Back to Journals » Infection and Drug Resistance » Volume 14
Landscape of Multidrug-Resistant Gram-Negative Infections in Egypt: Survey and Literature Review
Authors El-Kholy A , El-Mahallawy HA, Elsharnouby N, Abdel Aziz M, Helmy AM, Kotb R
Received 17 January 2021
Accepted for publication 26 March 2021
Published 24 May 2021 Volume 2021:14 Pages 1905—1920
DOI https://doi.org/10.2147/IDR.S298920
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Amani El-Kholy,1 Hadir A El-Mahallawy,2 Noha Elsharnouby,3 Mohamed Abdel Aziz,4 Ahmed Mohamed Helmy,5 Ramy Kotb6
1Department of Clinical Pathology, Faculty of Medicine, Cairo University, Cairo, Egypt; 2Clinical Pathology Department, National Cancer Institute, Cairo University, Cairo, Egypt; 3Department of Anesthesia, Intensive Care and Pain Management, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 4Pfizer Egypt, Levant & Iraq Medical Affairs, Cairo, Egypt; 5Pfizer Egypt Medical Affairs, Cairo, Egypt; 6Pfizer Africa & Middle East Medical Affairs, Dubai, United Arab Emirates
Correspondence: Amani El-Kholy
Department of Clinical Pathology, Faculty of Medicine, Cairo University, Cairo, Egypt
Tel +20 1001678063
Email [email protected]
Purpose: This article is the first to review published reports on the prevalence of multidrug-resistant (MDR) gram-negative infections in Egypt and gain insights into antimicrobial resistance (AMR) surveillance and susceptibility testing capabilities of Egyptian medical centers.
Materials and Methods: A literature review and online survey were conducted.
Results: The online survey and literature review reported high prevalence of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae (19– 85.24% of E. coli, and 10– 87% of K. pneumoniae), carbapenem-resistant Enterobacteriaceae (35– 100% of K. pneumoniae and 13.8– 100% of E. coli), carbapenem-resistant Acinetobacter baumannii (10– 100%), and carbapenem-resistant Pseudomonas aeruginosa (15– 70%) in Egypt. Risk factors for MDR Gram-negative infections were ventilated patients (67.4%), prolonged hospitalization (53.5%) and chronic disease (34.9%). Although antimicrobial surveillance capabilities were deemed at least moderate in most centers, lack of access to rapid AMR diagnostics, lack of use of local epidemiological data in treatment decision-making, lack of antimicrobial stewardship (AMS) programs, and lack of risk prediction tools were commonly reported by respondents.
Conclusion: This survey has highlighted the presence of knowledge gaps as well as limitations in the surveillance and monitoring capabilities of AMR in Egypt, with most laboratories lacking rapid diagnostics and molecular testing. Future efforts in Egypt should focus on tackling these issues via nationwide initiatives, including understanding the AMR trends in the country, capacity building of laboratories and their staff to correctly and timely identify AMR, and introducing newer antimicrobials for targeting emerging resistance mechanisms in Gram-negative species.
Keywords: Egypt, Gram-negative bacteria, hospital-acquired pneumonia, intra-abdominal infections, multidrug resistance, urinary tract infections
Introduction
Gram-negative species are ubiquitous in nature and associated with a plethora of infections in different body sites.1 Gram-negative bacteria are intrinsically resistant to many antibiotics.2 Antimicrobial resistance (AMR) in Gram-negative bacteria has become increasingly problematic for healthcare systems over the past 20 years, with escalating costs and mortality.1 Gram-negative species are frequently associated with nosocomial infections, including bloodstream infections (BSI), hospital-acquired pneumonia (HAP), urinary tract infections (UTI), skin and soft-tissue infections (SSTI) and complicated intra-abdominal infections (cIAI).3,4
Resistance becomes particularly problematic when organisms become multidrug-resistant (MDR) or extensively drug-resistant (XDR) as physicians are then limited by the treatment options they can use.5 In addition to the increasing resistance to available treatments, concerns regarding the limited number of new treatments under development have also been raised.6 In response to these concerns, the World Health Organization (WHO) published the global priority list of antibiotic-resistant bacteria, identifying resistant species deemed to be the most significant globally, and for which there is an urgent requirement for new antimicrobials to be developed.7 Three Gram-negative species were deemed “critical” under priority-one: carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Pseudomonas aeruginosa (CRPA), and carbapenem-resistant and third-generation cephalosporin-resistant Enterobacteriaceae.7
As new classes of antibiotics have been developed, Gram-negative bacteria have gained resistance through a variety of β-lactamase enzymes: initially through penicillinases, then cephalosporinases, extended-spectrum β-lactamases (ESBLs) and carbapenemases.8 Carbapenem resistance can result from the production of carbapenemase enzymes, or the combined activity of ESBL and efflux pumps, or porin mutations.1 Both intrinsic and acquired carbapenem resistance has been observed in Gram-negative bacteria, with acquired resistance in Enterobacteriaceae being mainly plasmid-mediated leading to horizontal gene transfer.8 Carbapenems are highly effective broad-spectrum antibiotics; there is therefore an urgent need for carbapenem-sparing strategies to reduce the spread of multidrug resistance globally.9
Treatment of MDR Gram-negative infections requires high levels of clinical and microbiological expertise as well as knowledge of the local epidemiology and patient risk factors.10 Commonly used antimicrobial agents to treat MDR Gram-negative infections include colistin, aminoglycosides, tigecycline, carbapenems and Fosfomycin.10–15 Novel treatment options for MDR gram-negative infections include the β-lactam/β-lactamase inhibitor (BL–BLI) combinations (ceftazidime–avibactam, meropenem–vaborbactam, ceftolozane–tazobactam and imipenem cilastatin relebactam), eravacycline and cefiderocol.10,16–19 Challenges in the treatment of MDR Gram-negative infections include the emergence of resistance to novel antimicrobials, such as ceftazidime–avibactam,20 meropenem–vaborbactam,21 ceftolozane–tazobactam,22 and cefiderocol.23 Implementing restrictions on the overuse of these antibiotics is therefore required whilst ensuring early and appropriate treatment for critically ill patients.10 Further challenges include the ineffectiveness of newer agents against CRAB24 and the need for carbapenemase identification prior to BL-BLI treatment.10 There is growing complexity surrounding the treatment of MDR Gram-negative infections, with the necessity to understand both national and local epidemiology, how to provide adequate empiric coverage, and have sufficient surveillance to identify opportunities to treat bacteria with emerging resistance mechanisms with new agents.
