Back to Journals » Infection and Drug Resistance » Volume 14
Extended-Spectrum β-Lactamase and Carbapenemase Producing Gram-Negative Bacilli Infections Among Patients in Intensive Care Units of Felegehiwot Referral Hospital: A Prospective Cross-Sectional Study
Authors Alebel M, Mekonnen F , Mulu W
Received 22 November 2020
Accepted for publication 8 January 2021
Published 2 February 2021 Volume 2021:14 Pages 391—405
DOI https://doi.org/10.2147/IDR.S292246
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sahil Khanna
Mekonnen Alebel,1 Feleke Mekonnen,2 Wondemagegn Mulu2
1Department of Clinical Laboratory Science, Chagni Hospital, Chagni, Ethiopia; 2Department of Medical Laboratory Science, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Wondemagegn Mulu
Department of Medical Laboratory Science, College of Medicine and Health Sciences, Bahir Dar University, P. O. Box: 79, Bahir Dar, Ethiopia
Email [email protected]
Background: Owing to the specific risk profile of its residents, intensive care units (ICUs) are the best place for selection pressure and the epicenter for resistance development and dissemination. Infections with β-lactamase releasing Gram-negative bacilli (GNB) at ICUs are an emerging global threat. This study dogged the magnitude of extended-spectrum β-lactamase (ESBL) and carbapenemase releasing Gram-negative bacilli infections and associated factors among patients in the ICUs of Felegehiwot Referral Hospital, Ethiopia.
Methods: A cross-sectional study was done through February to June 2020. Wound swabs, urine, blood and sputum samples were collected from patients in the ICUs symptomatic for infections while excluding those under coma and shock. Bacterial species were verified using standard microbiological methods. Carbapenemase and ESBL production were identified using modified carbapenem inactivation and combined disk diffusion methods, respectively. Multivariable analysis was calculated for factors associated with ESBL production. P-value < 0.05 was taken as cut-off for statistical significance.
Results: Out of 270 patients in the ICU, 67 (24.8%) and 14 (5.2%) had infections with ESBL and carbapenemase releasing GNB, respectively. The most frequent ESBL producing isolates were P. aeruginosa (100%), E. cloacae (100%), K. pneumoniae (82.8%) and E. coli (64%). The predominant carbapenemase producer isolates were K. pneumoniae (27.6%) and E. cloacae (33.3%). Overall, 77 (81.1%) of species were multi-drug resistant. All GNB species were 100% resistant to tetracycline and ampicillin. They are also resistant to cefuroxime, ceftazidime, sulfamethoxazole-trimethoprim and cefotaxime. Prior hospitalization (AOR = 5.5, CI = 2.63– 11.46), support with medical care devices (AOR = 23.7, CI = 4.6– 12) and arterial intravenous catheterization (AOR = 2.7, CI = 1.3– 5.3) had significant association with β-lactamase producing GNB infection.
Conclusion: Infection with ESBL and carbapenemase producing Gram-negative bacilli linked with an alarming degree of multi-drug resistant isolates is a major healthcare threat among patients in ICUs. Hence, strict adherence to infection prevention practices and wise use of antibiotics are recommended to slow the spread of antimicrobial resistance.
Keywords: ICU, GNB, ESBL, carbapenemase, multi-drug resistance, FHRH, Ethiopia
Background
Intensive care units (ICUs) are specially equipped units of hospitals which provide highly specialized care to patients who have suffered from a serious injury or illness.1 Patients are often hospitalized to ICUs following problems in their respiratory, cardiac, renal and brain, after surgery and major trauma complications.2,3
Patients hospitalized to ICUs are most at risk to develop infections from various sites.4 The most common infections that occurred in the ICU are ventilator-associated lower respiratory tract infections (LRTI), central line-associated bloodstream infections (BSI), and urinary tract infections (UTI) associated with catheter and surgical site infections (SSIs).5
Although the interventions are greatly improved and follow-ups are critical for patients in the ICU, morbidity and mortality due to infections are very high.6 Globally, infections with Gram-negative bacilli (GNB) are 5–10 times higher among patients in the ICU than those in the general wards.7 These, ICU-acquired infections are linked with underlying disease conditions, improper use of antimicrobials, invasive therapeutic interventions, mechanical ventilation, and central venous and urinary catheterization.8
Due to the specific risk profile of its residents, the ICU is the optimal place for selection pressure and is deemed the epicenter for resistance development, dissemination, and amplification of antimicrobial resistance (AMR).9 In the ICU, over 60% of patients get broad-spectrum antimicrobial treatments, and it is one of the major reasons for the rising and spreading of multi-drug resistant (MDR) strains in serious care units.9 Thus, infections among ICU patients are aggravated with the emergence of extended-spectrum beta-lactamases (ESBLs) and carbapenemases (CP) producing bacteria and the resistance of these microbes for drugs which save the lives of critically ill patients.10
The emergence of ESBLs and CP producing GNB infections among ICU patients are one of the recent alarming global health threats, worldwide.10 They are major causes for morbidity, longer hospital stays, and mortality in the ICU with limited therapeutic options and they utilize many resources to prevent, detect and respond to it.11 Therefore, GNB isolates from UTI, BSI, LRTI and wound infections (WI) in ICUs are superbugs and nightmare bacteria.12
Extended-spectrum beta-lactamases are enzymes generated by certain bacteria which can hydrolyze extended-spectrum third generation cephalosporins. While, carbapenemase are a type of beta-lactamase enzyme released from GNB to defend themselves against carbapenem antibiotics and tend to give resistance to all penicillins and cephalosporins.13
Enterobacterales family, Pseudomonas aeruginosa (P. aeruginosa) and Acinetobacter species are potential superbugs which can produce ESBLs and CP and are widely reported in ICU settings.6,14 Infections with ESBL producing bacteria are treated with the last resort antibiotic carbapenems. However, CP mediated resistance to carbapenem drugs are occurring.15 Members of the Enterobacterales family revealed dramatic increases in resistance to carbapenems.16 Therefore, ESBL producing Enterobacterales, carbapenemase expressing K. pneumoniae, carbapenem-resistant (CR) A. baumannii and MDR P. aeruginosa are the current critical organisms for research and innovation of new drugs.17
Although patients in the ICU are the epicenter for nosocomial infections and sources for AMR, information on the ESBLs and CP producing species from patients in ICUs are very scarce in sub-Saharan Africa. In Ethiopia, patients in the ICU suffer from healthcare associated infection with GNB pathogens and treatment failure. The majority of severely ill patients in the ICU are treated with randomly chosen broad-spectrum antibiotics and supported with invasive devices. The uses of common gloves for more than one patient by healthcare workers are also common practices in ICUs. These can induce substantial antibiotic pressure, AMR and the emergence and spreading of a high number of ESBLs and CP producing bacterial infections.18
The dearth of studies in ICU settings on beta-lactamase producing GNB infections in Ethiopia are associated with limited diagnostic and treatment facilities and habitual empirical treatments. Thus, genuine surveillance data on beta-lactamase production and related resistance to antibiotics among GNB is crucially required in developing countries including Ethiopia to alarm and create awareness for effective intervention measures, and emergence of GNB superbugs. Therefore, this study determined the prevalence of beta-lactamases (ESBLs and CP) producing Gram-negative bacilli infections and their associated factors among patients hospitalized in the ICU of Felegehiwot Referral Hospital, Ethiopia.
