Back to Journals » Infection and Drug Resistance » Volume 14
Prevalence and Associated Factors of Methicillin Resistance Staphylococcus aureus (MRSA) Among Urinary Tract Infection Suspected Patients Attending at Arba Minch General Hospital, Southern Ethiopia
Authors Mitiku A, Aklilu A , Biresaw G , Gize A
Received 19 March 2021
Accepted for publication 13 May 2021
Published 9 June 2021 Volume 2021:14 Pages 2133—2142
DOI https://doi.org/10.2147/IDR.S306648
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Asaye Mitiku,1 Addis Aklilu,2 Gelila Biresaw,2 Addisu Gize3
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Dilla University, Dilla, Ethiopia; 2Department of Medical Laboratory Science, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia; 3Department of Microbiology, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Correspondence: Addisu Gize 1271, Addis Ababa, Ethiopia
Email [email protected]
Background: Urinary tract infection (UTI) is a very frequent infection both in the community and hospital patients, and the emergence of methicillin-resistant Staphylococcus aureus (MRSA) in the community setting and infections with this pathogen become a prevalent problem among UTI patients. Therefore, the aim of this study was to determine prevalence and associated factors of methicillin resistance staphylococcus aureus (MRSA) among urinary tract infection suspected patients attending at Arba Minch General Hospital.
Methods: Facility-based cross-sectional study was done at Arba Minch General Hospital from July to October 2020. Midstream urine specimen was collected from outpatients, cultured and biochemical tests were performed to identify the intended pathogen, finally the antibiotic susceptibility pattern of MRSA was done and possible associated factors were determined. The cleaned data were entered and analyzed using SPSS version 21.
Results: Four hundred and twenty two (422) adult outpatients were enrolled in this study, of which males accounted for 238 (56.4%) of the participants. The mean and standard deviation age of the participants was 27.4 (SD 27.4 ± 15.6) years. A total of 54 S. aureus isolates were recovered from urine specimen. The prevalence of MRSA among the isolated S. aureus was 23/54 (42.59% (95% CI (35.0, 47.0)). Participants who had previous exposure to UTI (p < 0.002), presence of chronic disease (p < 0.029), and hospitalization (p < 0.006) were statically associated with the prevalence of MRSA. From all the MRSA isolates, 53.7% were resistant against Nitrofurantoin.
Conclusion: This study revealed that MRSA could be prevalent in isolates from patients suspected of urinary tract infection and exhibiting different resistance pattern for antibiotics commonly used for treatment of staphylococcal infections.
Keywords: methicillin resistance Staphylococcus aureus, urinary tract infection, Arba Minch General Hospital Southern Ethiopia
Background
Urinary tract infection (UTI) is the second most common site, after the respiratory tract, for bacterial infection in the human population,1 and it is a common worldwide problem. The world annual incidence is almost 250 million.2 It is estimated that about six million patients visit outpatient departments and 300,000 of them are treated in the wards every year, worldwide.3 Additionally, the infection is considered to affect all age categories, from young children up to elderly people in the community of hospitalized patients.4,5
Many groups of Gram positive and Gram negative bacteria have been implicated as causative agents of UTI. Staphylococcus aureus is the most important human staphylococcal pathogen and is a problematic pathogen in human medicine.6,7
The term “Methicillin-Resistant S. aureus” (MRSA) refers to the S. aureus that's able to resist the antibiotic listed, such as: methicillin, oxacillin, nafcillin, cephalosporins, imipenem, and other beta lactamase antibiotics.8 S. aureus, especially MRSA, is relatively ubiquitous and is the cause of many community infections.9 Another unique characteristic of MRSA is that it's a disease causing pathogen in the condition of nosocomial infection and also community-onset infections which has shown a widespread distribution in the last 40 years,10,11 while its control has been a global challenge. These MRSA associated infections create a serious problem in the case of health issues and also socio-economic costs by causing mortality and morbidity.12
The incidence of urinary tract infection is increasing because patients are more frequently fitted with various urinary catheters as endourology progresses technologically.13 In complicated urinary tract infections and hospitalized patients, Gram positive bacteria such as MRSA are comparatively more common.14,15