Aims
This article aims to:
- Review the prevalence of MDR Gram-negative infections in Egypt from published literature
- Gain a better understanding of the epidemiology of MDR Gram-negative infections as well as AMR surveillance and susceptibility testing capabilities of medical centers in Egypt through an online survey
We focused on the WHO priority-one MDR Gram-negative pathogens. This is the first publication of its kind to summarize published data concerning the prevalence of MDR Gram-negative infections in Egypt and to obtain additional insights using a survey.
Materials and Methods
Literature Search
A literature search of publications reporting prevalence of MDR Gram-negative human infections in Egypt was performed. Publications were considered if they: included full-text articles with data concerning infections from medical or surgical units of hospitals in Egypt between 2004 and 2020, were written in English, included data concerning multidrug resistance, carbapenem resistance (or carbapenemase production) or third-generation cephalosporin-resistant (or extended-spectrum beta-lactamase production) in Enterobacteriaceae, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii or Pseudomonas aeruginosa and if they contained data concerning infections in humans. References within the publications were also screened for potential inclusion.
A MedLine database search was conducted through PubMed using the search string and filters: (Egypt[Text Word] OR Egyptian[Text Word]) AND (Gram-negative OR Gram negative OR Enterobacteriaceae OR Escherichia coli OR Klebsiella pneumoniae OR Acinetobacter baumannii OR Pseudomonas aeruginosa) AND (infection OR bacteria OR organism OR pathogen) AND (multidrug resistant OR multidrug‐resistant OR multi‐drug resistant OR MDR OR carbapenem resistance OR carbapenem‐resistant OR carbapenemase OR extended-spectrum beta-lactamase OR ESBL OR third-generation cephalosporin resistant OR MBL OR metallo-beta-lactamase); filters: Full text, English, from 2004/1/1–2020/11/28; sorted by: First Author. The last search was conducted on the 28 November 2020. Titles and abstracts were first screened for inclusion, followed by a full-text review. Data was then extracted and tabulated for included publications.
Online Survey
The online survey was created using SurveyMonkey®. Questions were generated to focus on the most common bacteria causing nosocomial infections, especially the WHO “critical” priority pathogens,7 and on the capabilities of centers across Egypt and the barriers they face. Questions were all closed-ended questions with yes/no or multiple-choice answers only to ensure reliability of the survey. Survey results were collected anonymously, with only center names and specialty collected for the participating physicians to encourage honest reporting of information.
Validity of the survey was established using published methodologies:25,26 firstly, the survey was reviewed on two levels, by a group of experts in antimicrobial resistance who evaluated if the survey questions sufficiently captured the topic of interest and by a medical communications agency with experience in questionnaire development who evaluated the survey for clarity, consistency and errors. Secondly, a pilot was conducted on 8 participants to determine the acceptability and clarity of the questions, and to confirm its face validity. The questionnaire was modified accordingly, and the responses obtained in the pilot study were excluded from the study analysis.
For centers to participate in the survey, they had to meet the following eligibility criteria: (1) tertiary care hospital, (2) more than 30 intensive care unit (ICU) beds, (3) functional laboratory with supplies and personnel able to perform culture and full bacterial identification and antimicrobial susceptibility testing, and (4) infectious disease physicians or ICU physicians with expertise in infectious diseases and AMR, based on selection criteria for Egypt’s national hospital-acquired infections (HAI) surveillance program.27 The survey was circulated to physicians on September 21, 2020 with a follow-up sent on September 28, 2020. The survey was closed on October 20, 2020. Analysis included descriptive statistics of survey responses, median and interquartile range (IQR). Responses were summarized where a response was given, excluding missing values. All statistical analyses were performed using StataIC (StataCorp, Version 16).
The study protocol was approved by the Research Ethics Committee of Cairo University Medical School in accordance with the Declaration of Helsinki (Ethical approval number: N-13-2020). All physicians who responded to the survey consented to participation in the study and publication of the data.
Results
Literature Search
A total of 277 studies were screened. It was deemed that 201 publications were not eligible for inclusion. A total of 76 publications were included (Figure 1).
Prevalence of MDR Gram-negative infections in Egypt described in the literature is summarized in Figures 2–8, details of the studies are provided in Supplementary File 1 and additional data in Supplementary File 2. Reports of multidrug resistance in Gram-negative bacterial species are high in Egypt with 21–100% of P. aeruginosa, 30–100% of A. baumannii, 42.5–98.73% of K. pneumoniae and 22.8–96.07% of Escherichia coli defined as MDR.
 |
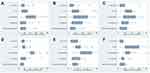
Figure 2 Summary of the prevalence (%) of reported carbapenem-resistant/carbapenemase-producing Gram-negative species: (A) A. baumanii; (B) P. aeruginosa; and (C) Enterobacteriaceae. Notes: Data from references 27,32–63. aAcinetobacter spp.; b% of CRAB; c% of P. aeruginosa isolates resistant to one or more β-lactams; d% of MDR P. aeruginosa; e% of carbapenem-non-susceptible K. pneumoniae; f% of CRKP |
 |
Figure 3 Summary of the prevalence (%) of ESBL-producing/third-generation cephalosporin-resistant Enterobacteriaceae (ESBL-E). Notes: Data from references 27,33,42,64–79. aKlebsiella spp. |
 |
Figure 4 Summary of the prevalence (%) of carbapenemase genes in A. baumannii. Notes:Data from references36–40,80–84.a% of imipenem-insusceptible A. baumannii; b% of A. baumannii. |
 |
Figure 5 Summary of the prevalence (%) of carbapenemase genes in P. aeruginosa. Notes: Data from references 37,40,44–47,49,51,80,85–92. a% of MBL-producing P. aeruginosa; b% of P. aeruginosa isolates resistant to one or more β-lactams; c% of XDR-CRPA; d% of imipenem-resistant P. aeruginosa; e% of carbapenemase genes |
 |
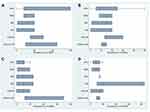
Figure 6 Summary of the prevalence (%) of carbapenemase and ESBL genes in Enterobacteriaceae. Notes: Data from references 52–55,64,72,93. a% of ESBL-E; b% of carbapenemase genes; c% of CRE |
 |