Methods
Study Design, Period and Setting
A cross-sectional study was performed through February to June, 2020 in the ICU of Felegehiwot Referral Hospital, Northwest Ethiopia. Felegehiwot Referral Hospital is one of the largest tertiary level referral hospitals in Ethiopia serving more than 7 million people. It currently delivers healthcare services in its different wards and ICUs. The ICUs have 2 rooms, 12 beds and serve more than 60 patients per week. On average, 60 patients are admitted weekly at the ICU for different admission cases to get treatment services. During data collection, ICUs have one neurologist, four intensives, four medical doctors, 5 clinical nurses and 3 pediatricians. All patients in the ICUs of Felegehiwot Referral Hospital and symptomatic for bacterial causes of UTI, BSI, LRTI or WI were the study population.
Variables of the Study
Beta-lactamases (extended-spectrum beta-lactamases and carbapenemase) producing Gram negative bacilli infections were the dependent variable while variables such as demographic (age, sex, residence, marital status, educational status and occupation), clinical related (admission for the last 12 months, patients ICU ward and patient’s referral), medical care related (urethral catheter, intravenous catheter, arterial intravenous and suprapubic catheter, support with medical care devices, mechanical ventilation status, ICU room cleanliness, room ventilation and hand hygiene practices of healthcare works) and treatment related (antibiotic treatment status, history of antibiotic use and history of antibiotic interruption) were the independent variables.
Inclusion and Exclusion Criteria
Patients of all ages and both genders (male and female) hospitalized in the ICU with symptoms for UTI, BSI, LRTI or WIs were included in the study. Conversely, patients in the ICU that were under coma and shock were excluded from the study.
Sample Size Determination and Sampling
A sample size of 270 patients was obtained via calculation with Epi info version 3.5.1 (public domain software, www.cdc.gov) at 95% level of confidence, 5% margin of error and a 0.227 proportion of ESBL production from a study in India.19 A convenient sampling technique was employed to include the study participants from the study population. All patients symptomatic for BSI, UTI, LRTI or WI were included until the compulsory sample size had been reached. Therefore, 101, 91, 44 and 34 patients in the ICUs presumptive for UTI, BSI, WI and LRTI, respectively were included in the study.
Data Collection
Data on demographic features and variables related to clinical, medical and treatment profiles of study participants were collected by the study team in consultation with the attending physician with face-to-face interviews and complementation with patient card reviews.
Specimen Collection and Processing
Specimens from study participants were collected following diagnosis by a physician. Urine (101), blood (91), wound swabs (44) and bronchial sputum (34) specimens were collected from ICU patients symptomatic for UTI, BSI, WI and LRTI, respectively following standard microbiological methods.20
Blood Specimen Collection and Processing
Blood of 10 mL, 5 mL and 1 mL were drawn from adult, children and infants, respectively and inoculated directly onto Tryptone Soya Broth (TSB) (Oxoid, UK) containing aerobic blood culture bottles. Inoculated blood culture bottles were incubated at 37 °C and inspected for 7 consecutive days. For those blood cultures that showed signs of microbial growth, sub-cultures were performed onto Blood Agar and MacConkey Agar (Oxoid, UK). All of the inoculated agar plates were incubated at 37 °C for 48 h.21
Urine Specimen Collection and Processing
Five mL of urine sample was collected with leak proof and sterile screw-capped plastic from non-catheterized patients. From catheterized patients, the same volumes of urine were aseptically collected via a urethral or supra pubic catheter line and ported to a sterile urine cup. Using a calibrated loop (0.002 mL), the urine sample then inoculated onto Cysteine Lactose Electrolyte Deficient Agar and incubated at 37 °C for 24 h. Colonies with significant bacteriuria: 105 CFU/mL for urine collected from non-catheterized patients and 103–105 CFU/mL for urine collected from catheterized patients were further processed and sub-cultured onto MacConkey and Blood Agar plates.20,21
Wound Swab Sample Collection and Processing
Swabs were collected from appropriate site of wound infection aseptically with sterile cotton tipped swab and were inoculated onto Blood Agar and MacConkey Agar at the time (Oxoid, UK). Inoculated plates were kept at 37 °C for 48 h.22
Sputum Sample Collection and Processing
Five mL of expectorated purulent sputum specimens were collected from participants with LRTI using dry wide necked clean leak proof containers. For those who had a suction tube, the same volumes of sputum were collected to a falcon tube.20,21 Upon collection, a loop full of specimen was streaked onto Blood Agar and MacConkey Agar (Oxoid, UK) and kept under aerobic condition at 37 ºC and bacterial growths were examined after 48 h of incubation.23
Bacterial Identification
Standard bacteriological methods were employed for identification of isolates.21,23 Gram-negative bacilli colonies were pre-identified with their colony appearance and Gram staining morphology. Pure cultures of isolates were further identified by necessary biochemical tests.21,23 Biochemical tests such as lactose fermentation, indole production, citrate utilization, gas production, motility and oxidases were used to identify members of the Enterobacterales, P. aeruginosa and A. baumannii. All the suspected isolates were further confirmed by an automated Vitek2 Compact (Biomerieux, France).