MRSA has been identified as a major pathogen in nosocomial infections. It is estimated that MRSA infections within the healthcare setting alone affected more than 150,000 patients annually in the European Union, with an additional cost of 380 million Euros.16 In the United States of America alone 80,461 and 11,285 infections of persistent MRSA and associated deaths occurred in 2011 respectively, and an estimated annual burden of between $1.4 billion and $13.8 billion was attributed to MRSA.17 Moreover, many African countries have experienced an increased burden of MRSA infections in which Ethiopia stands at first among those African countries that have high prevalence of MRSA infections with 42.8%18 followed by Nigeria (29.6%), Kenya (27.7%), Cameroon (21.3%), Cote D’Ivoire (16.8%), and Morocco (14.4%).19
MRSA is an emerged pathogen that is able to withstand the effect of different antimicrobials especially some strains which produce a biofilm both in hospital admitted patients as well as in the community, so rapid identification of this resistance can help to eliminate the infection effectively. Because of its ability to acquire resistance genes, S. aureus becomes resistant to broad types of antibiotics.20 The incidence of urinary tract infection caused by MRSA is increasing because patients are more frequently fitted with various urinary catheters.21,22 S. aureus is an etiologic agent of a wide range of diseases, from minor infections to life-threatening invasive diseases. MRSA caused more than 19,000 deaths and 278,000 hospitalizations though we have no written documents regarding this problem in the study area. Thus, this study was aimed to determine prevalence, antimicrobial susceptibility and associated factors of MRSA among urinary tract infection suspected patients attending Arba Minch General Hospital.
Materials and Methods
Study Settings
A cross-sectional study was conducted in Arba Minch General Hospital from July to October 2020. Arba Minch is situated between the Gamo highlands and the two known rift valley lakes: Abaya and Chamo. Arba Minch General Hospital is the biggest health institution in the Gamo zone. The hospital serves more than 1.5 million people by providing preventive, curative and rehabilitative care in outpatients, inpatients, pharmacy, and laboratory departments. According to data obtained from the hospital, the patient flow is outpatient department (OPD) = 89,320, inpatient = 11,737, surgery = 2146, medical ward = 1730, emergency OPD = 7072, and minor OPD = 6709.
All outpatients who visit the OPD of Arba Minch General Hospitals were the source population, whereas those adult outpatients who were suspected of having a urinary tract infection and visited the OPD of Arba Minch General Hospital during the study were the study population. Suspected urinary tract infection means a patient who has clinical signs and symptoms of urinary tract infection and requested a clinical urine sample to be cultured. History of antibiotic usage was defined as patients who had received any antibiotics prior to two weeks before the current study.
Sample Size and Sampling Technique
Since there is no previous study on MRSA among UTI suspected in Ethiopia, we used 50% of population proportion formula, and d (0.05) was the margin of error and adding 10% of the sample size for non-response rate, the total study sample size was calculated as 422 study subjects.
Systematic sampling was performed by estimating the total number of patients in the register of the same study period and study duration (3 months) in the previous year. K value was calculated by dividing total population by sample size. Then, the first participant was selected based on the value of K. Since k ≈ 1, data collection started from the first participant and continues using every other participant until the total sample size was completely filled.
All adult patients suspected of having a UTI and attended the OPD of AMGH during data collection were included and those patients who had received antibiotics within the last two weeks, aged less than 18 years and study participants who were not capable of responding to the questionnaire were not included in this study.
Data Collection Process
A structured questionnaire was used as a data collection tool. The questionnaire was translated into Amharic and was presented to the study participants to collect socio-demographic characteristics, and clinical features.