Figure 7 Summary of the prevalence (%) of carbapenemase and ESBL genes in E. coli. Notes: Data from references 40,55,75–78,80,94,95. a% of ESBL-producing/third-generation cephalosporin-resistant E. coli; b% of CREC; c% MDR E. coli |
 |
Figure 8 Summary of the prevalence (%) of carbapenemase and ESBL genes in K. pneumoniae. Notes: Data from references 40,55–62,70,80,94–99. a% of carbapenemase-resistant/-producing K. pneumoniae; b% of MDR K. pneumoniae; c% of ESBL-producing/third-generation cephalosporin-resistant K. pneumoniae; d% of ertapenem non-susceptible K. pneumoniae. |
Carbapenem resistance was reported in 26.6–100% of A. baumannii, 21–70% of P. aeruginosa and 28.8–69% of Enterobacteriaceae (K. pneumoniae 48.1–100% and E. coli 13.8–100%) in Egypt (Figure 2). ESBL production or third-generation cephalosporin resistance was reported in 16–48.93% of Enterobacteriaceae, 14–87% of E. coli, and 19–85.24% of K. pneumoniae, respectively (Figure 3). Carbapenemase genes New Delhi metallo-β-lactamase (NDM) and K. pneumoniae carbapenemase (KPC) were commonly reported in A. baumannii with a prevalence of 0–39.3% and 0–28.6%, respectively (Figure 4). Verona integron-encoded metallo-β-lactamase (VIM) and imipenemase metallo-β-lactamase (IMP) were commonly reported in P. aeruginosa with a prevalence of 0–100% and 0–42.8%, respectively (Figure 5). NDM and oxacillin carbapenemase 48 (OXA-48) genes were commonly reported in Enterobacteriaceae with a prevalence of 26.04–68.88% and 30–58.62%, respectively (Figure 6). NDM genes were also commonly reported in E.coli with a prevalence of 13.7–80.39% (Figure 7). In K. pneumoniae, there was a high prevalence of KPC, NDM and OXA-48 genes at 0–95.8%, 20.9–100%, and 0–80.65%, respectively (Figure 8). ESBL genes cefotaximase-Munich (CTX-M), sulphydryl variable (SHV) and Temoniera (TEM) were highly prevalent in 20.2–89.13%, 10.1–50% and 31.5–56.52% of Enterobacteriaceae, respectively (Figure 6). In E. coli, CTX-M and TEM prevalence was 32.8–100% and 4.2–100%, respectively (Figure 7). In K. pneumoniae, CTX-M, SHV and TEM prevalence was 38.9–100%, 0–81% and 0.4–57.1%, respectively (Figure 8).
Survey Results
Survey results are discussed below and provided in full in Supplementary File 3.
Participating Physicians
Of the 99 physicians who were sent the survey, 46 (46.5%) responded. Where specialty was specified (n = 40), 53.49% (n = 23) of the physicians were intensivists, 39.53% (n = 17) microbiologists, 4.65% (n = 2) infectious disease specialists, and 2.33% (n = 1) internal medicine specialists. Responding physicians were from 14 centers across Egypt.
Risk Factors and Types of HAI
Respondents reported the most common risk factors for MDR Gram-negative infections were ventilated patients (67.4%) or those experiencing prolonged hospitalization (53.5%). Chronic disease (34.9%), prior antibiotic use (32.6%) and elderly (23.3%) were also relatively common (Figure 9). The most common type of HAI in their centers was HAP (65.9%), followed by UTI (12.2%), and BSI (9.8%) (Figure 10, Figure 11, Figure 12, Figure 13).
 |
Figure 9 Risk factors for MDR Gram-negative infections. |
Prevalence of Gram-Negative MDR Species
Median (IQR) prevalence of Gram-negative species, Gram-negative MDR species and carbapenemases reported by responding centers are shown in Figures 11–13. Most respondents reported increased prevalence of MDR gram-negative infections over the past 5 years in their centers (95.83%), with most of these reporting an increase of 25–50% (60.87%). Many respondents predicted that prevalence of such infections is likely to increase over the next 10 years (58.33%).
Capabilities
Whilst 16.67% of respondents reported best-practice capabilities, the majority of respondents reported either good (37.50%) or moderate (29.17%) AMR surveillance capabilities in their centers, with more than half (58.33%) of respondents reporting that surveillance cultures were being performed for colonization at their center. In terms of antimicrobial susceptibility testing (AST), a large proportion of respondents reported that their centers performed culture antimicrobial susceptibility testing for all ICU patients (86.36%). One respondent reported that only patients at high risk of MDR infection were tested. Vitek® 2 was the most common automated AST system available to the respondents (50%). Other available automated AST systems or devices included Beckman Coulter Microscan® (5%), BD Phoenix™ Emerge™ (5%), BioFire® FilmArray® (20%), and other (15%). Most respondents reported that their centers were changing AST technology to take advantage of susceptibility panels with a greater range of more recent antimicrobial agents (81.82%). Only seven (35%) respondents reported having access to rapid AMR detection diagnostics, with singleplex PCR (n = 3), multiplex PCR (n = 2), microarray nanoparticle identification (n = 2), and GeneXpert® (n = 1) being the available platforms specified.
Clinical Practice Procedures
The Infectious Diseases Society of America (IDSA) was the most used source of guideline recommendations in clinical practice (65%), with national guidelines (40%), hospital guidelines (30%), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) (25%), WHO (20%), and The British Society for Antimicrobial Chemotherapy (5%) also reported. Half of physicians also reported other guidelines that significantly impacted on their clinical practice in treating Gram-negative infections. Most respondents reported that the guidelines implemented were either partially adequate (45%) or not adequate (40%) for managing MDR gram-negative infections. Whilst 60% of respondents reported hospital-specific protocols or standard operating procedures for the treatment of Gram-negative infections, the majority (75%) reported that Gram-negative infection risk prediction protocols were not used. Twelve respondents (65%) reported that local epidemiology data was used in treatment decision-making and contributed to antimicrobial stewardship (AMS), five respondents (25%) reported that it was not used. Eleven respondents (55%) reported the presence of a specific AMS program in their center.
Barriers and Challenges
More than half of respondents reported a lack of collaboration and communication between the laboratory and clinical staff (54.55%) as one of the key issues relating to AMR surveillance; incomplete data (36.36%) and lack of regulation governing surveillance (31.82%) were also key issues. Cost of tests (62.50%), knowledge gaps in clinicians of relevance to antimicrobial surveillance (56.25%), speed of test result reporting (43.75%), and availability of tests in laboratory (37.50%) were barriers to ideal susceptibility testing. The cost of newer treatments (55%), lack of access to newer antibiotics (50%), lack of formal AMS programs (45%), and clinical teams’ poor compliance with recommendations (30%) were considered barriers to successful management of MDR Gram-negative infections.
