Back to Journals » Infection and Drug Resistance » Volume 14
Prevalence, Clinical Characteristics and Changes of Antibiotic Resistance in Children with Nontyphoidal Salmonella Infections from 2009–2018 in Chongqing, China
Authors Wu L, Luo Y, Shi G, Li Z
Received 16 January 2021
Accepted for publication 17 March 2021
Published 13 April 2021 Volume 2021:14 Pages 1403—1413
DOI https://doi.org/10.2147/IDR.S301318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Li-juan Wu,1,* Yan Luo,2,* Guo-lu Shi,3,* Zhong-yue Li1
1Department of Gastroenterology, Ministry of Education Key Laboratory of Child Development and Disorders, National Clinical Research Center for Child Health and Disorders; China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Children’s Hospital of Chongqing Medical University, Chongqing Key Laboratory of Pediatrics, Chongqing, People’s Republic of China; 2Department of Pediatrics, Wushan County People’s Hospital of Chongqing, Chongqing, People’s Republic of China; 3Department of Pediatrics, Suining Central Hospital, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhong-yue Li
Department of Gastroenterology, Ministry of Education Key Laboratory of Child Development and Disorders, National Clinical Research Center for Child Health and Disorders; China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Children’s Hospital of Chongqing Medical University, Chongqing Key Laboratory of Pediatrics, No. 136, 2nd Zhongshan Road, Yuzhong District, Chongqing, People’s Republic of China
Tel +86-18225022855
Fax +86-68370225
Email [email protected]
Purpose: Nontyphoidal Salmonella (NTS) is a leading bacterial cause for acute gastroenteritis in children. With the increasing use of antibiotics worldwide, antibiotic resistance has become a global problem. However, data on NTS infections and changes in antibiotic resistance among children remain limited in China. We aimed to characterize the prevalence, clinical feature, serotype and the changes of antibiotic resistance of NTS in children in Chongqing.
Methods: 501 hospitalized children with NTS infections (confirmed by positive NTS culture) in Children’s Hospital of Chongqing Medical University from January 2009 to December 2018 were included. The clinical data and drug sensitivity test results were retrospectively reviewed and analyzed. Antibiotic resistance in NTS infections was compared between 2009– 2013 and 2014– 2018.
Results: A total of 501 isolates were detected. Most NTS infections occurred in children under three years old, which mainly occurred between July and October. The numbers of patients with diarrhea, fever, and vomiting were 472 (94.2%), 422 (84.2%) and 146 (29.1%), respectively. Serogroup B (67.5%) was the predominant serogroup isolates. And Salmonella Typhimurium was the most common serotype (79.2%). The study compared the drug resistance of NTS from 2009 to 2013 with that from 2014 to 2018. We found the drug resistance rates of NTS to cefazolin, cefotaxime, ciprofloxacin, levofloxacin and imipenem showed an upward trend. The drug resistance rates of NTS to chloramphenicol, ampicillin, ceftriaxone, cefepime and compound sulfamethoxazole decreased slightly, but still showed high drug resistance rates. And drug resistance rates of NTS to piperacillin/tazobactam and ceftazidime decreased significantly in the last ten years. Multi-drug resistance (MDR) isolates, were detected among 69 cases (13.7%) of 501 children with NTS infections.
Conclusion: The overall antibiotic resistance rates remained at a high level in Chongqing. Continuous surveillance of antibiotic resistance in NTS and control measures such as avoiding unnecessary antibiotic therapy in general NTS gastroenteritis are important. For severe or invasive infections caused by NTS infection in Southwest China, the use of ceftazidime is recommended until antibiotic sensitivity test results are available. And the choice of antibiotics should be based on the curative effect and the antibiotics sensitivity results.