Sample Collection and Processing
During patient urination time, mid-stream urine samples were collected using wide mouthed glass bottles. On the urine sample bottles the patient’s code, age, and time of urine collection were labeled. Study participants were well advised to sanitise their hands and their genital area with water before collection of 5–10 mL of clean catch mid-stream urine samples. Urine samples were transported to the microbiology laboratory, department of Medical Laboratory Science, College of Medicine and Health Sciences, Arba Minch University. Urine samples were processed, not longer than 2 hours after collection, if there was a delay to process within the 2 hours, samples were kept refrigerated at 4 °C until they were processed.23
Isolation and Identification
Urine specimens were inoculated on CLED and Mannitol Salt Agar (MSA) using a standard calibrated wire loop (1μL).24 A calibrated sterile plastic non-reusable wire loop that has a 1.0 mm diameter designed to deliver 1 μL (0.001 mL) of urine was used for the quantitative method and plating. Microscopic examination was done for all collected urine specimens to determine the presence of WBC, RBC, Pus cells, epithelial cells and bacteria. A loop full of the well mixed urine sample was inoculated with Mannitol Salt Agar (MSA) (Oxoid Ltd, England). All the streaked culture plates were then incubated at 37 °C for 24h to 48h. The plates were checked for growth of bacteria colony macroscopically.
The bacterial colonies were counted and multiplied by 1000 to give an estimated number of bacteria present per milliliter of urine. A significant bacterial count was taken for specimens that produced ≥105 colonies and subjected to further identification. A positive culture that produced more than 2 types of colonies was considered as due to contamination.
Identification of S. aureus was performed based on the typical characteristics of the organism, such as round colony, convex, golden yellow, mannitol fermenting colonies on MSA plates. Aseptically it was picked for further purification by repeated streaking on nutrient agar plates and characterized following established microbiological methods of Gram reaction, catalase and coagulase positive tests were used to ensure an organism of S. aureus.24
Antimicrobial Susceptibility Testing
Antimicrobial susceptibilities of the bacterial isolates were performed according to the criteria of the Clinical and Laboratory Standards Institute (CLSI), 2019 using the Kirby–Bauer disc diffusion method on Muller-Hinton Agar (Oxoid Ltd, UK). A 3–4 colony of bacteria was taken from a pure culture colony and transferred to a tube containing 5 mL sterile normal saline (0.85% NaCl) and mixed gently until it forms a homogenous suspension. The turbidity of the suspension was adjusted to the turbidity of McFarland 0.5 standard in a tube and swabbed on Muller Hinton.
The antibiotics used were Ciprofloxacillin (5 ug), Ampicillin (10 μg), Tobramycin (10 μg), Nitrofurantoin (300 μg), Piperacillin (100 μg), Cortimoxazole (25 μg), and Norfloxacin (10 µg). These antimicrobial drug discs were selected based on CLSI and also by considering the availability of these drugs in the study area for the treatment of UTI. After that the antibiotic discs were placed on Muller Hinton Agar, then left at room temperature to dry for 3 to 5 minutes and incubated at 37 °C for about 18 to 24 hours and the zones of inhibition were measured using a ruler. The results of the antimicrobial susceptibility test were interpreted according to the CLSI, 2019 guidelines.25
Detection of Methicillin Resistance
Detection of MRSA was performed using the Cefoxitin disc as a turning point. Briefly, isolates were subjected to Cefoxitin disc diffusion test using a 30 μg disc. A 0.5 McFarland standard suspension of the isolates was done and a culture was done on a Mueller Hinton agar plate. Plates were incubated at optimum temperature and time ie, 35 °C for 18–24 hours, then a zone of inhibition in mm was measured. If the zone of inhibition was ≤21 mm in diameter, it was reported as methicillin/oxacillin resistant and if it was ≥22 mm in diameter, it was considered as methicillin/oxacillin sensitive.
Data Quality Control
To ensure data quality, 5% of the sample size (n = 21) was pre-tested before the actual work. Two nurses and three laboratory data collectors were trained for one day. The implementation of quality control measures was applied throughout the data and specimen collection, the reliability of the research results was guaranteed. Reagents, media and antibiotic discs were checked for their quality and shelf life before use. After preparation, culture plates and antibiotic plates were also stored at the recommended refrigeration temperature (2–8 °C). Reference strains of S. aureus ATCC25923, was used as controls which were obtained from the Ethiopian public health institute.
In general, all laboratory procedures were applied based on recommended standard laboratory procedures by strictly following pre analytical, analytical and post-analytical stages of quality assurance which were incorporated in the standard operational procedures (SOPs) of microbiology and parasitology laboratory of Arba Minch University.