Discussion
The survey and the literature review both highlight the magnitude of the AMR problem across Egypt, with reports of very high rates of ESBL-E, CRE, CRAB, and CRPA. According to the survey, carbapenem resistance was reported in 20% (10‒45%) of E. coli, 35% (25‒60%) of K. pneumoniae, 15% (3‒40%) of P. aeruginosa, and 10% (5‒30%) of A. baumannii. ESBL production was reported in 20% (10‒60%) of E. coli, 10% (5‒50%) of K. pneumoniae, and 17% (7‒20%) of other Enterobacteriaceae. Key challenges faced by physicians within the country relate to a lack of access to the necessary tools, such as rapid diagnostics, molecular testing, local epidemiology data and newer antibiotics, as well as a lack of healthcare infrastructure, stewardship, regulation, and collaboration.
A recent review of the epidemiology of MDR infections across the Arab League has shown that Egypt reports high resistance levels compared to its neighbors.28 ESBL-E prevalence was 4‒25% in the Gulf Cooperation Council (GCC), 31‒66% in the Levant, and 9‒35% in the rest of the African countries versus 55% in Egypt.28 CRE prevalence was 0‒1% in the GCC, 1‒22.5% in the Levant, and <2% in the rest of the African countries versus 28% in Egypt. CRPA prevalence was 3‒21% in the GCC, 28‒93% in the Levant, and 19‒56% in the rest of the African countries versus 51% in Egypt. Finally, CRAB prevalence was 36‒100% in the GCC, 64‒89% in the Levant, and 75‒88% in the rest of the African countries versus 93% in Egypt.28 Such high levels of resistance in Egypt compared to neighboring countries are thought to be the result of challenges in infection prevention and control,29 high levels of antibiotic consumption,30 and use in non-human populations.31 The levels of resistance reported in our online survey are lower than those reported within this review, potentially a result of differences in the populations or hospitals included in both studies.
The survey and the literature review also highlight the large variation in prevalence of MDR infections across hospitals or centers. Some of this variability might be a result of many studies reporting prevalence within a small number of centers or hospitals (typically between one and six hospitals) or with small number of samples or isolates. However, three published studies have described prevalence of MDR Gram-negative HAI via Egypt’s national HAI surveillance program, reporting data from up to 310 ICUs from 72 hospitals.27,32,33 Whilst historically Egypt has reported limitations in its surveillance and microbiology capabilities,29 the national HAI surveillance program paves the way to obtaining epidemiological data which will help to inform infection control and prevention strategies.27 A further potential reason for the variability in prevalence may be the nature of the Egyptian healthcare system: a complex and fragmented network of Ministry of Health and Population facilities, private hospitals, university hospitals, and military hospitals, as well as other ministry-associated hospitals with varied levels and availability of medical services.29 This poses significant challenges in terms of AMS and AMR control initiatives. Furthermore, in contrast to many countries, the infectious disease specialty is almost absent in Egypt, which impedes prudent use of antimicrobials, adequate cross-specialty communication, institution-wide infectious disease leadership, and the development and management of AMS systems. Finally, the process of data collection varies between different centers, with no single authority responsible for organizing data collection concerning AMR. A further gap in MDR Gram-negative infection reporting in Egypt includes the lack of data concerning community acquired MDR infections.
The survey has provided valuable information concerning MDR Gram-negative infections in Egypt and has collected a broad range of data concerning prevalence of such infections, capabilities of centers, and key barriers and issues faced in AMR surveillance, susceptibility testing, and management of MDR Gram-negative infections. The survey was anonymous to encourage honest reporting of data by physicians; nevertheless, the accuracy of the data reported cannot be verified. Furthermore, only tertiary care hospitals with capabilities to perform bacterial identification and AST were included in the survey; this has the potential to overestimate the prevalence of MDR Gram-negative species across the country. The number of responses to questions relating to carbapenemase prevalence within the survey was low, resulting from the lack of molecular testing capabilities within the country. Therefore, cautious interpretation of carbapenemase prevalence is required. Future efforts in Egypt should focus on collecting additional data concerning molecular characterization of resistance genes across a variety of healthcare centers in Egypt. Such molecular data will provide a true understanding of the resistance patterns in MDR Gram-negative infections in the country, facilitate future use of novel antimicrobials such as BL-BLIs and, therefore, is required to combat recent trends of increasing antimicrobial resistance. Future steps in the fight against AMR in Egypt should include antimicrobial stewardship to optimize the use of antibiotics, improve laboratory capacities and national initiatives to control antimicrobial resistance.
Conclusion
The survey has provided valuable information concerning the epidemiology and resistance patterns of MDR Gram-negative infections in Egypt, whilst highlighting the challenges and barriers faced by physicians. Collective efforts to overcome these challenges and reduce the burden of MDR Gram-negative infections across the country are urgent and critical to preventing the spread of multidrug resistance. Nationwide initiatives are needed for understanding the AMR trends in the country, capacity building of laboratories and staff to correctly and timely identify AMR as well as introducing newer antimicrobials for treatment of MDR Gram-negative infections.
Acknowledgments
We thank all participating physicians who completed the survey. Medical writing support was provided by Dr. Abigail Holland of Connect Communications, Dubai.
Funding
This work, including medical writing support, was supported by Pfizer, Egypt.
Disclosure
Professor Noha Elsharnouby reports non-financial support from Pfizer, during the conduct of the study. Dr Mohamed Abdel Aziz, Ahmed Mohamed Helmy, and Ramy Kotb report being employees of Pfizer during the conduct of the study and outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Eichenberger EM, Thaden JT. Epidemiology and mechanisms of resistance of extensively drug resistant Gram-negative bacteria. Antibiotics (Basel). 2019;8(2). doi:10.3390/antibiotics8020037
2. Poole K. Multidrug resistance in Gram-negative bacteria. Curr Opin Microbiol. 2001;4(5):500–508. doi:10.1016/S1369-5274(00)00242-3
3. Gaynes R, Edwards JR; National Nosocomial Infections Surveillance S. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–854. doi:10.1086/432803
4. Sartelli M, Catena F, Ansaloni L, et al. Complicated intra-abdominal infections worldwide: the definitive data of the CIAOW Study. World J Emerg Surg. 2014;9(1):37. doi:10.1186/1749-7922-9-37
5. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
6. Kaye KS, Pogue JM. Infections caused by resistant Gram-negative bacteria: epidemiology and management. Pharmacotherapy. 2015;35(10):949–962. doi:10.1002/phar.1636
7. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics; 2017. Available from: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/.