Keywords: nontyphoidal Salmonella, pediatric, prevalence, antibiotic resistance
Introduction
In China, food safety was recognized as the second largest risk of diseases.1 70–80% of food poisoning incidents were caused by Salmonella in China.2 Even though sanitary measures, water treatment and food safety standards have improved in the past ten years, NTS infections in children remains a global public health concern.3 WHO estimates that 93.8 million cases of gastroenteritis worldwide are linked to Salmonella infections each year, and Salmonella infections caused 155,000 deaths.4 In the United States, NTS causes 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths every year, respectively.5 In china, during 2000–2009, NTS annually caused 9.87 million gastroenteritis cases and 792 deaths.6 In view of the seriousness of the situation, laboratory surveillance for NTS infections has been strengthened in China in recent years.7
Salmonellae is a group of gram-negative, facultative anaerobic bacteria. The genus Salmonella, named for Dr. Daniel Salmon, was first described in 1866 by Dr. Theobald Smith.8 There are three kinds of surface antigens of Salmonella, which are body (O) antigens, flagella (H) antigens and capsule (VI) antigens. According to the “O” antigens, different serogroups of Salmonella can be classified. And according to the “H” antigens, different serotypes can be further divided. Salmonella comprises more than 2600 serotypes which belong to 42 serogroups.9,10 And Salmonella can be divided into Salmonella typhi and NTS. Salmonella typhi are divided into typhoid bacillus and paratyphosus bacillus, which can cause typhoid and paratyphoid. Other serotypes, known as NTS, can cause acute gastroenteritis, chronic enteritis, septicemia and other diseases. Previous researches have shown that young children are the most vulnerable group to NTS.11,12 Immature immunity may account for the high risk of infection at this stage.13 A national study reported that 34% of diarrheal patients infected with NTS were aged <5 years old in China in 2008.7 The pathogenicity of Salmonella is mainly related to the virulence factors that it carries, including Salmonella pathogenicity islands (SPIs), virulence plasmids, pili, and enterotoxins.14 The pathogenic factors of NTS will be released to induce the host to develop a mucosal inflammation response after NTS infections.15 When the host tries to eliminate the bacteria, it may cause “collateral damage” that destroys the human intestinal microecological balance, resulting in clinical symptoms like diarrhea.13 NTS gastroenteritis is mostly a self-limiting disease and resolves within one week in healthy children.16 However, a proportion of cases can lead to invasive disease in human, including systemic infections such as bacteremia, vasculitis, intracranial infections, respiratory infections, urinary tract infections and osteomyelitis. Invasive NTS infections can be life-threatening, and it usually occurs in infants, the elderly and patients with immune deficiency.17 And antibiotics therapy is indicated only if it causes severe infections or invasive infection.18,19 Antibiotic regimens recommended by clinicians against human Salmonella infections include third generation cephalosporins, quinolones, and macrolides.20 Considering the side effects of drug, third generation cephalosporins are recommended for use in children. The misuse of antibiotics is an important factor responsible for high resistance to antibiotics. And antibiotic resistance is especially problematic in these systemic infections, where antibiotic therapy can be life-saving.21 The bacterial strains resistant to three or more than three antimicrobial drug classes are defined as multi-drug resistant (MDR). It was reported that about 17–20% strains had antibiotic resistance, and the incidence of MDR was increasing in abroad.22,23 A study in Shanghai, China, showed antimicrobial susceptibility displays 60.5% of isolates resistant to one clinically important antibiotics.24 Another research in Shanghai found that only 1.1% strains were sensitive to all 16 antibiotics, and the antibiotic resistance rates of the third and fourth generation cephalosporin (cefotaxime and cefepime) were 10% and 8.1%.25 Besides, a study in Guangzhou showed annual resistance rates of ampicillin are relatively stable while the resistance rates of NTS to ceftazidime in 2015 (31,43%) were significantly higher than that in 2014 (16%). And the rates of antibiotic resistance to ampicillin between serotype Typhimurium and Enteritidis isolates were significantly higher than those in other serotypes.26 The drug resistance rates of Salmonella to cephalosporin and cefepime were 22.3% and 13.1%, respectively in 4 hospitals in Shenzhen,27 which were all higher than the results of previous studies in China.28,29 This phenomenon shows that the antibiotic resistance is not optimistic. Regarding the mechanisms of antibiotic resistance, it suggests that the corresponding resistance genes are commonly located on either plasmids, transposons, gene cassettes or variants of the Salmonella genomic islands SGI1 and SGI2.30 However, researches about NTS infections and the change of antibiotic resistance in pediatrics is limited in China. We aimed to summarize the clinical features, serotype, serogroup and the changes of antibiotic resistance during 2009 to 2018 in Chongqing, Southwest China. To enhance the realization of the disease, accordingly improve the ability of diagnosis and treatment in clinical.
Materials and Methods
Study Design and Data Collection
This was a retrospective study between January 2009 and December 2018 at the Children’s Hospital of Chongqing Medical University, one of the largest children’s hospitals in China. The annual outpatient visits of our hospital are nearly 3 million, and the annual inpatient visits are about 80,000. Clinical data of children with positive NTS culture were collected in this study. Other clinical data such as age, sex, duration of hospitalization and antibiotic susceptibility test results of NTS were retrospectively analyzed for patients under 18 years (removal of newborns).