Statistical Analysis
Data were entered and analyzed using SPSS statistical software version 21. Logistic regression was used to see the relationship between dependent and independent variables. In all cases a p-value less than 0.05 was used as statistically significant of the test. The strength of the association was interpreted using an odds ratio in a 95% confidence interval. Finally, the results were presented as words, percentages, graphs and tables. Variables that were found with a statistically significant association (p < 0.05) at univariate logistic analysis were entered and analyzed by multiple logistic regression analysis.
Ethical Considerations
Ethical clearance was obtained from the institutional review board. The objective of the study was clearly described to the study participants including the benefits and risk, and confidentiality was maintained using identification codes. Considering the objective of the study and the demographic characteristics of the participants, the institutional review board gave a permission to take both verbal or written consent. So both verbal or written informed consents were taken from respondents prior to enrollment and collection of data. Verbal consent was taken from some respondents who are not able to read and write, and written consent was taken from those who can put their signature, and conducted in accordance with the Declaration of Helsinki.
Results
Socio-Demographic Characteristics
A total of 422, urinary tract infection suspected adults were investigated during the study period, of which 238 (56.4%) were females. The ages ranged from 18 to 89 years with the mean ± SD age of participants being 27.4 ± 15.6 years, Table 1.
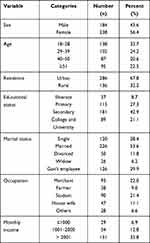 |
Table 1 Socio-Demographic Characteristics Among UTI Suspected Patients Attending at Arba Minch General Hospital, Southern Ethiopia, 2020 (n = 422) |
Among a total of 422, about 249/422 (59.0%) of the study participants had a previous history of antibiotic usage in the last 6 months while nearly half of study participants, 221/422 (52.4%) had previous histories of UTI. From 422 study participants, 153/422 (36.3%) had a chronic underlying disease and 148/422 (35.1%) had exposure to hospitalization within the last 12 months.
Prevalence of S. aureus and MRSA
Out of a total 422 study participants’ urine cultures, 12.6% (53/422) S. aureus were isolated, and 5.9% (n = 25/422) of the culture positives were from males and 6.6% (n = 28/422) were from females. The isolation rate of S. aureus was the highest in the 18–28 years group (35.8% (19/53)) whereas 47.2% (25/53) was isolated from married study participants.
Out of the 53 S. aureus recovered, 43.4% (23/53) were found to be MRSA and the remaining (56.6%) were MSSA. The overall prevalence of MRSA among study participants was 43.4% [95% CI (35.0, 47.0)]. MRSA in females was slightly higher than in males 22.6% (12/53) versus 20.8% (11/53). Meanwhile the highest 30.4% (7/23) MRSA was detected in the age group 18–28 years followed by age group 29–39 years 26.1% (6/23). However, MRSA prevalence did not differ significantly comparing males to females and in these age groups respectively.
Antimicrobial Susceptibility Pattern
The highest resistance pattern of S.aureus was seen in Ampicillin 96.5% (51/53) followed by Piperacillin (94.3%), Tobramycin (88.7%), Ciprofloxacin (81.1%), Norfloxacine (73.6%), Cefoxitin (43.4%) whereas highest sensitivity was seen in Nitrofurantoin (75.5%) followed by Cortimoxazole (58.6%). Amongst common antibiotics used for treatment of urinary tract infections, Nitrofurantoin was the most effective against S. aureus. Among the total number of isolated S. aureus, n = 23 were found to be resistant to Cefoxitin (MRSA) while the remaining n = 30 were sensitive to Cefoxitin (MSSA), Figure 1.
 |
Figure 1 Antimicrobials resistance of MRSA among UTI suspected patients attending at Arba Minch General Hospital, Southern Ethiopia. |
Both bivariate and multivariable logistic regression analyses were done to determine factors associated with the prevalence of MRSA. All variables including socio-demographic characteristics were assessed to determine whether they were a factor or not for the prevalence of MRSA with taking P-value less than or equal to 0.25 in bivariate analysis as a cut-off point.