Antimicrobial Susceptibility Testing
All the identified isolates were subjected to susceptibility testing against amoxicillin-clavulanic acid (30 µg), ampicillin (10 µg), cefepime (30 µg), cefotaxime (30 µg), ceftazidime (30 µg), ceftriaxone (30 µg), cefuroxime (30 µg), ciprofloxacin (5 µg), gentamicin (10 µg), imipenem (10 µg), meropenem (10 µg), nitrofurantoin (10 µg), piperacillin (100 µg), sulfamethoxazole-trimethoprim (1.25/23.75 µg), and tetracycline (30 µg) (Oxoid, UK) using the Kirby-Bauer method on Mueller Hinton Agar (MHA) (Oxoid, UK). Zone sizes from the Clinical and Laboratory Standard Institute guidelines were employed to interpret the results. Bacterial isolates which were resistant to three or more antibiotics from different classes were considered as MDR.24
Screening and Confirmation of Extended-Spectrum β-Lactamase Producers
ESBL producing isolates were screened using the Kirby Bauer disk diffusion test.24 A 0.5 McFarland adjusted bacterial suspension was properly streaked onto MHA. Ceftriaxone (30 µg), cefotaxime (30 µg) and ceftazidime (30 µg) disks were placed onto the inoculated MHA plate and incubated overnight at 37 °C. Isolates that revealed ≤22 mm inhibition zone size for ceftazidime and/or ≤27 mm for cefotaxime and/or ≤25 mm for ceftriaxone were considered as ESBL positive.24 These isolates were then subjected to double disk synergy confirmatory test according to the 2019 CLSI guidelines.24 Organisms suspended in normal saline were inoculated onto MHA plate. Ceftazidime (30 µg), cefotaxime (30 µg) and ceftriaxone (30 µg) disks were placed around amoxicillin-clavulanic acid (20/10µg) 20 mm apart. After incubation at 37 °C for 24 h, isolates that revealed ≥5 mm increase in the growth inhibition zone for any antimicrobial associated with amoxicillin-clavulanic acid was considered as positive and the isolates were interpreted as an ESBL producer.24
Carbapenemase Detection Methods
Bacterial isolates exhibiting a growth inhibition zone size of ≤19 mm to meropenem and imipenem disks in the Kirby Bauer disk diffusion test were subjected to a modified carbapenem inactivation method (mCIM) test.20 Briefly, the bacterial isolates were emulsified in tryptic soya broth with meropenem disk (10 µg) and incubated for 4 h. McFarland standard equivalent suspension carbapenem sensitive indicator organism (E. coli ATCC®25,922) evenly swabbed onto MHA, and then the meropenem in the tryptic soya broth was dispensed and incubated at 37 °C for 24 h. The zone of inhibition for meropenem was measured. A zone diameter of 6–15 mm or presence of pinpoint colonies within a 16–18 mm zone was considered as CP producer.24
Quality Control
A questionnaire was prepared in English and translated into the national language (Amharic) and back to English to check its consistency. The questionnaire was checked for completeness during and after data collection. The qualities of the sputum sample were evaluated for proper culture according to the criteria given by the American Society for Microbiology.25 The sterility of culture media were checked with overnight incubation of 5% prepared media and observed for the presence of any growth. American Type Culture Collection (ATCC) standard reference strains (E. coli ATCC-25922 and P. aeruginosa ATCC-27853) were used to check the performance of the culture media. K. pneumoniae ATCC 700603 and E. coli ATCC 25922 control strains were used for quality control of ESBL detection. K. pneumoniae BAA1705 and E. coli ATCC 25922 control strains were taken as a positive and negative quality control, respectively for carbapenemase detection.
Data Analysis
Data were coded and analyzed using IBM SPSS statistics for Windows, Version 25 (IBM Corp, Armonk, NY, USA). Descriptive statistics were calculated. Variables were fitted to the bivariable logistic regression. Variables with a P-value of ≤ 0.2 in the bivariable analysis were then further entered into a logistic regression model to identify independent predictors of beta-lactamases producing bacterial infection. Backward stepwise logistic regression techniques were fitted in the multivariable analysis and confounding and multicollinearity were controlled. A P-value < 0.05 was considered as cut-off for statistical significance. Crude and adjusted odds ratio with 95% confidence intervals were calculated. Hosmer and Lemeshow gardens-of-fit test was used to check the fulfillment of the necessary assumptions for the implementation of logistic regression. A P-value > 0.005 was considered a good fit.
Ethical Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from Bahir Dar University College of Medicine and Health Sciences Institutional Review Board (IRB) dated on 05/02/2020 with the registration number of 002. Before data collection, permission was taken from APHI and Felegehiwot Referral Hospital. From the study participants whose age was 18 years and above, written informed consents were taken. For children, written assents were obtained from caregivers before they are asked to give data and sample. Participation in the study was fully voluntary. All information obtained from this study was kept confidential at all levels and utilized only for the study. Positive findings were reported to the physicians attending in the ICU for the proper management of patients.