8. Codjoe FS, Donkor ES. Carbapenem resistance: a review. Med Sci (Basel). 2017;6(1). doi:10.3390/medsci6010001
9. Corcione S, Lupia T, Maraolo AE, Mornese Pinna S, Gentile I, De Rosa FG. Carbapenem-sparing strategy: carbapenemase, treatment, and stewardship. Curr Opin Infect Dis. 2019;32(6):663–673. doi:10.1097/QCO.0000000000000598
10. Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR Gram-negative bacteria. Front Med (Lausanne). 2019;6:74. doi:10.3389/fmed.2019.00074
11. Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6(9):589–601. doi:10.1016/S1473-3099(06)70580-1
12. Panidis D, Markantonis SL, Boutzouka E, Karatzas S, Baltopoulos G. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest. 2005;128(2):545–552. doi:10.1378/chest.128.2.545
13. Zusman O, Avni T, Leibovici L, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57(10):5104–5111. doi:10.1128/AAC.01230-13
14. Grabein B, Graninger W, Rodriguez Bano J, Dinh A, Liesenfeld DB. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect. 2017;23(6):363–372. doi:10.1016/j.cmi.2016.12.005
15. Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2014;58(9):5598–5601. doi:10.1128/AAC.02435-13
16. Montravers P, Bassetti M. The ideal patient profile for new beta-lactam/beta-lactamase inhibitors. Curr Opin Infect Dis. 2018;31(6):587–593. doi:10.1097/QCO.0000000000000490
17. Lucasti C, Vasile L, Sandesc D, et al. Phase 2, Dose-Ranging Study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal infection. Antimicrob Agents Chemother. 2016;60(10):6234–6243. doi:10.1128/AAC.00633-16
18. Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 2013;68(10):2286–2290. doi:10.1093/jac/dkt178
19. Wright H, Bonomo RA, Paterson DL. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect. 2017;23(10):704–712. doi:10.1016/j.cmi.2017.09.001
20. Shields RK, Chen L, Cheng S, et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2017;61(3).
21. Sun D, Rubio-Aparicio D, Nelson K, Dudley MN, Lomovskaya O. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61(12). doi:10.1128/AAC.01694-17
22. MacVane SH, Pandey R, Steed LL, Kreiswirth BN, Chen L. Emergence of ceftolozane-tazobactam-resistant pseudomonas aeruginosa during treatment is mediated by a single AmpC structural mutation. Antimicrob Agents Chemother. 2017;61(12). doi:10.1128/AAC.01183-17
23. Malik S, Kaminski M, Landman D, Quale J. Cefiderocol resistance in acinetobacter baumannii: roles of beta-lactamases, siderophore receptors, and penicillin binding protein 3. Antimicrob Agents Chemother. 2020;64(11). doi:10.1128/AAC.01221-20
24. Isler B, Doi Y, Bonomo RA, Paterson DL. New treatment options against carbapenem-resistant acinetobacter baumannii infections. Antimicrob Agents Chemother. 2019;63(1). doi:10.1128/AAC.01110-18
25. Bolarinwa OA. Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Niger Postgrad Med J. 2015;22(4):195–201. doi:10.4103/1117-1936.173959
26. Chiwaridzo M, Chikasha TN, Naidoo N, et al. Content validity and test-retest reliability of a low back pain questionnaire in Zimbabwean adolescents. Arch Physiother. 2017;7:3. doi:10.1186/s40945-017-0031-y
27. Talaat M, El-Shokry M, El-Kholy J, et al. National surveillance of health care-associated infections in Egypt: developing a sustainable program in a resource-limited country. Am J Infect Control. 2016;44(11):1296–1301. doi:10.1016/j.ajic.2016.04.212
28. Moghnieh RA, Kanafani ZA, Tabaja HZ, Sharara SL, Awad LS, Kanj SS. Epidemiology of common resistant bacterial pathogens in the countries of the Arab League. Lancet Infect Dis. 2018;18(12):e379–e394. doi:10.1016/S1473-3099(18)30414-6
29. Talaat M, Kandeel A, Rasslan O, et al. Evolution of infection control in Egypt: achievements and challenges. Am J Infect Control. 2006;34(4):193–200. doi:10.1016/j.ajic.2005.05.028
30. Talaat M, Saied T, Kandeel A, et al. A point prevalence survey of antibiotic use in 18 hospitals in Egypt. Antibiotics (Basel). 2014;3(3):450–460. doi:10.3390/antibiotics3030450
31. Dahshan H, Abd-Elall AM, Megahed AM, Abd-El-Kader MA, Nabawy EE. Veterinary antibiotic resistance, residues, and ecological risks in environmental samples obtained from poultry farms, Egypt. Environ Monit Assess. 2015;187(2):2. doi:10.1007/s10661-014-4218-3
32. Kotb S, Lyman M, Ismail G, et al. Epidemiology of Carbapenem-resistant Enterobacteriaceae in Egyptian intensive care units using National Healthcare-associated infections surveillance data, 2011–2017. Antimicrob Resist Infect Control. 2020;9:2. doi:10.1186/s13756-019-0639-7
33. See I, Lessa FC, ElAta OA, et al. Incidence and pathogen distribution of healthcare-associated infections in pilot hospitals in Egypt. Infect Control Hosp Epidemiol. 2013;34(12):1281–1288. doi:10.1086/673985
34. Abdulzahra AT, Khalil MAF, Elkhatib WF. First report of colistin resistance among carbapenem-resistant Acinetobacter baumannii isolates recovered from hospitalized patients in Egypt. New Microbes New Infect. 2018;26:53–58. doi:10.1016/j.nmni.2018.08.007