In bivariate logistic regression analysis different socio-demographic and clinical factors such as marital status (married), previous exposure to UTI, previous antibiotic usage, presence of chronic underlying disease and hospitalization, were shown to have a statistical significant association with prevalence of MRSA, Table 2.
 |
Table 2 Bivariate Analysis of UTI Suspected Patients Attending at Arba Minch General Hospital, Southern Ethiopia, 2020 (= 422) |
In multivariate logistic regression analysis; previous exposure to UTI, presence of chronic disease and hospitalization were statistically associated. Those patients who have a history of hospitalization were 2 times more likely to develop MRSA compared to those who were not exposed (AOR: 2.43; 95% CI: 1.30–4.55), Table 3.
 |
Table 3 Multivariable Analysis of Factors Associated to MRSA Among UTI Suspected Patients Attending Arba Minch General Hospital, Southern Ethiopia, 2020 |
Discussion
MRSA is a global public health problem and MRSA infections can be found in both hospitals and the community.26 It is known as an important bacterial pathogen that can cause community and hospital acquired urinary tract infections with a high morbidity and mortality rate in spite of the use of antibiotics.27 In our study we found a 12.6% prevalence for S. aureus, and of this 43.4% was MRSA.
In the present study, the prevalence rate of S. aureus from UTI suspected clients was lower than from a similar study conducted in Debre Markos hospital,28 and from the other study in northeast Ethiopia.29 In the present study, 23 of MRSA isolates were recovered from 53 total isolates of S. aureus. The overall proportion of MRSA among study participants was 43.4% [95% CI (35.0, 47.0)] which was slightly higher than the study conducted in India.30 However, this finding was lower than that of the study reported from Debre Markos University, Ethiopia28 and from other country studies like; Nepal with a prevalence of 82.0%,31 Iran with a prevalence of 55.6%,32 and 64.3% reported from Nigeria.33 This difference in isolation rate of MRSA could be due to the isolation technique (multiple screens) and there might be specific risk factors which are not assessed by this study that might decrease the proportion of MRSA among S. aureus in the current study, sample collection method (they used more than one clinical specimen), variation in study participants (since they collected from patients with a confirmed UTI whereas this study is from UTI suspected participants only).
The overall proportion of MRSA among isolated S. aureus in this study was elevated from previous studies conducted in Iran (25.8%),34 Nepal (30.8%),35 Uganda (33.3%).36 and Nigeria (13%).37 This increased proportion of MRSA might be due to differences in geographic area, MRSA becoming a global nosocomial pathogen with rapid spread to health care as well as community and urinary tract infection associated factors may also play an important role in increasing the prevalence of MRSA in the community.
The highest level of antimicrobial resistance among S. aureus isolates in this study was observed in Ampicillin (96.5%) followed by Piperacillin (94.3%), Tobramycin (88.7%), Ciprofloxacin (81.1%), Norfloxacine (73.6%), and Cefoxitin (43.4%) whereas the highest sensitivity was seen in Nitrofurantoin (75.5%) followed by Cortimoxazole (58.6%). This report was in agreement among studies done in Iran38 and India.39
In a recent study the resistance of MRSA to Ampicillin and Tobramycin was 100%. This result was nearly consistent with the study done in northeast Ethiopia,29 meanwhile the resistance pattern of MRSA for commonly prescribed antibiotics was Nitrofurantoin (53.7%), Cortimoxazole (64.9%), Norfloxacine (76.3%) and Ciprofloxacin (69.4%), and this was similar with a previous study.40
Urinary tract infection's associated factors may play an important role in increasing the prevalence of UTIs in the study population. In the present study, a number of factors were assessed to determine whether they are an important factor or not for the prevalence of MRSA among UTI suspected patients. Those patients who have a history of hospitalization were 2 times more likely to acquired MRSA as compared to study participants who do not have a history of hospitalization (AOR: 2.43; 95% CI: 1.30–4.55). This finding was supported by studies conducted in Ethiopia, Jimma University,41 Egypt,42 China,43 Ireland44 and Brazil.45 The possible explanation may be due to the speed of S. aureus in becoming resistant to Methicillin, a similar method of isolation of MRSA, and rapid colonization of MRSA.
