65%; Function: 608/936, 65%) (Table 4).
Adverse Events
No adverse events related to the administration or use of VS were reported in any of the patients during the study period.
Discussion
Viscosupplementation is a viable and well-utilized treatment modality for osteoarthritis. While the evidence supporting its efficacy in general is mounting, the evidence regarding the cumulative effect of multiple courses of treatment, especially in real-world settings, remains somewhat sparse. To examine the effect of repeated courses of treatment on pain and function, we used real-world data and compared results following a single course of treatment with multiple courses, and further compared the relative effects of repeated courses on pain and function. We noted that repeated courses provided greater improvements over baseline than did a single course of treatment. We further noted that, in patients receiving multiple courses of treatment, there was a continued improvement following successive courses of treatment, with pain and functional abilities scores improving through the fourth course of treatment.
The effect of repeated courses of VS treatment has been examined by several authors, and while a consensus on the point at which improvement plateaus remains elusive, there is compelling evidence from several studies that echo our findings that improvements continue to be noted up to and beyond 4 courses of treatment. Recent recommendations from the EUROVISCO group support the retreatment of symptomatic patients following an initial course of treatment,13 and reviews of real-world evidence have commented on the value of repeated treatment in improving function and reducing the need for analgesics.11 Among the most powerful data on this effect is a large study by Altman et al,20 who examined the IMS Health PharMetrics Plus database for data from patients who received between 1 and >5 courses of VS treatment and found that incremental and statistically significant improvements were noted following each successive course of treatment. When combined with results from Navarro-Sarabia and the AMELIA study,6 which found that the beneficial effects of multiple courses of treatment lasted up to one year following a fourth course of treatment, these results suggest that there is an incremental and long-term benefit to repeated courses of VS treatment. More recently, Altman et al7 sought to move closer to a consensus finding on repeated use of VS and completed a systematic review of 17 randomized trials and observational studies with greater than 1 repeated course of HA treatment. In their analysis, they found that up to 4 repeated courses of treatment were effective at minimizing knee pain and improving function. The included studies varied widely regarding the number of repeated courses provided, however, making comparisons of the effects based on the number of courses difficult. Cumulatively, the results from these various studies provide important commentary on the value of repeated courses of treatment with VS; however, this evidence, while compelling, is from tightly controlled settings or patient registries and does not adequately reflect real-world settings. As such, our results from a multisite, real-world setting represent an important addition to the literature.
In our study, we noted two important findings: 1) multiple courses provide superior results over a single course, and 2) improvements continued with repeated courses, as evident from the improvements in change-from-baseline with each subsequent course, which continued through the third and fourth courses of treatment for pain and function scores. Interesting amongst our findings, though, were the differences noted in our between-groups analysis as to exactly when treatment effects plateau or maximize, based on whether final or averaged scores were used in the calculation. When final, post-treatment scores were used, the effect was noted after the third course; however, when averaged scores were used, improvements were maintained into the fourth course and beyond, especially for WOMAC Pain and Function domains. We also noted that, when comparing results from differing numbers of courses in the MC group, there was a continued improvement in the fourth courses when compared with two courses. As such, our data confirms the value of continued courses of treatment, but the differences noted between averaged and final scores leaves open questions of how the time between courses impacts patient improvement. Further investigation into this variable is warranted and may be a key to gaining an improved perspective on the cumulative effect of repeated courses.
Also important in our study was the observation that the continued effect was noted not just in raw pain and function scores but also in responder rates. We noted that scores for VAS and all domains of WOMAC saw improvements in responder rates following the second course, improvements that were improved-upon in the third and fourth courses. Statistically, improvements beyond the second course were not significant; however, the decreasing sample size in the latter courses may be partially responsible for the lack of statistical significance. The ability of repeated courses to continue to improve outcomes is a valuable finding and, when combined with other observations that repeated treatment can in fact turn non-responders into responders,6 suggests that early stoppage of treatment due to slow response may not be beneficial, and treatment should continue, to allow time for the carry-over effect to be observed.
The level of improvement in pain and function score observed in our study mirror those of similar studies. We noted improvements of 38% over baseline in VAS and 34–37% over baseline in WOMAC scores in patients receiving a single course of treatment, improvements which increased to >40% for all scales following the second course of treatment. These findings mirror those of others, where improvements ranging from 28–54% in pain scores and up to 32% in function scores have been noted.11,21 We also noted similarities in responder rates with other studies that have examined several repeated courses of treatment. Navarro-Sarabia et al6 noted that 70.5% of participants saw a >20% improvement in function scores following their fourth course of treatment, findings that mirror our observations that 70–78% of patients achieved a clinically important improvement after their third or fourth course of treatment. Our results differ, though, when considering pain scores, where we observed that an MCID was achieved in 62% of cases, while other studies saw up to 79.2% of patients achieve an overall pain reduction of 20%.6 This discrepancy may be explained by the fact that we calculated MCID using a >30% change to indicate a clinically important change and may also reflect the varied time between treatment courses in our study. Indeed, when recalculated as a >20% change, the proportion of patients in our study seeing a clinically important improvement exceeds 70%.