35. Al-Hassan L, El Mehallawy H, Amyes SG. Diversity in Acinetobacter baumannii isolates from paediatric cancer patients in Egypt. Clin Microbiol Infect. 2013;19(11):1082–1088. doi:10.1111/1469-0691.12143
36. Benmahmod AB, Said HS, Ibrahim RH. Prevalence and mechanisms of carbapenem resistance among acinetobacter baumannii clinical isolates in Egypt. Microb Drug Resist. 2019;25(4):480–488. doi:10.1089/mdr.2018.0141
37. El-Kholy AA, Elanany MG, Sherif MM, Gad MA. High prevalence of VIM, KPC, and NDM expression among surgical site infection pathogens in patients having emergency surgery. Surg Infect (Larchmt). 2018;19(6):629–633. doi:10.1089/sur.2018.088
38. El-Sayed-Ahmed MA, Amin MA, Tawakol WM, Loucif L, Bakour S, Rolain JM. High prevalence of bla(NDM-1) carbapenemase-encoding gene and 16S rRNA armA methyltransferase gene among Acinetobacter baumannii clinical Isolates in Egypt. Antimicrob Agents Chemother. 2015;59(6):3602–3605. doi:10.1128/AAC.04412-14
39. Fouad M, Attia AS, Tawakkol WM, Hashem AM. Emergence of carbapenem-resistant Acinetobacter baumannii harboring the OXA-23 carbapenemase in intensive care units of Egyptian hospitals. Int J Infect Dis. 2013;17(12):e1252–1254. doi:10.1016/j.ijid.2013.07.012
40. Makharita RR, El-Kholy I, Hetta HF, et al. Antibiogram and genetic characterization of carbapenem-resistant Gram-negative pathogens incriminated in healthcare-associated infections. Infect Drug Resist. 2020;13:3991–4002. doi:10.2147/IDR.S276975
41. Nageeb W, Kamel M, Zakaria S, Metwally L. Phenotypic characterization of Acinetobacter baumannii isolates from intensive care units at a tertiary-care hospital in Egypt. East Mediterr Health J. 2014;20(3):203–211. doi:10.26719/2014.20.3.203
42. Raouf M, Ghazal T, Kassem M, Agamya A, Amer A. Surveillance of surgical-site infections and antimicrobial resistance patterns in a tertiary hospital in Alexandria, Egypt. J Infect Dev Ctries. 2020;14(3):277–283. doi:10.3855/jidc.12124
43. Sultan AM, Seliem WA. Identifying risk factors for healthcare-associated infections caused by carbapenem-resistant acinetobacter baumannii in a neonatal intensive care unit. Sultan Qaboos Univ Med J. 2018;18(1):e75–e80. doi:10.18295/squmj.2018.18.01.012
44. Basha AM, El-Sherbiny GM, Mabrouk MI. Phenotypic characterization of the Egyptian isolates “extensively drug-resistant Pseudomonas aeruginosa” and detection of their metallo-β-lactamases encoding genes. Bull Natl Res Cent. 2020;44:117. doi:10.1186/s42269-020-00350-8
45. El-Mahdy R, El-Kannishy G. Virulence factors of carbapenem-resistant pseudomonas aeruginosa in hospital-acquired infections in Mansoura, Egypt. Infect Drug Resist. 2019;12:3455–3461. doi:10.2147/IDR.S222329
46. Farhan SM, Ibrahim RA, Mahran KM, Hetta HF, Abd El-Baky RM. Antimicrobial resistance pattern and molecular genetic distribution of metallo-beta-lactamases producing Pseudomonas aeruginosa isolated from hospitals in Minia, Egypt. Infect Drug Resist. 2019;12:2125–2133. doi:10.2147/IDR.S198373
47. Gaballah A, Elbaradei A, Elsheredy A, Kader O. Emergence of blaVEB and blaGES among VIM-producing Pseudomonas aeruginosa clinical isolates in Alexandria, Egypt. Acta Microbiol Immunol Hung. 2019;66(1):131–142. doi:10.1556/030.65.2018.044
48. Hashem H, Hanora A, Abdalla S, Shawky A, Saad A. Carbapenem susceptibility and multidrug-resistance in pseudomonas aeruginosa isolates in Egypt. Jundishapur J Microbiol. 2016;9(11):e30257. doi:10.5812/jjm.30257
49. Hashem H, Hanora A, Abdalla S, Shaeky A, Saad A. Dissemination of metallo-beta-lactamase in Pseudomonas aeruginosa isolates in Egypt: mutation in blaVIM-4. APMIS. 2017;125(5):499–505. doi:10.1111/apm.12669
50. Khalifa HO, Soliman AM, Ahmed AM, et al. High carbapenem resistance in clinical Gram-negative pathogens isolated in Egypt. Microb Drug Resist. 2017;23(7):838–844. doi:10.1089/mdr.2015.0339
51. Zafer MM, Al-Agamy MH, El-Mahallawy HA, Amin MA, Ashour MS. Antimicrobial resistance pattern and their beta-lactamase encoding genes among Pseudomonas aeruginosa strains isolated from cancer patients. Biomed Res Int. 2014;2014:101635. doi:10.1155/2014/101635
52. ElMahallawy H, Zafer MM, Amin MA, Ragab MM, Al-Agamy MH. Spread of carbapenem resistant Enterobacteriaceae at tertiary care cancer hospital in Egypt. Infect Dis (Lond). 2018;50(7):560–564. doi:10.1080/23744235.2018.1428824
53. Helal SF, El-Rachidi NG, AbdulRahman EM, Hassan DM. The presence of blaKPC-mediated resistance in Enterobacteriaceae in Cairo University hospitals in Egypt and its correlation with in vitro carbapenem susceptibility. J Chemother. 2014;26(2):125–128. doi:10.1179/1973947813Y.0000000099