Specimens Collection, Bacterial Culture and Drug Sensitivity Test
We collected different samples according to the clinical symptoms and clinician’s judgment. Samples of feces, blood, bone marrow, cerebrospinal fluid and secretions were included in our study. Fecal specimens were collected in dry, clean, leak-proof specimen collect containers. The blood and bone marrow specimens were placed into a special blood culture bottle immediately after collected. Specimens of cerebrospinal fluid were collected and placed into sterile tubes for bacterial culture. Excretions were collected by sterile pharyngeal swab tube. Chromogenic Salmonella Agar has been used for rapid isolation and identification of Salmonella (Shanghai Comagal Microbial Technology CO., LTD). To increase the culture rates of bacteria, the specimens and preproliferative culture medium were placed into 36°C±1°C constant temperature incubator for 4–6 hours enrichment. The preproliferative solution was inoculated into the selenite cystine culture medium for secondary proliferation for 18–24 hours. Then the proliferation solution was inoculated on agar plate for 18–24 hours. If there were no typical or suspicious colonies, we usually continue to culture for another 24 hours. The serotyping, serogrouping and antibiotic susceptibility test were further conducted for patients with positive culture of NTS. We exclude same kind of specimens from the same patient in case repetition. Antibiotic susceptibility tests were evaluated by the Kirby–Bauer disk diffusion method (Oxoid, UK). Isolates were classified as resistant, intermediate, and susceptible according to the Clinical Laboratory Standards Institute (CLSI) guidelines.31 Escherichia coli ATCC25922 was used for quality control in disk diffusion test.
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 data editor. Count data were described using frequencies and their proportions, and Chi-square (χ2) test was used for comparison. Measurement data were described using median, average ± standard deviation and quartile, compared using nonparametric Mann–Whitney test. For all analyses, we regarded statistical significance as p<0.05.
Results
Prevalence and Clinical Characteristics
Over the past ten years, 501 cases of NTS infections were confirmed, among which 459 were only positive in feces culture, 21 were only positive in blood culture, 2 were only positive in pus culture and 1 was only positive in bone marrow culture. Both fecal and blood culture were positive in 14 cases. Both blood and bone marrow culture were positive in 2 cases. Both blood and cerebrospinal fluid culture were positive in 2 cases. Although cases of NTS infections occurred all around the year, the peak occurred from summer to autumn, mainly in July (13.4%), August (16.7%), September (17.0%) and October (17.4%) (Figure 1). Among these 501 patients infected by NTS, 306 cases were male and 195 were female, for a male to female ratio of 1.5:1. The main age of onset was one month to three years old (84.8%), in which 44.9% were younger than 1 year old. Among these 501 patients, 84.2% patients had fever, which was mainly moderate to high fever. The median temperature was 39.5°C (range 37.6°C-42°C, mean 39.5±0.04°C), and the median thermal duration was 3 days (range 1–41 days, mean 4.47±0.20 days). 472 patients (94.2%) had diarrhea. Their stools were mostly watery or sticky. Among the children with diarrhea, feces only contained mucus in 218 cases (46.2%), only contained blood in 31 cases (6.6%), contained both mucus and blood in 18 cases (3.8%). And 25 cases (5.3%) showed typical mucopurulent bloody stools. The others (38.1%) had no mucus and blood in stools. The median frequencies of diarrhea were 7 times/day (ranging from 2 to 30 times/day, with an average of 7.67±0.18 times/day). And the median duration of diarrhea was 10 days (ranging from 1 to 240 days, with an average of 12.0±0.59 days). Other concomitant symptoms were vomiting, convulsion, abdominal pain and distension. Laboratory tests showed that PCT, CRP and leukocyte elevation accounted for 94.6%, 57.1% and 37.3%, respectively.
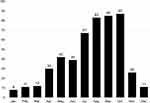 |
Figure 1 Distribution of 501 cases of children with nontyphoidal Salmonella infections by month. Y axis: number of cases. |
Serogroup and Serotype
Serogroup B (67.5%) was the predominant serogroup isolates, followed by serogroup C (11.8%), D (11.6%), E (4.2%), ungrouped (5%) and one case was the polyvalent serum group. Serotype identification was performed on 284 isolates. In total, 12 serotypes were identified among the isolates. Salmonella Typhimurium was the most common serotype, accounting for 79.2% (225/284), followed by Salmonella Enteritidis (9.5%, 27/284). Other rare Salmonella serotypes included Salmonella Derby, Stanley, Dublin, London, Aragorn, Newport, Bovis morbifificans, Sao Paulo, Weltevreden, Anatum and one case was the polyvalent serotype (Table 1).