Results
Demographic and Clinical Characteristics
A total of 270 patients in the ICU with symptoms of UTI (101, 37.4%), BSI (91, 33.7%), WI (44, 16.3%) and LRTI (34, 12.6%) were included during the study. Of the 270 patients, 147 (54.4%) were males. The median age of study participants was 54 years and the majority (32.2%) were older than 60 years. One hundred thirty eight (51.1%) of the study participants were rural residents (Table 1).
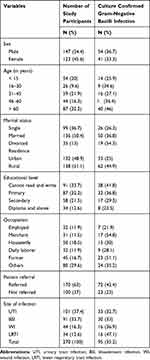 |
Table 1 Culture Confirmed Gram-Negative Bacilli Infections and Demographic Characteristics of Study Participants |
Gram-Negative Bacilli Infections
Table 1 illustrates the prevalence of infection with GNB. Overall, 95 (35.2%) patients had culture confirmed GNB infection. The percentage of culture confirmed UTI, BSI, WI and LRTI was 33 (32.7%), 30 (33%), 16 (36.9%) and 16 (47.1%), respectively. The percentage of GNB infection was higher among rural (44.9%) than urban (25%) inhabitants. Referred patients had higher percentages (42.4%) of infection than the counter (23%).
Prevalence of β-Lactamase Producing GNB Infection
Overall, 67 (24.8%) patients had infection with ESBL producing GNB. The percentage of UTI, BSI, LRTI and WI with ESBL producing GNB was 23 (22.8%), 18 (19.8%), 12 (35.3%) and 14 (31.8%), respectively. The prevalence of carbapenemase producing GNB infection was 14 (5.2%). The percentage of CP production was 5 (14.7%), 5 (5.5%), 2 (4.5%) and 2 (1.98%) in LRTI, BSI, WI and UTI, correspondingly (Figure 1).
 |
Figure 1 Prevalence of ESBL and carbapenemase producing Gram-negative bacilli infections. |
Frequency of Bacterial Isolates
Table 2 illustrates the frequency of GNB isolates. Overall, K. pneumoniae 29 (30.5%) was the most predominant isolate with E. coli 25 (26.3%), Acinetobacter spp. 13 (13.7%) and Enterobacter spp. 6 (6.3%). The most common isolated bacteria from UTI were E. coli 15 (45.5%) and K. pneumoniae 10 (30.3%). P. aeruginosa 6 (37.5%) was the most common organism from wound infections with K. pneumoniae 3 (18.8%) and Acinetobacter spp. 3 (18.8%). From BSI, K. pneumoniae 9 (30%) followed by E. coli 6 (20%) and Acinetobacter spp. 5 (16.7%) were the most common isolates. Similarly, K. pneumoniae 7 (43.8%) was the leading isolate with Acinetobacter spp. 4 (25%) from LRTI.
 |
Table 2 Isolation Rate of Bacterial Species Among Patients in the ICU |
Frequency of Extended-Spectrum β-Lactamase and Carbapenemase Production
All P. aeruginosa and E. cloacae isolates were found to be ESBL producers. The majority of K. pneumoniae (82.8%) and E. coli (64%) species were ESBL producers. ESBL production was found in 56.6–81.3% of isolates from various specimens with the highest percentage recorded among isolates from wound (81.3%) and sputum samples (75%). The proportion of CP production was 8 (27.6%), 2 (18.2%) and 2 (8%) among K. pneumoniae, P. aeruginosa and E. coli species, respectively. The proportion of CP production was, 4 (40%), 3 (18.8%), 5 (15.2%) and 2 (12.5%) from blood, wound, urine and sputum sample isolates, respectively (Table 3).
 |
Table 3 Distribution of ESBL and CP Among Bacterial Isolates of Clinical Specimens at ICU |
Antimicrobial Resistance Profiles of the Bacterial Isolates
All GNB species were 100% resistant to tetracycline and ampicillin. They revealed 83.3–100% resistance towards piperacillin. GNB showed a high percentage of resistance toward cefuroxime (83.2%), ceftazidime (77.9%), sulfamethoxazole-trimethoprim (77.9%) and cefotaxime (75.8%). The least percentage of resistance was found toward ciprofloxacin (51.6%), imipenem (16.8%) and meropenem (21%) (Table 4).
 |
Table 4 Antimicrobial Resistance Profiles of Bacterial Species Among Patients at the ICU |
All P. aeruginosa and E. cloacae isolates showed resistance to sulfamethoxazole-trimethoprim, cefuroxime, cefotaxime, and ceftazidime. K. pneumoniae isolates revealed 72.4–89.7% of resistance toward ciprofloxacin, cefepime, ceftriaxone, nitrofurantoin, sulfamethoxazole-trimethoprim, cefotaxime and amoxicillin clavulanic acid, respectively. P. aeruginosa isolates showed 90.9–100% of resistance toward cefepime, nitrofurantoin, amoxicillin, piperacillin, cefuroxime, ceftazidime, cefotaxime, ceftriaxone, gentamicin, sulfamethoxazole-trimethoprim and amoxicillin-clavulanic acid, respectively. Acinetobacter spp. showed 76.9−100% of resistance toward ceftazidime, ciprofloxacin, sulfamethoxazole-trimethoprim, nitrofurantoin, cefuroxime and piperacillin, respectively. Moreover, E. cloacae revealed 83.3–100% of resistance toward gentamicin, nitrofurantoin, piperacillin, ceftriaxone, sulfamethoxazole-trimethoprim, cefotaxime, ceftazidime, and cefuroxime, respectively (Table 4).
