Back to Journals » Patient Preference and Adherence » Volume 14
Moving the Dial on Heart Failure Patient Adherence Rates
Authors Makris E, Hu L, Jones GB , Wright JM
Received 22 September 2020
Accepted for publication 20 November 2020
Published 4 December 2020 Volume 2020:14 Pages 2407—2418
DOI https://doi.org/10.2147/PPA.S283277
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Eleanna Makris,1 Lucy Hu,1 Graham B Jones,1,2 Justin M Wright1
1TRD Innovation Group, Novartis Pharmaceuticals, East Hanover, NJ 07936, USA; 2Clinical and Translational Science Institute, Tufts University Medical Center, Boston, MA 02111, USA
Correspondence: Graham B Jones Email [email protected]
Introduction: Heart failure remains a substantive contributor to patient morbidity and mortality rates worldwide and represents a significant burden on the healthcare ecosystem. Faced with persistent physical symptoms and debilitating social consequences, patients follow complex treatment regimens and often have difficulty adhering to them.
Purpose: In this manuscript, we review factors which contribute to low adherence rates and advance potential single- and multi-factor-based interventions. It is hoped that these observations can lead to improvements in managed care of this vulnerable population of patients.
Methods: A narrative review of the primary literature was performed on contributing factors with primary focus on the period 2015– 2020 using available databases and search engines. Adherence pain points identified were mapped against a series of potential solutions which are presented.
Results: Enhancement of treatment adherence relies on two approaches viz. single-factor and multi-factor solutions. Single factors identified include electronic reminders, enhanced health education, financial incentives, gamification strategies, community drivers, persona-based modeling, and burden relief of poly pharmacy. Multi-factor solutions combine two or more of the seven approaches offering the potential for flexible interventions tailored to the individual.
Discussion and Conclusion: Heart failure patients with poor adherence have increased mortality, hospitalization needs, and healthcare costs. This review highlights current single-factor and multi-factor adherence methods. Against a backdrop of diversity of approaches, multi-factor solutions cast the widest net for positively influencing adherent behaviors. A key enabler lies in the development and leveraging of patient personas in the synthesis of successful intervention methods. Deployable solutions can also be envisioned in clinical trials where adherence tracking represents an essential component.
Keywords: heart failure, cardiac disease, treatment adherence, intervention, connected health
Introduction
Heart failure (HF) affects nearly 7 million adults in the USA, contributing to 1 in every 8 deaths.1 While mortality rates have declined over time due to improved patient education, surgical interventions, and the introduction of new medications, HF still presents a major financial burden on the healthcare system. This exceeded $30 billion in 2012 and is expected to more than double by 2030.1 HF also presents greater impact on a patient’s perception of their health and quality of life than most other chronic conditions and is associated with impaired physical and social functioning.2 In terms of disability-adjusted life years (DALY), heart failure collectively contributes a burden of >62,000 healthy life years lost.3
Approximately 80% of HF patients are aged 65 years and older, a population that is at higher risk for comorbidities and the adverse effects of polypharmacy.4 Common comorbidities include other heart conditions such as hypertension, coronary artery disease, and atrial fibrillation, as well as conditions such as diabetes mellitus and chronic obstructive pulmonary disease.5 Current treatment protocols for HF include self-care (exercise, smoking cessation, calorific restriction, lowered sodium intake) and a variety of chemotherapeutics which include statins, beta blockers, diuretics, and ACE inhibitors in conjunction with other drugs.6 Significantly, while HF primarily impacts middle-aged to elderly populations, it remains a contributing factor to the top five leading causes of death in every age category.5
Adherence is vital to the success of any prescribed treatment plan, yet non-adherence for chronic cardiac conditions increases over time and can reach as high as 60% after 3 years.7 Non-adherence has been identified as a lead contributor in hospital readmissions, high morbidity and mortality rates, and high medical expenditures.8 Non-adherence can be unintentional; patients may forget to take medications, forget to refill a prescription, or may not take a medication correctly.9 However, some patients may intentionally choose to not adhere to treatment due to lack of motivation or autonomy, adverse side effects, desire for control, or perceived stigmas associated with taking medication.10 These issues may be exacerbated for patients requiring polypharmacy (40–50% of elderly patients in high-income countries) where >25% of elderly patients have a chronic intake of 10+ medicines.11,12 Polypharmacy can impact adherence through myriad drivers including increased financial burden, unwanted drug–drug interactions, and complex/difficult dosing regimens. Studies have shown that a patient's perceptions on the number of prescribed medications have a stronger correlation with low adherence than the actual number, making polypharmacy a complex issue to tackle.13
In order to develop effective non-adherence solutions, accurate adherence measurement tools are required as self-recording presents evident limitations. Morawski reported that self-recording as a measuring metric increased the likelihood of patients recording they were adherent without truly changing their medication behavior.14 In this regard, there has been natural interest in the deployment of technologies to improve outcomes. An early example was the introduction of the so-called “smart” pill bottle. Its concept is to verify adherence by tracking physical movements in the preamble to dispensing and taking medication. Approaches include sensing of pill bottle movement, removal of its cap, and pouring of the pill into one hand.15–17 Myriad technologies and sensors have been evaluated including collar switch activation and mass analysis pre/post event. However, a limitation lies in that they only imply intent to take medications. Second-generation versions have been developed with a secondary tracking component. One such example is in combination with a wrist-based sensor which follows the hand to mouth motion usually associated with oral medication administration (while also monitoring if the medication was removed from the container).18,19 A more recent example is in the form of a wearable digital smart necklace. This technology is able to decipher if a user has swallowed saliva, food, or a pill through an inbuilt piezoelectric sensor.16,20 Other approaches have deployed invasive technologies, the most widely publicized being ingestible sensors. With this approach, RFID tags are embedded into the gelatin capsule of the medication which emits a signal when liberated by gastric fluids.21 A limiting factor has been the need for patients to wear an RFID tag detector on body, either on the abdomen or on a lanyard type device, which may deter adoption of this innovative technology.20