 |
Table 1 Prevalence, Clinical Features, Laboratory Tests, Serogroup and Serotype in 501 Patients with NTS Infections |
Antibiotic Susceptibility Tests
In our study, the majority of patients (95.6%) received antibiotics therapy. And most of patients (92.42%) had clinic resolution through appropriate therapy. Antibiotic sensitivity results showed that the highest rates of antibiotic resistance were recorded in relation to cefazolin and aminoglycoside antibiotics (amikacin, gentamicin, tobramycin), 93.8%, 94.1%, 98.2%, 95.1%, respectively, followed by ampicillin (82.7%), chloramphenicol (59.3%), cotrimoxazole (44.2%), ciprofloxacin (18.5%), levofloxacin (7.2%). Among the third generation cephalosporins, the antibiotic resistance rates of cefotaxime, ceftriaxone ceftazidime were (33.3%), (32.5%), (18.7%), respectively. The antibiotic resistance rate of cefepime was 20.8%. The antibiotic resistance rate of piperacillin-tazobactam was about 7.1% and imipenem’s resistance rate was less than 1% (Table 2). Antibiotic susceptibility tests showed that NTS had highest resistance rates to aminoglycoside antibiotics, high resistance rates to ampicillin and cefazolin, and relatively low resistance rates to amoxicillin-clavulanate potassium, piperacillin-tazobactam and ceftazidime. The resistance rates to quinolones antibiotics (ciprofloxacin, levofloxacin) were lower than 20%, among which levofloxacin was the lowest (less than 10%).
 |
Table 2 Antimicrobial Susceptibility Results of NTS Isolated from 501 Patients |
Drug Resistance Changes Over the Past Decade
Our study compared the antibiotic resistance rates of NTS between 2009 to 2013 and 2014 to 2018 (Figure 2). We found that the antibiotic resistance rates of NTS against cefazolin (from 77.1% to 95.9%), cefotaxime (from 28.3% to 34.4%), ciprofloxacin (from 12.3% to 19.2%) and levofloxacin (from 1.9% to 7.9%) were on the rise. The antibiotic resistance rates to chloramphenicol (from 66.7% to 56.9%), ampicillin (from 56.1% to 42.8%), ceftriaxone (from 44.2% to 32.1%), cefepime (from 27.3% to 20.1%) and compound sulfamethoxazole (from 89.6% to 81.7%) showed a downward trend. The antibiotic resistance rates of NTS to piperacillin-tazobactam (from 21.4% to 5.4%) and ceftazidime (from 30.6% to 17.1%) decreased significantly. But the overall antibiotic resistance rates were still high.
MDR Isolates
By the late 1980s, multiple countries had reported MDR strains of Salmonella. In our study, 69 strains (13.8%) of MDR were isolated from 501 children with NTS infections. The proportion of MDR isolates showed a decreasing trend (Figure 3).
 |
Figure 3 The proportion of MDR isolates in each year. Notes:The black column indiciates MDR, the grey column indicates NTS. |
Prevalence and Antibiotic Resistance of Salmonella Typhimurium
Salmonella Typhimurium was the most common serotype among the NTS infections. We found that the prevalence of Salmonella Typhimurium was higher in recent years. Salmonella Typhimurium infections accounted for 79.2% of the total NTS infections in our hospital (Figure 4). The incidence of antibiotic resistance of Salmonella Typhimurium is displayed in Table 3. Our study detected that the trend of the antibiotic resistance changes of Salmonella Typhimurium was basically corresponding to that of the overall antibiotic resistance of NTS.