Multi-Drug Resistance Profile of the Bacterial Isolates
Overall, 77 (81.05%) bacterial species were MDR. All of P. aeruginosa, Acinetobacter spp., Citrobacter spp. and E. cloacae were found to be MDR. The MDR rate of E. coli and K. pneumoniae isolates were 27 (93.1%) and 16 (60%), respectively (Figure 2).
 |
Figure 2 MDR profile among bacterial isolates from intensive care units. Abbreviation: MDR, multi-drug resistance. |
Multivariable Analysis
Under multivariable analysis, ESBL production was significantly associated with a history of admission for the last 12 months (AOR = 5.5, CI = 2.63–11.46), support with medical care devices (AOR = 23.7, CI = 4.6–12), with arterial intravenous catheterization (AOR = 2.7, CI = 1.3–5.3) and hospitalization in surgical ICU (AOR = 6.69, CI = 1.2–36.5). Participants who had a history of admission in the last 12 months were 5.5 times more likely to have an ESBL producing bacterial infection compared to others. Likewise, patients hospitalized in surgical ICU had 6.7 times the risk of developing ESBL producing bacterial infection than those admitted to medical and neonatal ICU. Participants who had support with medical care devices were 23.7 times more likely to develop ESBL producing bacterial infections compared to those who had no the support. Moreover, participants who had arterial IV catheters were 2.7 times more likely to be infected with ESBL producing bacterial infections as compared to their counterparts (Table 5).
 |
Table 5 Bivariable and Multivariable Analysis of Factors Associated with β-Lactamase Producing GNB Infection Among Patients in the Intensive Care Units |
Discussion
Although there is lack of preliminary baseline data in Ethiopia on culture confirmed bacterial infections in the ICU, the overall Gram-negative bacilli infections among ICU patients in the present study is alarmingly high. This could be due to poor adherence to infection prevention practices in the study area. Study participants referred from other healthcare facilities had a higher percentage of infection than their counterparts. Similar results were found in Uganda.6 This could be due to circulations of pathogens among healthcare worker's attire, inpatients, hospital equipment, or interventional procedures.
The prevalence of GNB infection (35.2%) among ICU patients in the present study was analogous with studies from Uganda (32%)6 and India (37%).26 However, it was higher than other studies done in Ethiopia (25.13%),27 Mexico (19%),28 Nipa (19.5%)29 and Nepal (12.6%).30 Conversely, a high percentage of infections had been reported in Bosnia (65.2%),31 India (62%)32 and Nigeria (50.9%).33
The most prevalent cultured confirmed infections in the present study were lower respiratory tract infections and wound infections which are reliable with studies from Mexico28 and Southern India,34 where most of the infections were lower respiratory tract infections and wound infections. This might be due to the high chance of exposure to hospital pathogens during care and following the use of mechanical ventilation and other supportive devices for intensive care. Moreover, respiratory and wound infections are one of the most predominant clinical syndromes worldwide.
Taken as a whole, 24.8% of patients in the ICU had infections with ESBL producing Gram-negative bacilli in the present study. This is a major threat for patients and healthcare workers. Because patients in the ICU are critically ill and immunocompromised this requires intensive care services. Healthcare workers have frequent contact with patients for intensive care. Moreover, infections with ESBL producing organisms are resistant to third generation cephalosporins and require more complex treatments, prolonged hospitalization and intravenous carbapenem antibiotics. The prevailing percentage of ESBL infections in the current study was concurrent with former findings from India (21.4%),26 France (25%),35 Qatar (26%)36 and Nepal (28.2%).30 However, lower than a study from India (35.2%).37
A higher percentage of infections with ESBL producing GNB was found among patients with lower respiratory tract infections (35.3%) and wound infections (19.8%). Fairly similar findings were reported in India where 34.3% of patients in the ICU had LRTI with ESBL producing bacteria26 and Saudi Arabia where 38.8% of ICU patients had LRTI with ESBL producing bacteria.38
E. cloacae and P. aeruginosa isolates were the leading ESBL producers with K. pneumoniae and E. coli. This is consistent with findings reported in Indonesia,39 Algeria40 and India37 where E. cloacae was the predominant ESBL producing isolate. In reports from Uganda,6 Nepal,30 Brazil41 and Mexico,28 E. coli and K. pneumoniae were also the most common ESBL producing isolates. Moreover, 56.6–81.3% of GNB isolates from blood, urine, sputum and wound samples of the present study are ESBL producers. This showed that, Enterobacterales, P. aeruginosa and Acinetobacter are the predominant ESBL producers. This might be due to continuous exposure of these bacteria to a variety of β-lactams that leads to the production of beta-lactamases.42 Moreover, plasmid and chromosomal gene mediated beta-lactamase enzymes are major reasons for antibiotic resistance.30
Though data on carbapenemase producing GNB infection among patients in ICUs is very limited in Ethiopia, the present study prevalence (5.2%) is higher than a report from Addis Ababa (2%), Ethiopia.27 This is because, the former study included both ICU and non-ICU patients. It is a fact that non-ICU patients have a lower risk and lower chance of acquiring CP producing bacteria than patients in the ICU. Previous studies in Nigeria,33 India,37 Taiwan,43 Indonesia,39 Nepal30 and Romania44 reported 34.5%, 34.5%, 15.4%, 13.7%, 11.2% and 21.6% of CP production, respectively which are much higher than the present finding. This could be due to heterogeneity in region, ICU settings, bacterial species, and methods of carbapenemase detection, local antibiotic use and infection control systems.