54. Kamel NA, El-Tayeb WN, El-Ansary MR, Mansour MT, Aboshanab KM. Phenotypic screening and molecular characterization of carbapenemase-producing Gram-negative bacilli recovered from febrile neutropenic pediatric cancer patients in Egypt. PLoS One. 2018;13(8):e0202119. doi:10.1371/journal.pone.0202119
55. Tawfick MM, Alshareef WA, Bendary HA, Elmahalawy H, Abdulall AK. The emergence of carbapenemase blaNDM genotype among carbapenem-resistant Enterobacteriaceae isolates from Egyptian cancer patients. Eur J Clin Microbiol Infect Dis. 2020;39(7):1251–1259. doi:10.1007/s10096-020-03839-2
56. El-Badawy MF, El-Far SW, Althobaiti SS, Abou-Elazm FI, Shohayeb MM. The First Egyptian report showing the co-existence of bla NDM-25, bla OXA-23, bla OXA-181, and bla GES-1 among carbapenem-resistant K. pneumoniae clinical isolates genotyped by BOX-PCR. Infect Drug Resist. 2020;13:1237–1250. doi:10.2147/IDR.S244064
57. ElMahallawy H, Zafer MM, Al-Agamy M, et al. Dissemination of ST101 blaOXA-48 producing Klebsiella pneumoniae at tertiary care setting. J Infect Dev Ctries. 2018;12(6):422–428. doi:10.3855/jidc.9789
58. Ghaith DM, Zafer MM, Said HM, et al. Genetic diversity of carbapenem-resistant Klebsiella Pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur J Clin Microbiol Infect Dis. 2020;39(3):583–591. doi:10.1007/s10096-019-03761-2
59. Khalil MAF, Elgaml A, El-Mowafy M. Emergence of multidrug-resistant New Delhi Metallo-beta-Lactamase-1-producing klebsiella pneumoniae in Egypt. Microb Drug Resist. 2017;23(4):480–487. doi:10.1089/mdr.2016.0003
60. Khalil MAF, Hager R, Abd-El Reheem F, et al. A Study of the Virulence Traits of carbapenem-resistant klebsiella pneumoniae Isolates in a Galleria mellonella model. Microb Drug Resist. 2019;25(7):1063–1071. doi:10.1089/mdr.2018.0270
61. Mohamed ER, Ali MY, Waly N, Halby HM, El-Baky RMA. The Inc FII plasmid and its contribution in the transmission of blaNDM-1 and blaKPC-2 in Klebsiella pneumoniae in Egypt. Antibiotics (Basel). 2019;8(4). doi:10.3390/antibiotics8040266
62. Ragheb SM, Tawfick MM, El-Kholy AA, Abdulall AK. Phenotypic and genotypic features of klebsiella pneumoniae harboring carbapenemases in Egypt: OXA-48-like carbapenemases as an investigated model. Antibiotics (Basel). 2020;9(12). doi:10.3390/antibiotics9120852
63. Wasfi R, Elkhatib WF, Ashour HM. Molecular typing and virulence analysis of multidrug resistant Klebsiella pneumoniae clinical isolates recovered from Egyptian hospitals. Sci Rep. 2016;6:38929. doi:10.1038/srep38929
64. Abdallah HM, Wintermans BB, Reuland EA, et al. Extended-spectrum beta-lactamase- and carbapenemase-producing enterobacteriaceae isolated from Egyptian patients with suspected blood stream infection. PLoS One. 2015;10(5):e0128120. doi:10.1371/journal.pone.0128120
65. Bouchillon SK, Johnson BM, Hoban DJ, et al. Determining incidence of extended spectrum beta-lactamase producing Enterobacteriaceae, vancomycin-resistant Enterococcus faecium and methicillin-resistant Staphylococcus aureus in 38 centres from 17 countries: the PEARLS study 2001–2002. Int J Antimicrob Agents. 2004;24(2):119–124. doi:10.1016/j.ijantimicag.2004.01.010
66. Fam N, Leflon-Guibout V, Fouad S, et al. CTX-M-15-producing Escherichia coli clinical isolates in Cairo (Egypt), including isolates of clonal complex ST10 and clones ST131, ST73, and ST405 in both community and hospital settings. Microb Drug Resist. 2011;17(1):67–73. doi:10.1089/mdr.2010.0063
67. Zafer MM, El-Mahallawy HA, Abdulhak A, Amin MA, Al-Agamy MH, Radwan HH. Emergence of colistin resistance in multidrug-resistant Klebsiella pneumoniae and Escherichia coli strains isolated from cancer patients. Ann Clin Microbiol Antimicrob. 2019;18(1):40. doi:10.1186/s12941-019-0339-4
68. Abd-Elmonsef MME, Elsharawy D, Abd-Elsalam AS. Mechanical ventilator as a major cause of infection and drug resistance in intensive care unit. Environ Sci Pollut Res Int. 2018;25(31):30787–30792. doi:10.1007/s11356-017-8613-5
69. Awad HA, Mohamed MH, Badran NF, Mohsen M, Abd-Elrhman AS. Multidrug-resistant organisms in neonatal sepsis in two tertiary neonatal ICUs, Egypt. J Egypt Public Health Assoc. 2016;91(1):31–38. doi:10.1097/01.EPX.0000482038.76692.3
70. El-Domany RA, Awadalla OA, Shabana SA, El-Dardir MA, Emara M. Analysis of the correlation between antibiotic resistance patterns and virulence determinants in pathogenic klebsiella pneumoniae isolates from Egypt. Microb Drug Resist. 2020. doi:10.1089/mdr.2020.0236
71. El-Kholy A, Saied T, Gaber M, et al. Device-associated nosocomial infection rates in intensive care units at Cairo University hospitals: first step toward initiating surveillance programs in a resource-limited country. Am J Infect Control. 2012;40(6):e216–220. doi:10.1016/j.ajic.2011.12.010
72. Galal L, Abdel Aziz NA, Hassan WM. Defining the relationship between phenotypic and genotypic resistance profiles of multidrug-resistant enterobacterial clinical isolates. Adv Exp Med Biol. 2019;1214:9–21.