 |
Table 3 Antimicrobial Susceptibility Profile of Salmonella Typhimurium |
Discussion
In China, NTS is a major enteric pathogen for acute gastroenteritis in children.7,24 In the past ten years, 501 patients were detected with NTS infections. The main symptoms at admission were fever, diarrhea and mucous stools, while vomiting and bloody stools were less common. In our study, the majority of the cases occurred in infants, which consistent with a retrospective study carried out in a tertiary children’s hospital in Guangzhou.26 It was reported that NTS infections are usually caused by commercially produced food contaminated by animal stools.32 NTS, as one of the most common zoonotic pathogens,33 can be attributed to animals such as layers, pigs, cattle, reptiles, and broilers.34 Infants are more likely to be exposed to polluted environments at this stage because this is the time when they learned to climb, walk and run. So, the intensified control programs of NTS in animal reservoirs, especially in poultry, and better hygiene practices throughout the food production chain are necessary. Besides, outbreaks of Salmonella associated with infant milk products have been recorded worldwide.35–38 And it has been confirmed that breastfeeding could decrease the risk of sporadic salmonellosis among infants, because exclusive breastfeeding could help infants avoid contacting to contaminated food. Furthermore, some studies showed that some components of breast milk could refrain the adhesion and invasiveness of Salmonella to human intestinal cells.39,40 So, we recommend breastfeeding in infancy. Although NTS infections happened over the year, the highest incidence rates occurred in the summer months to autumn months (July to October), which coincides with a torrid and rainy weather in Chongqing. A study from Kazakhstan and Central Asia showed that an increase of 1°C in temperature was associated with an increase of cases by 5·5%, and a 1 mm increase in precipitation was associated with a 0·6% increase in salmonellosis counts in the same months.41 Heavy rainfall results in contamination of drinking water and environment may be a plausible explanation for the positive association. Our findings were corresponding with the study from Shanghai and Zhejiang.24,42
Among these 501 patients infected by NTS, serogroup B was the most common serogroup isolates. A study conducted in Taiwan between 2000 and 2009 also showed that serogroup B was the predominant serogroup.43 In total, 12 serotypes were identified among 284 isolates. Salmonella Typhimurium was the predominant serotype (79.2%), followed distantly by Salmonella Enteritidis (9.5%). Other studies in China also displayed that Salmonella Typhimurium was the most common serotype.14,26,44,45 And Salmonella Typhimurium was also the most common serotype in North America and Oceania.46 However, some other studies showed that Salmonella Enteritidis was the most common serotype in China.17,24,47 In Japan, Salmonella Ser. infantis was the most frequently detected serotype of Salmonella in seafood.46 While in US, Serotype Dublin was the predominant isolates.23 In a word, the distribution of salmonellae has strong regional characteristics and the prevalence of serotypes is diverse in different areas.
In recent years, the detection rates of NTS infections in inpatients increased significantly, which is related to the increase of poultry farming, the improvement of transportation, and the increasing maturity of detection methods. Since 2015, Chromogenic Salmonella Agar has been used for rapid isolation and identification of Salmonella, which significantly increased the detection rates of Salmonella. The increased detection rates of NTS also indicate NTS infections in children remains a serious public health problem. Antibiotic treatment is not recommended to general gastroenteritis resulting from NTS infections in healthy children.16 Because antibiotic treatment has been reported to prolong the duration of the bacterial excretion from the intestinal tract.48 However, the majority of patients (95.6%) received antibiotics in our study, which could attribute to the regional and population variation. As a tertiary children’s hospital center in Southwest China, our hospital received many patients from surrounding districts. Most of them were still symptomatic at the time of admission, although they had been treated in other hospitals. Nevertheless, the usage of antibiotic was much higher than our expectation. By grouping and comparing the antibiotic resistance rates over the past ten years, we found that the drug resistance rates of quinolones were relatively low on the whole, which could attribute to the relatively lower utilization rates of quinolones in children, considering the side effects on children. The third generation cephalosporins were the first recommended choice in children. Its resistance rates were between 17.1% and 34.4%, which were higher than all previous studies in China.25,27 Extended-spectrum cephalosporins, especially ceftriaxone, have previously been suggested as the drugs of first-line choice for treating children with serious nontyphoidal Salmonella infections. However, the drug resistance rates of ceftriaxone remained at a high level in the past ten years, which was significantly higher than Guangdong12 and Hangzhou14 in China. Therefore, the drug resistance of ceftriaxone in southwest China is alarming. But the antibiotic resistance rates of NTS to piperacillin-tazobactam (from 21.4% to 5.4%) and ceftazidime (from 30.6% to 17.1%) decreased significantly. Maybe ceftazidime is a better choice in consideration of the higher drug resistance of ceftriaxone. In our research, 69 strains of MDR (13.8%) were isolated from 501 children with NTS infections. The proportion of MDR isolates showed a downward trend, which may be related to the restriction of antibiotic use in animals. Antibiotic resistance in NTS arises in large part because of antibiotic use in animal husbandry.21 In recent years, in order to ensure the quality and safety of animal products and public health, some restrictions on the use of antibiotics have been issued.49 Although carbapenem, as a special class of antibiotics, its resistance rates were less than 1%, the drug resistance still increased in recent years. And we detected three resistant strains against to imipenem during 2014–2018. However, we did not find strain was resistant to the carbapenem antibiotics during 2009–2013. This phenomenon indicated that the situation of antibiotic resistance is serious. It is noteworthy that this trend may increase in the future. Therefore, in clinical practice, we should avoid the abuse of antibiotics. The high rates of drug resistance to third generation cephalosporins and increasing resistance to quinolones among children with NTS infections in Chongqing are a serious public health problem that requires continuous monitoring and rational usage of antibiotics.