Another important factor which was statistically associated with prevalence of MRSA was the presence of a chronic underlying disease. Those study participants who have chronic underlying diseases were 2 times more susceptible to having MRSA relative to those who had not had a chronic underlying disease [AOR = 2.03, 95% CI: (1.07–3.83)]. This correlation was similar to a study conducted in Uganda.36 In addition study participants who had a history of previous exposure to UTI were 3 times more likely to develop MRSA relative to those who were not exposed to UTI previously (AOR: 2.55; 95% CI: 1.56–7.03).
Limitation of the Study
This study has a limitation that lack of susceptibility testing for clindamycin and levofloxacin, both of them which were potential options for treatment of MRSA, an appropriate CFU/mL cut-off to identify clinically significant S. aureus bacteriuria and too small sample to analyze resistance patterns of MSSA or MRSA.
Conclusion
The overall finding of MRSA in the current study was high. Ampicillin and Tobramycin should be no longer considered in the first line of drugs for UTI treatment because of high resistance. Health information dissemination about UTI and appropriate antibiotic use should be given to clients more specifically for UTI patients. Effective methods of infection prevention control should be utilized to help reduce this high prevalence of S. aureus and MRSA infections. Further phenotypic and genotypic studies are needed to establish and clarify the genetic mechanism behind antibiotic resistance and prevalence.
Abbreviations
ATCC, American Type Culture Collection; AOR, adjusted odds ratio; CLSI, Clinical Laboratory Standard Institute; OR, odds ratio; MRSA, methicillin resistance Staphylococcus aureus; MSA, Mannitol Salt Agar; SPSS, Statistical Package Social Sciences; UTI, Urinary Tract Infection; WHO, World Health Organization.
Data Sharing Statement
Data that support the findings of the study are found in the manuscript file.
Ethics Approval and Consent to Participate
Ethical clearance was secured from the College of Medicine and Health Sciences, Arba Minch University review committee and submitted to Arba Minch General Hospital. Confidentiality was maintained during data collection and analysis by using non-identifier codes. Regarding ethical issues, the study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
We would like to also acknowledge study participants, and Arba Minch General Hospital staffs for their help in selecting study targeted participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation or in all of these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article was submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests in this work.
References
1. Custovic A, Smajlovic J, Hadzic S, Ahmetagic S, Tihic N, Hadzagic H. Epidemiological surveillance of bacterial nosocomial infections in the surgical intensive care unit. Mater Sociomed. 2014;26(1):7. doi:10.5455/msm.2014.26.7-11
2. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi:10.1038/nrmicro3432
3. Renuart AJ, Goldfarb DM, Mokomane M, et al. Microbiology of urinary tract infections in Gaborone, Botswana. PLoS One. 2013;8(3):e57776. doi:10.1371/journal.pone.0057776
4. Geerlings SE. Clinical presentations and epidemiology of urinary tract infections. UTI. 2017;27–40.
5. Sasirekha B. Prevalence of ESBL, AmpC β-lactamases and MRSA among uropathogens and its antibiogram. EXCLI J. 2013;12:81.
6. Coates R, Moran J, Horsburgh MJ. Staphylococci: colonizers and pathogens of human skin. Future Microbiol. 2014;9(1):75–91. doi:10.2217/fmb.13.145
7. Miao J, Chen L, Wang J, et al. Current methodologies on genotyping for nosocomial pathogen methicillin-resistant Staphylococcus aureus (MRSA). Microb Pathog. 2017;107:17–28. doi:10.1016/j.micpath.2017.03.010
8. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–687.
9. Faden A. Methicillin-resistant Staphylococcus aureus (MRSA) screening of hospital dental clinic surfaces. Saudi J Biol Sci. 2019;26(7):1795–1798. doi:10.1016/j.sjbs.2018.03.006
10. Azeez-Akande O. Global trend of methicillin-resistant Staphlococcus aureus and emerging challenges for control. Afr J Clin Exp Microbiol. 2010;11(3). doi:10.4314/ajcem.v11i3.57771
11. Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22(5):416–422. doi:10.1016/j.cmi.2015.12.002
12. Tong SY, Chen LF, Fowler VG. Colonization, pathogenicity, host susceptibility, and therapeutics for Staphylococcus aureus: what is the clinical relevance?. In Seminars in immunopathology. Springer-Verlag. 2012;34(2):185–200.