73. Saied T, Elkholy A, Hafez SF, et al. Antimicrobial resistance in pathogens causing nosocomial bloodstream infections in university hospitals in Egypt. Am J Infect Control. 2011;39(9):e61–65. doi:10.1016/j.ajic.2011.04.009
74. Talaat M, Hafez S, Saied T, Elfeky R, El-Shoubary W, Pimentel G. Surveillance of catheter-associated urinary tract infection in 4 intensive care units at Alexandria university hospitals in Egypt. Am J Infect Control. 2010;38(3):222–228. doi:10.1016/j.ajic.2009.06.011
75. Abd El-Baky RM, Masoud SM, Mohamed DS, et al. Prevalence and some possible mechanisms of colistin resistance among multidrug-resistant and extensively drug-resistant pseudomonas aeruginosa. Infect Drug Resist. 2020;13:323–332. doi:10.2147/IDR.S238811
76. Abdelaziz MO, Bonura C, Aleo A, Fasciana T, Cala C, Mammina C. Cephalosporin resistant Escherichia coli from cancer patients in Cairo, Egypt. Microbiol Immunol. 2013;57(5):391–395. doi:10.1111/1348-0421.12046
77. Al-Agamy MHM, El-Din Ashour MS, Wiegand I. First description of CTX-M beta-lactamase-producing clinical Escherichia coli isolates from Egypt. Int J Antimicrob Agents. 2006;27(6):545–548. doi:10.1016/j.ijantimicag.2006.01.007
78. El-Badawy MF, Tawakol WM, Maghrabi IA, Mansy MS, Shohayeb MM, Ashour MS. Iodometric and molecular detection of ESBL production among clinical isolates of E. coli fingerprinted by ERIC-PCR: the First Egyptian Report Declares the Emergence of E. coli O25b-ST131clone Harboring blaGES. Microb Drug Resist. 2017;23(6):703–717. doi:10.1089/mdr.2016.0181
79. Hassan WM, Hashim A, Domany R. Plasmid mediated quinolone resistance determinants qnr, aac(6ʹ)-Ib-cr, and qep in ESBL-producing Escherichia coli clinical isolates from Egypt. Indian J Med Microbiol. 2012;30(4):442–447. doi:10.4103/0255-0857.103766
80. Abdulall AK, Tawfick MM, El Manakhly AR, El Kholy A. Carbapenem-resistant Gram-negative bacteria associated with catheter-related bloodstream infections in three intensive care units in Egypt. Eur J Clin Microbiol Infect Dis. 2018;37(9):1647–1652. doi:10.1007/s10096-018-3294-7
81. Abouelfetouh A, Torky AS, Aboulmagd E. Phenotypic and genotypic characterization of carbapenem-resistant Acinetobacter baumannii isolates from Egypt. Antimicrob Resist Infect Control. 2019;8:185. doi:10.1186/s13756-019-0611-6
82. Al-Agamy MH, Khalaf NG, Tawfick MM, Shibl AM, El Kholy A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int J Infect Dis. 2014;22:49–54. doi:10.1016/j.ijid.2013.12.004
83. Alkasaby NM, El Sayed Zaki M. Molecular Study of Acinetobacter baumannii Isolates for Metallo-beta-Lactamases and extended-Spectrum-beta-Lactamases Genes in Intensive Care Unit, Mansoura University Hospital, Egypt. Int J Microbiol. 2017;2017:3925868. doi:10.1155/2017/3925868
84. El Bannah AMS, Nawar NN, Hassan RMM, Salem STB. Molecular epidemiology of carbapenem-resistant acinetobacter baumannii in a Tertiary Care Hospital in Egypt: clonal spread of blaOXA-23. Microb Drug Resist. 2018;24(3):269–277. doi:10.1089/mdr.2017.0057
85. Abaza AF, El Shazly SA, Selim HSA, Aly GSA. Metallo-Beta-Lactamase producing pseudomonas aeruginosa in a Healthcare setting in Alexandria, Egypt. Pol J Microbiol. 2017;66(3):297–308. doi:10.5604/01.3001.0010.4855
86. Abbas HA, El-Ganiny AM, Kamel HA. Phenotypic and genotypic detection of antibiotic resistance of Pseudomonas aeruginosa isolated from urinary tract infections. Afr Health Sci. 2018;18(1):11–21. doi:10.4314/ahs.v18i1.3
87. El Zowalaty ME, Gyetvai B. Effectiveness of antipseudomonal antibiotics and mechanisms of multidrug resistance in pseudomonas aeruginosa. Pol J Microbiol. 2016;65(1):23–32. doi:10.5604/17331331.1197272
88. El-Domany RA, Emara M, El-Magd MA, Moustafa WH, Abdeltwab NM. Emergence of imipenem-resistant pseudomonas aeruginosa clinical isolates from Egypt Coharboring VIM and IMP carbapenemases. Microb Drug Resist. 2017;23(6):682–686. doi:10.1089/mdr.2016.0234
89. El-Mahdy TS. Expression of ampC, oprD, and mexA, outer membrane protein analysis and carbapenemases in multidrug resistant clinical isolates of Pseudomonas aeruginosa from Egypt. J Chemother. 2014;26(6):379–381. doi:10.1179/1973947814Y.0000000195
90. Raouf MR, Sayed M, Rizk HA, Hassuna NA. High incidence of MBL-mediated imipenem resistance among Pseudomonas aeruginosa from surgical site infections in Egypt. J Infect Dev Ctries. 2018;12(7):520–525. doi:10.3855/jidc.9936
91. Soliman AM, Zarad HO, Nariya H, Shimamoto T, Shimamoto T. Genetic analysis of carbapenemase-producing Gram-negative bacteria isolated from a university teaching hospital in Egypt. Infect Genet Evol. 2020;77:104065. doi:10.1016/j.meegid.2019.104065
92. Zafer MM, Al-Agamy MH, El-Mahallawy HA, Amin MA, El din ashour S. Dissemination of VIM-2 producing Pseudomonas aeruginosa ST233 at tertiary care hospitals in Egypt. BMC Infect Dis. 2015;15:122. doi:10.1186/s12879-015-0861-8
93. Poirel L, Abdelaziz MO, Bernabeu S, Nordmann P. Occurrence of OXA-48 and VIM-1 carbapenemase-producing Enterobacteriaceae in Egypt. Int J Antimicrob Agents. 2013;41(1):90–91. doi:10.1016/j.ijantimicag.2012.08.015
94. El-Kholy AA, Girgis SA, Shetta MAF, Abdel-Hamid DH, Elmanakhly AR. Molecular characterization of multidrug-resistant Gram-negative pathogens in three tertiary hospitals in Cairo, Egypt. Eur J Clin Microbiol Infect Dis. 2020;39(5):987–992. doi:10.1007/s10096-020-03812-z
95. Helmy OM, Kashef MT. Different phenotypic and molecular mechanisms associated with multidrug resistance in Gram-negative clinical isolates from Egypt. Infect Drug Resist. 2017;10:479–498. doi:10.2147/IDR.S147192
96. Ahmed SH, Daef EA, Badary MS, Mahmoud MA, Abd-Elsayed AA. Nosocomial blood stream infection in intensive care units at Assiut University Hospitals (Upper Egypt) with special reference to extended spectrum beta-lactamase producing organisms. BMC Res Notes. 2009;2:76. doi:10.1186/1756-0500-2-76
97. Azzab MM, El-Sokkary RH, Tawfeek MM, Gebriel MG. Multidrug-resistant bacteria among patients with ventilator associated pneumonia in an emergency intensive care unit, Egypt. East Mediterr Health J. 2016;22(12):894–903. doi:10.26719/2016.22.12.894
98. Hassuna NA, AbdelAziz RA, Zakaria A, Abdelhakeem M. Extensively-drug resistant Klebsiella pneumoniae recovered from neonatal sepsis cases from a major NICU in Egypt. Front Microbiol. 2020;11:1375. doi:10.3389/fmicb.2020.01375
99. Metwally L, Gomaa N, Attallah M, Kamel N. High prevalence of Klebsiella pneumoniae carbapenemase-mediated resistance in K. pneumoniae isolates from Egypt. East Mediterr Health J. 2013;19(11):947–952. doi:10.26719/2013.19.11.947
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.