Conclusion
In conclusion, the majority of patients with NTS infections were under three years old, mainly from July to October. We should pay more attention to food safety for children and prevent them from eating food contaminated with NTS, especially in summer and autumn. Salmonella Typhimurium was the most common serotype. The antibiotic resistance rates of NTS to piperacillin-tazobactam, ceftriaxone, ceftazidime and cefepime showed a downward trend in the past ten years, among which piperacillin-tazobactam and ceftazidime decreased significantly. So, in Southwest China, ceftazidime is recommended for severe or invasive infections caused by NTS infections until drug sensitivity test results are available. However, the whole antibiotic resistance rates were at a high level. And 69 strains of MDR (13.8%) were detected from 501 isolates. Therefore, continuous surveillance of drug resistance in NTS and control measures such as avoiding unnecessary antibiotic therapy in general NTS gastroenteritis are important in our country.
Ethical Approval
This study was approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University. All data were anonymized to maintain patients’ privacy, and the study was conducted in accordance with the Declaration of Helsinki. In light of the retrospective and anonymous nature of the study, the Ethics Committee did not require written informed consent provided by patients.
Acknowledgment
Li-juan Wu, Yan Luo, Guo-lu Shi should be considered co-first authors.
Funding
No funding sources.
Disclosure
The authors report no conflicts of interest.
References
1. Paudyal N, Pan H, Liao X, et al. A meta-analysis of major foodborne pathogens in Chinese food commodities between 2006 and 2016. Foodborne Pathog Dis. 2018;15(4):187–197. doi:10.1089/fpd.2017.2417
2. Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the united states–major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi:10.3201/eid1701.p11101
3. Magwedere K, Rauff D, De Klerk G, Keddy KH, Dziva F. Incidence of nontyphoidal salmonella in food-producing animals, animal feed, and the associated environment in south africa, 2012–2014. Clin Infect Dis. 2015;61(Suppl 4):S283–S289. doi:10.1093/cid/civ663
4. Majowicz SE, Musto J, Scallan E, et al. The global burden of nontyphoidal salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–889. doi:10.1086/650733
5. Medalla F, Gu W, Mahon BE, et al. Estimated incidence of antimicrobial drug–resistant nontyphoidal salmonella infections, United States, 2004–2012. Emerg Infect Dis. 2016;23(1):29–37. doi:10.3201/eid2301.160771
6. Mao X, Hu JF, Liu XM. Estimation on disease burden of foodborne non-typhoid salmonellosis in china using literature review method. Chin J Dis Control Prevention. 2011;15(7):622–625.
7. Ran L, Wu S, Gao Y, et al. Laboratory-based surveillance of nontyphoidal salmonella infections in china. Foodborne Pathog Dis. 2011;8(8):921–927. doi:10.1089/fpd.2010.0827
8. Schultz M. Theobald smith. Emerg Infect Dis. 2008;14(12):1940–1942. doi:10.3201/eid1412.081188
9. Desai PT, Porwollik S, Long F, et al. Evolutionary genomics of salmonella enterica subspecies. MBIO. 2013;4(2). doi:10.1128/mBio.00579-12
10. Marzel A, Desai PT, Goren A, et al. Persistent infections by nontyphoidal salmonella in humans: epidemiology and genetics. Clin Infect Dis. 2016;62(7):879–886. doi:10.1093/cid/civ1221
11. Wang P, Goggins WB, Chan E. Associations of salmonella hospitalizations with ambient temperature, humidity and rainfall in hong kong. Environ Int. 2018;120:
12. Liang Z, Ke B, Deng X, et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal salmonella from human patients in Guangdong province, China, 2009–2012. BMC Infect Dis. 2015;15:
13. Fan Q, Yi M, Liu H, et al. The impact of age and pathogens type on the gut microbiota in infants with diarrhea in dalian, china. Can J Infect Dis Med Microbiol. 2020;2020:
14. Yue M, Li X, Liu D, Hu X. Serotypes, antibiotic resistance, and virulence genes of salmonella in children with diarrhea. J Clin Lab Anal. 2020;34(12):e23525. doi:10.1002/jcla.23525
15. Fritsch J, Abreu MT. The microbiota and the immune response: what is the chicken and what is the egg? Gastrointest Endosc Clin N Am. 2019;29(3):381–393. doi:10.1016/j.giec.2019.02.005
16. Bula-Rudas FJ, Rathore MH, Maraqa NF. Salmonella infections in childhood. Adv Pediatr. 2015;62(1):29–58. doi:10.1016/j.yapd.2015.04.005
17. Katiyo S, Muller-Pebody B, Minaji M, et al. Epidemiology and outcomes of nontyphoidal salmonella bacteremias from england, 2004 to 2015. J Clin Microbiol. 2019;57(1). doi:10.1128/JCM.01189-18
18. Wen SC, Best E, Nourse C. Non-typhoidal salmonella infections in children: review of literature and recommendations for management. J Paediatr Child Health. 2017;53(10):936–941. doi:10.1111/jpc.13585
19. Onwuezobe IA, Oshun PO, Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal salmonella infection. Cochrane Database Syst Rev. 2012;11:CD001167. doi:10.1002/14651858.CD001167.pub2
20. Wang X, Biswas S, Paudyal N, et al. Antibiotic resistance in salmonella typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front Microbiol. 2019;10:
21. McDermott PF, Zhao S, Tate H. Antimicrobial resistance in nontyphoidal salmonella. Microbiol Spectr. 2018;6(4). doi:10.1128/microbiolspec.ARBA-0014-2017
22. Williamson DA, Lane CR, Easton M, et al. Increasing antimicrobial resistance in nontyphoidal salmonella isolates in australia from 1979 to 2015. Antimicrob Agents Ch. 2018;62(2). doi:10.1128/AAC.02012-17
23. Angelo KM, Reynolds J, Karp BE, Hoekstra RM, Scheel CM, Friedman C. Antimicrobial resistance among nontyphoidal salmonella isolated from blood in the united states, 2003–2013. J Infect Dis. 2016;214(10):1565–1570. doi:10.1093/infdis/jiw415
24. Li Y, Xie X, Xu X, et al. Nontyphoidal salmonella infection in children with acute gastroenteritis: prevalence, serotypes, and antimicrobial resistance in shanghai, china. Foodborne Pathog Dis. 2014;11(3):200–206. doi:10.1089/fpd.2013.1629
25. Wei ZQ, Chang HL, Li YF, Xu XB, Zeng M. Clinical epidemiology and antimicrobial resistance of nontyphoidal salmonella enteric infections in children: 2012–2014. Chin J Pediatr. 2016;54(7):489–495. doi:10.3760/cma.j.issn.0578-1310.2016.07.003
26. Liang B, Xie Y, He S, et al. Prevalence, serotypes, and drug resistance of nontyphoidal salmonella among paediatric patients in a tertiary hospital in guangzhou, china, 2014–2016. J Infect Public Heal. 2019;12(2):252–257. doi:10.1016/j.jiph.2018.10.012
27. Dong-ling L, Jie-hong W, Jing-song WU, et al. Characteristics of drug resistance and molecular typing for salmonella in diarrhea patients from four hospitals in shenzhen. Chin J Zoonoses. 2017;33(10):897–902. doi:10.3969/j.issn.1002-2694.2017.10.009
28. Hopkins KL, Kirchner M, Guerra B, et al. Multiresistant salmonella enterica serovar 4, [5], 12: i:-in europe: a new pandemic strain? Euro Surveill. 2010;15(22):19580. doi:10.1016/j.cmi.2016.07.033
29. Da-wei L, E-hong LJ, Ng-xiao JL, et al. Pattern of antimicrobial resistance and molecular typing for salmonella isolated from diarrhea cases in guangzhou. Modern Preventive Med. 2016;43(4).
30. Michael GB, Schwarz S. Antimicrobial resistance in zoonotic nontyphoidal salmonella: an alarming trend? Clin Microbiol Infect. 2016;22(12):968–974. doi:10.1016/j.cmi.2016.07.033
31. Wayne PA. Clinical and laboratory standards institute. Perform Stand Antimicrob Susceptibility Testing. 2011.
32. Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin Microbiol Rev. 2015;28(4):901–937. doi:10.1128/CMR.00002-15
33. Team CEE. The 2013 joint ECDC/EFSA report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks published. Eurosurveillance. 2015;20(4).