13. Gilmore B, Hamill T, Gorman S, Jones D. Catheter-based drug–device combination products: an overview. Drug-Device Combination Prod. 2010;61–92.
14. Bereket W, Hemalatha K, Getenet B. Update on bacterial nosocomial infections. Eur Rev Med Pharmacol Sci. 2012;16(8):1039–1044.
15. Kosmidis CI, Chandrasekar PH. Management of gram-positive bacterial infections in patients with cancer. Leuk Lymphoma. 2012;53(1):8–18. doi:10.3109/10428194.2011.602770
16. Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi:10.1016/S1473-3099(18)30605-4
17. Dilnessa T, Bitew A. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolated from clinical samples at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia. BMC Infect Dis. 2016;16(1):1–9. doi:10.1186/s12879-016-1742-5
18. Sahile T, Esseye S, Beyene G, Ali S. Post-surgical infection and antibiotic susceptibility patterns of bacteria isolated from admitted patients with signs of infection at Jimma University specialized hospital, Jimma, Ethiopia. Int J Trop Dis Health. 2016;17(4):1–12. doi:10.9734/IJTDH/2016/27253
19. Kesah C, Ben Redjeb S, Odugbemi T, et al. Prevalence of methicillin‐resistant Staphylococcus aureus in eight African hospitals and Malta. Clin Microbiol Infect. 2003;9(2):153–156. doi:10.1046/j.1469-0691.2003.00531.x
20. Shahmoradi M, Faridifar P, Shapouri R, Mousavi SF, Ezzedin M, Mirzaei B. Determining the biofilm forming gene profile of staphylococcus aureus clinical isolates via multiplex colony PCR method. Rep Biochem Mol Biol. 2019;7(2):181–188.
21. Saïd-Salim B, Mathema B, Kreiswirth BN. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen. Infect Control Hosp Epidemiol. 2003;24(6):451–455. doi:10.1086/502231
22. Soto SM. Importance of biofilms in urinary tract infections: new therapeutic approaches. Adv Biol. 2014;2014:2014. doi:10.1155/2014/157895
23. Miller JM, Miller SA. A Guide to Specimen Management in Clinical Microbiology. John Wiley & Sons; 2017.
24. Cheesbrough M. District Laboratory Practice in Tropical Countries. Part 2. New York:Cambridge University Press; 2006:300–301.
25. Humphries RM, Abbott AN, Hindler JA. Understanding and addressing CLSI breakpoint revisions: a primer for clinical laboratories. J Clin Microbiol. 2019;57(6). doi:10.1128/JCM.00203-19
26. Bhatta DR, Cavaco LM, Nath G, et al. Association of Panton Valentine Leukocidin (PVL) genes with methicillin resistant Staphylococcus aureus (MRSA) in Western Nepal: a matter of concern for community infections (a hospital based prospective study). BMC Infect Dis. 2016;16(1):1–6. doi:10.1186/s12879-016-1531-1
27. Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G. Ciprofloxacin resistance in community-and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2015;15(1):1–16. doi:10.1186/s12879-015-1282-4
28. Kahsay A, Mihret A, Abebe T, Andualem T. Isolation and antimicrobial susceptibility pattern of Staphylococcus aureus in patients with surgical site infection at Debre Markos Referral Hospital, Amhara Region, Ethiopia. Arch Public Health. 2014;72(1):1–7. doi:10.1186/2049-3258-72-16
29. Kibret M, Abera B. Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia. Asian Pac J Trop Biomed. 2014;4(2):164–168. doi:10.1016/S2221-1691(14)60226-4
30. Mendem SK, Gangadhara TA, Shivannavar CT, Gaddad SM. Antibiotic resistance patterns of Staphylococcus aureus: a multi center study from India. Microb Pathog. 2016;98:167–170. doi:10.1016/j.micpath.2016.07.010
31. Shrestha LB, Baral R, Khanal B. Comparative study of antimicrobial resistance and biofilm formation among Gram-positive uropathogens isolated from community-acquired urinary tract infections and catheter-associated urinary tract infections. Infect Drug Resist. 2019;12:957. doi:10.2147/IDR.S200988
32. Yousefi M, Fallah F, Arshadi M, Pourmand M, Hashemi A, Pourmand G. Identification of tigecycline-and vancomycin-resistant Staphylococcus aureus strains among patients with urinary tract infection in Iran. New Microbes New Infect. 2017;19:8–12. doi:10.1016/j.nmni.2017.05.009
33. Ibe C, Onyeagba R, Charles S, et al. Prevalence and antibiotic susceptibility patterns of methicillin-resistant Staphylococcus aureus (MRSA) isolated from healthy inhabitants of Uturu rural communities, Abia state, Nigeria. J Nat Sci Res. 2013;3(10):85–91.