Our study has limitations. The retrospective design of the study may be considered a limitation, but the large sample size and multisite setting provide valuable data on the real-world effects of VS. The low sample size of patients receiving 3 or more courses of treatment, the non-standardized administration of VS products, plus the variability in the number of courses received – due to physician discretion in prescribing treatment – and the variability with regards to physiotherapy, rehabilitation and medication may also limit the strength of the conclusions somewhat. These factors, however, represent real-world variables and therefore our study provides data that may more closely reflect the circumstances in typical patient populations. While our study demonstrated definitive improvements with multiple courses of treatment, the comparison of post-treatment scores without factoring in the time between courses may introduce selection bias into the study and somewhat diminish the evaluation of a carry-over effect from previous courses of treatment. Future studies are planned that will take into account these factors, including the time between courses of treatment, to identify predictors of treatment success. Finally, the lack of a comparator group representing alternate treatment modalities (eg physical therapy) may be considered a weakness, although the goal of the study was to compare the effects of courses of VS in a real-world setting, and not to evaluate its effectiveness relative to alternate treatments.
Conclusions
Our study determined that multiple courses of treatment provide greater improvements in pain and function scores than does a single course of treatment, and that there is an increase in improvements with successive courses of treatment, until a plateau effect is noted following the fourth course of treatment. While more information is required to establish the relationship between carry-over and plateau effects, and to identify predictors for treatment success, the data provides a strong basis for consideration of repeat courses of treatment in patients where a single course fails to provide suitable relief and improvement.
Acknowledgments
The authors would like to thank Mr. Aidan Muir for his assistance with data analysis.
Disclosure
Karen Brown reports personal fees from Motion Research, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
1. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors for knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29:100587.
2. Benazzo F, Perticarini L, Padolino A, et al. A multi-centre, open label, long-term follow-up study to evaluate the benefits of a new viscoelastic hydrogel (Hymovis®) in the treatment of knee osteoarthritis. Eur Rev Med Pharmacol Sci. 2016;20(5):959–968.
3. Heger R, Paulsen G, Fickert U, Kresmann M. Open-label study of initial and repeat treatment cycles of Hylan G-F 20 in patients with symptomatic knee osteoarthritis. Open Rheumatol J. 2016;10:88–100. doi:10.2174/1874312901610010088
4. Petrella RJ, Wakeford C. Pain relief and improved physical function in knee osteoarthritis patients receiving ongoing hylan G-F 20, a high-molecular-weight hyaluronan, versus other treatment options: data from a large real-world longitudinal cohort in Canada. Drug Des Devel Ther. 2015;9:5633–5640. doi:10.2147/DDDT.S88473
5. Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: results of an extensive critical literature review. Semin Arthritis Rheum. 2019;48(4):563–572. doi:10.1016/j.semarthrit.2018.06.002
6. Navarro-Sarabia F, Coronel P, Collantes E, et al. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70(11):1957–1962. doi:10.1136/ard.2011.152017
7. Altman R, Hackel J, Niazi F, Shaw P, Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum. 2018;48(2):168–175. doi:10.1016/j.semarthrit.2018.01.009
8. Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis–meta-analysis. Osteoarthritis Cartilage. 2011;19(6):611–619. doi:10.1016/j.joca.2010.09.014
9. Concoff A, Sancheti P, Niazi F, Shaw P, Rosen J. The efficacy of multiple versus single hyaluronic acid injections: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18(1):542. doi:10.1186/s12891-017-1897-2
10. Bannuru RR, Brodie CR, Sullivan MC, McAlindon TE. Safety of repeated injections of sodium hyaluronate (SUPARTZ) for knee osteoarthritis: a systematic review and meta-analysis. Cartilage. 2016;7(4):322–332. doi:10.1177/1947603516642271
11. Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S28–33. doi:10.1016/j.semarthrit.2015.11.008
12. Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45(2):140–149. doi:10.1016/j.semarthrit.2015.04.011
13. Raman R, Henrotin Y, Chevalier X, et al. Decision algorithms for the retreatment with viscosupplementation in patients suffering from knee osteoarthritis: recommendations from the EUROpean VIScosupplementation COnsensus Group (EUROVISCO). Cartilage. 2018;9(3):263–275. doi:10.1177/1947603517693043
14. Strand V, Lim S, Takamura J. Evidence for safety of retreatment with a single intra-articular injection of Gel-200 for treatment of osteoarthritis of the knee from the double-blind pivotal and open-label retreatment clinical trials. BMC Musculoskelet Disord. 2016;17:240. doi:10.1186/s12891-016-1101-0
15. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
16. CMS.gov. Local coverage determinations; 2021. Available from: https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs.
17. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840.
18. Lee JS, Hobden E, Stiell IG, Wells GA. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med. 2003;10(10):1128–1130. doi:10.1197/S1069-6563(03)00372-5
19. Maredupaka S, Meshram P, Chatte M, Kim WH, Kim TK. Minimal clinically important difference of commonly used patient-reported outcome measures in total knee arthroplasty: review of terminologies, methods and proposed values. Knee Surg Relat Res. 2020;32(1):19. doi:10.1186/s43019-020-00038-3
20. Altman R, Lim S, Steen RG, Dasa V. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: evidence from a large U.S. health claims database. PLoS One. 2015;10(12):e0145776. doi:10.1371/journal.pone.0145776
21. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:Cd005321.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