The predominant carbapenemase producing bacteria were isolated from LRTI (14.7%) and BSI (5.5%) in the present study. It is true that P. aeruginosa and K. pneumoniae are common etiologies of LRTI and BSI. These isolates are the major carbapenemase producers in the present study. Similarly, carbapenemase producing bacteria were isolated from LRTI among ICU patients.26
The principal carbapenemase producing bacteria in the present study were P. aeruginosa (27.6%) and K. pneumoniae (18.2%). In spite of percentage differences, K. pneumoniae (4.21%) and P. aeruginosa (2.6%) were the predominant carbapenemase producing isolates in Algeria.40 Similarly, K. pneumoniae (91%) and P. aeruginosa (66%) were the commonest carbapenemase producing organisms in Taiwan.45
The burden of beta-lactamase producing bacterial infections among ICU patients is aggravated by the high percentage (81.5%) of multi-drug resistant strains. The prevailing prevalence of MDR is in accord with studies done in Uganda (58%),6 India (72.5%),26 Nepal (62.1% and 79%)30,46 and Mexico (70.96%).28 The high burden of MDR isolates in the present study could be associated with empirical and non-selective use of antibiotics, irrational dose regimens, and the transfer of MDR determining genes among the isolates. Furthermore, mutations in the chromosome might result in over production of ESBLs, over-expression of efflux pumps, target modifications and permeability alterations.
In this study, all the bacterial isolates were resistant to ampicillin and tetracycline. These antibiotics are frequently used for a first line of treatment for common infections. Comparable results were reported in Ethiopia where bacterial isolates were 100% resistant to ampicillin and 81.9% resistant to sulfamethoxazole-trimethoprim.27
Besides the high burden of beta-lactamase production and MDR, resistance of GNB towards third generation cephalosporins is a serious issue and poses a major challenge in the management of patients in the ICU. Antimicrobial susceptibility testing of isolates revealed a high percentage of resistance to cefuroxime (83.1%), ceftazidime (77.9%), cotrimoxazole (77.9%) and cefotaxime (75.8%) in this study. A study from Mexico reported 84.17% resistance to cefuroxime, 82.93% resistance to piperacillin, 78.1% resistance to cefotaxime and 77.4% resistance to ceftriaxone.28 Another study from Nepal reported 66.1% resistance to ceftazidime.46
Strong resistance towards third generation cephalosporins occurs among P. aeruginosa, E. cloacae, K. pneumoniae and E. cloacae isolates in the present study as all P. aeruginosa and E. cloacae were resistant against sulfamethoxazole-trimethoprim, cefuroxime, cefotaxime, and ceftazidime. The findings are comparable with a study from Algeria where P. aeruginosa and E. cloacae isolates were 100% resistant to sulfamethoxazole-trimethoprim, cefuroxime, cefotaxime, and ceftazidime.40
In general, the reason for the observed resistance to different antibiotics among GNB isolates could be due to the syndromic management of patients and non-selective prescription of broad spectrum antibiotics to treat various sites of bacterial infections. In Ethiopia, syndromic management of patients and use of antibiotics without definite diagnosis and prescription are common practices which results in overuse and misuse of drugs that greatly contributes to the emergence and spreading of drug resistance.
A previous history of hospital admission within the past year, and use of arterial intravenous catheters are predictor variables for beta-lactamase producing bacterial infection in the present study. This is consistent with studies from India47 and Poland.3 In the present study, the use of a supportive medical care device is strongly associated with the occurrence of a beta-lactamase producing bacterial infection. This is consistent with a report from Poland.3 Hospitalization in a surgical ICU is also a predictor variable in our finding, and the finding is in line with a previous study.48 This might be due to inappropriate infection prevention control, and biofilm formation of pathogens which have become resistant to many drugs.
Conclusions
The alarming prevalence of beta-lactamase producing GNB infections among patients in the ICU is obtained. These beta-lactamase producing isolates are extremely MDR and revealed a high burden of resistance to cephalosporin, penicillins, tetracycline and anti-metabolite classes of antibiotics. Thus, to slow the threat of these superbugs, treatment of ICU patients has to be guided with regular drug susceptibility testing and intensive care services should be given in accord with strict infection prevention practices. Further, similar studies that include identification of ESBL and CP determining genes among patients in ICU are recommended in Ethiopia.
Strengths and Limitations
Findings from this study will be an important touchstone for future, larger, studies to explore the distribution of carbapenemase and ESBL organisms and associated factors in intensive care unit settings. As the present study was based on phenotypic detection of ESBL, carbapenemase and AMR, some beta-lactamases, carbapenemases and pathogenic strains might have been missed. Thus, molecular characterizations are recommended for future studies.
Abbreviations
APHI, Amhara Public Health Institute; ATCC, American Type Culture Collection; AMR, antimicrobial resistance; BSI, blood stream infection; CLSI, Clinical and Laboratory Standards Institute; CP, carbapenemase; ESBL, extended-spectrum β-lactamase; GNB, Gram-negative bacilli; ICU, intensive care unit; LRTI, lower respiratory tract infection; MDR, multi-drug resistance; UTI, urinary tract infection.
Data Sharing Statement
Results of this study are generated from the data collected and analyzed based on stated materials and methods. All data concerning to this study are in the manuscript. All data are documented in the manuscript and there is no supplementary file.
Acknowledgments
This study was supported by Bahir Dar University. We are grateful to Ethiopian Public Health Institute for bringing bacterial reference strains. Our gratitude extends to Felegehiwot Referral Hospital for conducting this study in their facility. We are indebted to ICU healthcare workers for their role in identification of patients and support during sample collection. We acknowledge the study participants for their participation in the study.
Authors’ Information MA is Medical Microbiologist in the department of Clinical Laboratory Science, Chagni Hospital, Ethiopia.
FM is assistant professor of Medical Microbiology in the department of Medical Laboratory Science, College of Medicine and Health Sciences, Bahir Dar University. WM is Associate Professor of Medical Microbiology in the department of Medical Laboratory Science, College of Medicine and Health Sciences, Bahir Dar University, Ethiopia.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; took part in drafting the article and revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
The study was financed by Bahir Dar University. The finance was used for purchasing chemicals, data collection, analysis and interpretation.