34. Mughini-Gras L, Enserink R, Friesema I, Heck M, van Duynhoven Y, van Pelt W. Risk factors for human salmonellosis originating from pigs, cattle, broiler chickens and egg laying hens: a combined case-control and source attribution analysis. PLoS One. 2014;9(2):e87933. doi:10.1371/journal.pone.0087933
35. Jourdan-da SN, Fabre L, Robinson E, et al. Ongoing nationwide outbreak of salmonella agona associated with internationally distributed infant milk products, france, december 2017. Euro Surveill. 2018;23(2). doi:10.2807/1560-7917.ES.2018.23.2.17-00852
36. Jones G, Pardos DLGM, Herrera-Leon L, et al. Outbreak of salmonella enterica serotype poona in infants linked to persistent salmonella contamination in an infant formula manufacturing facility, france, august 2018 to february 2019. Euro Surveill. 2019;24(13). doi:10.2807/1560-7917.ES.2019.24.13.1900161
37. Cahill SM, Wachsmuth IK, Costarrica ML, Ben EP. Powdered infant formula as a source of salmonella infection in infants. Clin Infect Dis. 2008;46(2):268–273. doi:10.1086/524737
38. Yang B, Zhao H, Cui S, et al. Prevalence and characterization of salmonella enterica in dried milk-related infant foods in Shaanxi, China. J Dairy Sci. 2014;97(11):6754–6760. doi:10.3168/jds.2014-8292
39. Coppa GV, Facinelli B, Magi G, et al. Human milk glycosaminoglycans inhibit in vitro the adhesion of escherichia coli and salmonella fyris to human intestinal cells. Pediatr Res. 2016;79(4):603–607. doi:10.1038/pr.2015.262
40. Liu B, Yu Z, Chen C, Kling DE, Newburg DS. Human milk mucin 1 and mucin 4 inhibit salmonella enterica serovar typhimurium invasion of human intestinal epithelial cells in vitro. J Nutr. 2012;142(8):1504–1509. doi:10.3945/jn.111.155614
41. Grjibovski AM, Kosbayeva A, Menne B. The effect of ambient air temperature and precipitation on monthly counts of salmonellosis in four regions of kazakhstan, central asia, in 2000–2010. Epidemiol Infect. 2014;142(3):608–615. doi:10.1017/S095026881300157X
42. Ke Y, Lu W, Liu W, Zhu P, Chen Q, Zhu Z. Non-typhoidal salmonella infections among children in a tertiary hospital in ningbo, zhejiang, china, 2012–2019. PLoS Negl Trop Dis. 2020;14(10):e0008732. doi:10.1371/journal.pntd.0008732
43. Hung Y, Lay C, Wang C, Koo M. Characteristics of nontyphoidal salmonella gastroenteritis in Taiwanese children: a 9-year period retrospective medical record review. J Infect Public Heal. 2017;10(5):518–521. doi:10.1016/j.jiph.2016.09.018
44. Ren L, Yang M, Geng L, et al. Nontyphoidal salmonella gastroenteritis in a tertiary children’s hospital in southern china: characteristics and dietary considerations. Gastroent Res Pract. 2018;2018:
45. Yang X, Jin K, Yang F, et al. Nontyphoidal salmonella gastroenteritis in baoshan, shanghai, china, 2010 to 2014: an etiological surveillance and case-control study. J Food Prot. 2017;80(3):482–487. doi:10.4315/0362-028X.JFP-16-309
46. Aoki Y, Kitazawa K, Kobayashi H, et al. Clinical features of children with nontyphoidal salmonella bacteremia: a single institution survey in rural japan. PLoS One. 2017;12(6):e0176990. doi:10.1371/journal.pone.0176990
47. Zhang S, Zhou Y, Tian L, et al. Antibiotic resistance and molecular characterization of diarrheagenic escherichia coli and non-typhoidal salmonella strains isolated from infections in southwest china. Infect Dis Poverty. 2018;7(1). doi:10.1186/s40249-018-0427-2
48. Murase T, Yamada M, Muto T, Matsushima A, Yamai S. Fecal excretion of salmonella enterica serovar typhimurium following a food-borne outbreak. J Clin Microbiol. 2000;38(9):3495–3497. doi:10.1128/JCM.38.9.3495-3497.2000
49. Bo H, Wei W, Zhi-xing F, Jing Y. On the status of antibiotics usage and the progress in antibiotic-free breeding in poultry farming. Food Ind. 2017;38(08):216–220.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