34. Rahimi F, Katouli M, Karimi S. Biofilm production among methicillin resistant Staphylococcus aureus strains isolated from catheterized patients with urinary tract infection. Microb Pathog. 2016;98:69–76. doi:10.1016/j.micpath.2016.06.031
35. Shrestha B, Pokhrel B, Mohapatra T. Study of nosocomial isolates of Staphylococcus aureus with special reference to methicillin resistant S. aureus in a tertiary care hospital in Nepal. Nepal Med Coll. 2009;11(2):123–126.
36. Bahati J, Stephen BM, Joseph N, Asiphas O, Musa K, Taseera K. Prevalence and bacteriology of symptomatic urinary tract infection among pregnant women at Mbarara Regional Referral Hospital, South-western Uganda. J Interpers Violence. 2020;35(17–18):3286–3307. doi:10.1177/0886260517708407
37. Mofolorunsho CK, Ocheni M, Omatola CA, Agieni AG. Staphylococcus Aureus prevalence and antibiotic susceptibility profile in anyigba, north-central Nigeria. Am J Infect Dis. 2015;11(4):93. doi:10.3844/ajidsp.2015.93.97
38. Yousefi M, Pourmand MR, Fallah F, Hashemi A, Mashhadi R, Nazari-Alam A. Characterization of Staphylococcus aureus biofilm formation in urinary tract infection. Iran J Public Health. 2016;45(4):485.
39. Singh D, Chand A, Goel S. Prevalence of MRSA among Staphylococcus aureus isolated from patients of urinary tract infection along with its antibiogram. Int J. 2019;2(4):364.
40. Hussain M, Basit A, Khan A, et al. Antimicrobial sensitivity pattern of methicillin resistant Staphylococcus aureus isolated from hospitals of Kohat district. Pak J Inf Mol Biol. 2013;1(1):13–16.
41. Kejela T, Bacha K. Prevalence and antibiotic susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) among primary school children and prisoners in Jimma Town, Southwest Ethiopia. Ann Clin Microbiol Antimicrob. 2013;12(1):1–11. doi:10.1186/1476-0711-12-11
42. Eldegla H, Eldars W, Mesbah MR, et al. Healthcare associated infections and patterns of antibiotic resistance in tropical medicine department in Egypt. Int J Curr Microbiol Appl Sci. 2016;5(6):1–15. doi:10.20546/ijcmas.2016.506.001
43. Mao P, Peng P, Liu Z, Xue Z, Yao C. Risk factors and clinical outcomes of hospital-acquired mrsa infections in Chongqing, China. Infect Drug Resist. 2019;12:3709. doi:10.2147/IDR.S223536
44. Looney AT, Redmond EJ, Davey NM, et al. Methicillin-resistant Staphylococcus aureus as a uropathogen in an Irish setting. Medicine. 2017;96(14):14. doi:10.1097/MD.0000000000004635
45. Santos HB, Machado DP, Camey SA, Kuchenbecker RS, Barth AL, Wagner MB. Prevalence and acquisition of MRSA amongst patients admitted to a tertiary-care hospital in Brazil. BMC Infect Dis. 2010;10(1):1–7. doi:10.1186/1471-2334-10-328
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