Disclosure
The authors declared that they have no conflicts of interest for this work.
References
1. Marshall JC, Bosco L, Adhikari NK, et al. What is an intensive care unit? A report of the task force of the world federation of societies of intensive and critical care medicine. J Crit Care. 2017;37:270–276. doi:10.1016/j.jcrc.2016.07.015
2. Dereli N, Ozayar E, Degerli S, Sahin S, Koç F. Three-year evaluation of nosocomial infection rates of the ICU. Rev Bras Anestesiol. 2013;63(1):79–84. doi:10.1590/S0034-70942013000100006
3. Deptuła A, Trejnowska E, Ozorowski T, Hryniewicz W. Risk factors for healthcare-associated infection in light of two years of experience with the ECDC point prevalence survey of healthcare-associated infection and antimicrobial use in Poland. J Hosp Infect. 2015;90(4):310–315. doi:10.1016/j.jhin.2015.03.005
4. Stocchetti N, Carbonara M, Citerio G, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452–464. doi:10.1016/S1474-4422(17)30118-7
5. Khan HA, Baig FK, Mehboob R. Nosocomial infections: epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7(5):478–482. doi:10.1016/j.apjtb.2017.01.019
6. Agaba P, Tumukunde J, Tindimwebwa JV, Kwizera A. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: a cross sectional study. BMC Res Notes. 2017;10(1):349. doi:10.1186/s13104-017-2695-5
7. Akhtar N. Hospital acquired infections in a medical intensive care unit. J Coll Physicians Surg Pak. 2010;20(6):386–390.
8. Kołpa M, Wałaszek M, Gniadek A, Wolak Z, Dobroś W. Incidence, microbiological profile and risk factors of healthcare-associated infections in intensive care units: a 10 year observation in a provincial hospital in Southern Poland. Int J Environ Res Public Health. 2018;15(1):112. doi:10.3390/ijerph15010112
9. Lai CC, Wang CY, Chu CC, et al. Correlation between antibiotic consumption and resistance of Gram-negative bacteria causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009. J Antimicrob Chemother. 2011;66(6):1374–1382. doi:10.1093/jac/dkr103
10. Sabir R, Alvi SF, Fawwad A. Antimicrobial susceptibility pattern of aerobic microbial isolates in a clinical laboratory in Karachi-Pakistan. Pak J Med Sci. 2013;29(3):851. doi:10.12669/pjms.293.3187
11. Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4(148):148ra116. doi:10.1126/scitranslmed.3004129
12. Akova M, Daikos GL, Tzouvelekis L, Carmeli Y. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin Microbiol Infect. 2012;18(5):439–448. doi:10.1111/j.1469-0691.2012.03823.x
13. É R, Woerther PL, Barbier F. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann Intensive Care. 2015;5(1):21.
14. Mehrad B, Clark MN, Zhanel GG, Lynch JP. Antimicrobial resistance in hospital-acquired Gram-Negative bacterial infections. Chest. 2015;147(5):1413–1421. doi:10.1378/chest.14-2171
15. Yamachika S, Sugihara C, Kamai Y, Yamashita M. Correlation between penicillin-binding protein 2 mutations and carbapenem resistance in Escherichia coli. J Med Microbiol. 2013;62(3):429–436. doi:10.1099/jmm.0.051631-0
16. Nordmann P. Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Et Maladies Infect. 2014;44(2):51–56. doi:10.1016/j.medmal.2013.11.007
17. Rello J, Eshwara VK, Lagunes L, et al. A global priority list of the top ten resistant microorganisms (TOTEM) study at intensive care: a prioritization exercise based on multi-criteria decision analysis. Eur J Clin Microbiol Infect Dis. 2019;38(2):319–323. doi:10.1007/s10096-018-3428-y
18. Venkatasubramanian P, Balasubramani SP, Patil R. “Planetary Health” perspectives and alternative approaches to tackle the AMR challenge. Antimicrob Resist. 2020;165–188.
19. Arora A, Jain C, Saxena S, Kaur R. Profile of drug resistant Gram negative bacteria from ICU at a tertiary care center of India. Asian J Med Health. 2017;18:1–7. doi:10.9734/AJMAH/2017/31434
20. Walia K, Madhumathi J, Veeraraghavan B, et al. Establishing antimicrobial resistance surveillance & research network in India: journey so far. Indian J Med Res. 2019;149(2):164. doi:10.4103/ijmr.IJMR_226_18
21. Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. Cambridge university press; 2006.
22. Zowawi HM, Harris PNA, Roberts MJ, et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 2015;12(10):570. doi:10.1038/nrurol.2015.199
23. Khushbu Y, Satyam P. Bacteriological profile of lower respiratory tract infection (LRTI) among HIV seropositive cases in Central Terai of Nepal. Int J Curr Microbiol Appl Sci. 2015;4(11):431–442.
24. Wayne PA. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing.
25. Miller JM, Binnicker MJ, Campbell S, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67(6):e1–94.
26. Gupta R, Malik A, Rizvi M, Ahmed M. Presence of metallo-beta-lactamases (MBL), extended-spectrum beta-lactamase (ESBL) & AmpC positive non-fermenting Gram-negative bacilli among Intensive Care Unit patients with special reference to molecular detection of blaCTX-M & bla AmpC genes. Indian J Med Res. 2016;144(2):271. doi:10.4103/0971-5916.195043
27. Beyene D, Bitew A, Fantew S, Mihret A, Evans M. Multidrug-resistant profile and prevalence of extended spectrum β-lactamase and carbapenemase production in fermentative Gram-negative bacilli recovered from patients and specimens referred to National Reference Laboratory, Addis Ababa, Ethiopia. PLoS One. 2019;14(9):e0222911. doi:10.1371/journal.pone.0222911
28. Uc-Cachón AH, Gracida-Osorno C, Luna-Chi IG, Jiménez-Guillermo JG, Molina-Salinas GM. High prevalence of antimicrobial resistance among Gram-negative isolated bacilli in Intensive Care Units at a Tertiary-Care Hospital in Yucatán Mexico. Med. 2019;55(9):588.
29. Singh N, Pattnaik D, Neogi DK, Jena J, Mallick B. Prevalence of ESBL in Escherichia coli isolates among ICU patients in a tertiary care hospital. JCDR. 2016;10(9).
30. Kayastha K, Dhunge B, Karki S, et al. Extended-Spectrum β-Lactamase-producing Escherichia coli and Klebsiella Species in pediatric patients visiting international friendship children’s Hospital, Kathmandu, Nepal. Infect Dis Res Treat. 2020;13:1–7.
31. Kovacevic P, Zlojutro B, Kovacevic T, Baric G, Dragic S, Momcicevic D. Microorganisms profile and antibiotics sensitivity patterns in the only medical intensive care unit in Bosnia and Herzegovina. Microb Drug Resist. 2019;25(8):1176–1181. doi:10.1089/mdr.2018.0458
32. Siddique SG, Bhalchandra MH, Wyawahare AS, Bansal VP, Mishra JK, Naik SD. Prevalence of MRSA, ESBL and Carbapenemase producing isolates obtained from endotracheal and tracheal tubes secretions of ICU patient at tertiary care centre. Int J Curr Microbiol App Sci. 2017;6(4):288–299. doi:10.20546/ijcmas.2017.604.032
33. Kano CU. Carbapenem-Resistant Enterobacteriaceae (CRE) in Intensive Care Units and surgical wards of hospitals with no history of carbapenem usage in Kano, North West Nigeria. Niger J Microbiol. 2015;27(1):2612–2618.
34. Moolchandani K, Sastry AS, Deepashree R, Sistla S, Harish BN, Mandal J. Antimicrobial resistance surveillance among Intensive Care Units of a Tertiary Care Hospital in Southern India. J Clin Diagn Res. 2017;11(2):DC01–DC07. doi:10.7860/JCDR/2017/23717.9247
35. Pilmis B, Zahar JR. Ventilator-associated pneumonia related to ESBL-producing gram negative bacilli. Ann Transl Med. 2018;6(21):424. doi:10.21037/atm.2018.09.34
36. Sid Ahmed MA, Bansa D, Acharya A, et al. Antimicrobial susceptibility and molecular epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae from intensive care units at Hamad Medical Corporation, Qatar. Antimicrob Resist Infect Control. 2016;5:4. doi:10.1186/s13756-016-0103-x
37. Oberoi L, Singh N, Sharma P, Aggarwal A. ESBL, MBL and Ampc β lactamases producing superbugs–Havoc in the Intensive Care Units of Punjab India. JCDR. 2013;7(1):70. doi:10.7860/JCDR/2012/5016.2673
38. Saeed NK, Kambal AM, El-Khizzi NA. Antimicrobial-resistant bacteria in a general intensive care unit in Saudi Arabia. Saudi Med J. 2010;31(12):1341–1349.
39. Saharman YR, Lestari DC. Phenotype characterization of beta-lactamase producing enterobacteriaceae in the intensive care unit (ICU) of CiptoMangunkusumo hospital in 2011. Acta Med Indonesiana. 2016;45(1).
40. Hecini-Hannachi A, Bentchouala C, Lezzar A, et al. Multidrug-resistant bacteria isolated from patients hospitalized in Intensive Care Unit in University Hospital of Constantine, Algeria. Afr J Microbiol Res. 2016;10(33):1328–1336. doi:10.5897/AJMR2016.8257
41. Leite CA, Oizumi KY, Caleffi-Ferracioli KR, et al. β - lactamase producing Gram-negative bacteria in an intensive care unit in southern Brazil. Brazilian J Pharm Sci. 2017;53(2).
42. Shrestha UT, Shrestha S, Adhikari N, et al. Plasmid profiling and occurrence of β-lactamase enzymes in multidrug-resistant uropathogenic Escherichia coli in Kathmandu, Nepal. Infect Drug Resist. 2020;13.
43. Chang YY, Chuang YC, Siu LK, et al. Clinical features of patients with carbapenem non-susceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect. 2015;48(2):219–225. doi:10.1016/j.jmii.2014.05.010
44. Zaha DC, Kiss R, Hegedűs C, et al. Recent advances in investigation, prevention, and management of healthcare-associated infections (HAIs): resistant multidrug strain colonization and its risk factors in an Intensive Care Unit of a University Hospital. Biomed Res Int. 2019;2019:2019. doi:10.1155/2019/2510875
45. Liao CH, Lee NY, Tang HJ, et al. Antimicrobial activities of ceftazidime-avibactam, ceftolozane- tazobactam, and other agents against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa isolated from intensive care units in Taiwan: results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan in 2016. Infect Drug Resist. 2019;12:545. doi:10.2147/IDR.S193638
46. Muktan B, Shrestha UT, Dhungel B, et al. Plasmid mediated colistin resistant mcr-1 and co-existence of OXA-48 among Escherichia coli from clinical and poultry isolates: first report from Nepal. Gut Pathog. 2020;12:44. doi:10.1186/s13099-020-00382-5
47. Daef EA, Elsherbiny NM. Clinical and microbiological profile of nosocomial infections in adult intensive care units at Assiut University hospitals, Egypt. J Am Sci. 2012;8(12):1239–1250.
48. Wilson J. Surgical site infection: the principles and practice of surveillance. Part 1: key concepts in the methodology of SSI surveillance. J Infect Prev. 2013;14(1):6–12. doi:10.1177/1757177412471147
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