While these and other technologies may hold promise, there are additional over-arching factors that need to be considered for adoption in managed care, including familiarity with e- and m-health devices and comfort levels in sharing medical information. The American Heart Association conducted a study to assess patient acceptance of e-Health devices to monitor cardiac-related conditions. Gratifyingly some 86% of patients were comfortable in sending heart rhythm information from smartwatches to their healthcare provider.22 In addition, 91% of healthcare providers were willing to prescribe anticoagulation medication stemming from smartphone-based detection of atrial fibrillation.22 Interestingly, another study concluded that patients were more likely to enter honest medical information to a device rather than in person.23 If scalable, this suggests increasing trust in the use of technology for healthcare purposes from both patients and providers.24 This said, HF and other cardiovascular diseases are largely found within older populations. A frequently mentioned barrier for e- and m-Health tools in cardiovascular disease is the lack of knowledge on how to use technology, especially among older age groups.25 Research however shows that older adults express the most willingness to learn how to use m-Health devices and even have higher adherence rates (74%) than younger patients when technology is incorporated into adherence routines.25,26 Multiple pilot studies indicate that both e-Health and m-Health methods have positive results for managing chronic conditions and changing health behaviors.27,28 Specific improvements have been noted in i) patient self-monitoring, ii) building social networks, iii) sharing information between patients and providers, iv) indirect feedback interactions, v) tailored education, and vi) improved communication.28 If matured and deployed appropriately, there exists the potential for a large positive impact on decreasing HF-related emergency department visits using technology, hence the impetus for this review.29
Methods
Comprehensive primary literature review was conducted using PubMed, SciFinder Scholar and Google Scholar databases. Search terms included heart failure, cardiac disease, medicine adherence, treatment adherence, multi-factor and multifaceted interventions, multidimensional solutions and combined intervention. Primary weighting was applied to the period 2015–2020, general reviews, and higher powered clinical studies. Secondary research was conducted on identified adherence pain points and factors identified in the primary trawl, and combined with relevant materials derived from websites and related social media portals (Figure 1).
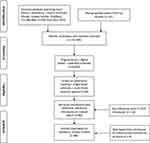 |
Figure 1 Study flowchart |
Findings
A rich and growing literature on adherence factors for HF is developing, reflecting the importance as a pivotal component of managed patient care. Additionally, there is a logical drive on the part of health benefit providers (end payers) to identify interventions and reimbursement models based on adherence and outcome measures. For interventions involving medications and lifestyle factors, improving adherence rates will remain an area of considerable emphasis, as will mean to accurately assess such rates. Based on analysis of the published literature, studies on enhancement of treatment adherence can be placed in two categories – single-factor and combination/multi-factor approaches. Herein we summarize key studies and utilize findings to propose new strategies and future areas of research to increase adherence.
Single-Factor Solutions
Single-factor solutions for adherence can be effectively grouped according to seven general approaches (vide infra). Some of these are either disease agnostic or pertain to indications with relationships to HF but as such are deemed relevant based on known co-morbidities.
Adherence Reminders
The advent of widely available digital communication devices has made automated reminder systems among the most widely studied adherence driver. The most common tool is currently SMS text as it is easy to integrate, inexpensive, and simple to understand for non-technology savvy patients.30 Apps on smartphones and other Bluetooth compatible devices are also widely used, in an effort to link adherence events to patient calendars.30 Much pioneering work on use of mobile technologies to help improve patients’ health behaviors was conducted in oncology and related chronic diseases31,32 and is now being extrapolated to HF patient care. There is compelling evidence to support the use of SMS prompts with adherence rate increases of up to 18% reported.33 This said, the absence of personalized two-way communication is a noted limitation and has led to development of chatbot variants.
Health Education
Patient health literacy is the strongest predictor of medication adherence and also positively correlates with knowledge of particular medications.34 However, in one study only 14% of HF patients felt assured in their understanding of their condition after receiving reading materials or verbal instructions.35 Lower health literacy is also linked to poorer standards of self-care and comprehension of labelling materials, factors that can lead to increased hospitalization rates and higher mortality risk.36 HF patients are subjected to a considerable distress during the diagnosis period and may not absorb all educational information presented initially, favoring approaches which mature once patients are acclimated to their new routine and condition.35,37 In their transition to independence, provider–patient interactions have been shown to be important for improving adherence9 as are interactive educational formats which promote knowledge retention more effectively than reading-material and workbook-based resources.37,38 There is also evidence that learning and coping strategies can improve rehabilitation adherence, especially for HF patients with lower levels of education and socio-economic status.39 Optimal approaches appear to involve a combination of inpatient and outpatient education,40 which can also extend to caregivers (often close family members)37 who often lack relevant healthcare knowledge.41
Financial Incentives
Insight to this interesting model was first obtained in the antihypertensive and anti-hyperlipidemic arena, revealing the most effective approach incentivizes both patients and their clinicians.42,43 A study examining patient views showed that 71% believed that the financial incentives positively impacted adherent behaviors.44 Monetary incentive programs can also be personalized to the patient and the factors that motivate them.45 One study (which recorded positive outcomes relative to control arms of the study) assessed the impact in group settings and with the option for charitable contributions to be rendered based on adherence goals being met.46 Another interesting study examined the comparative efficacy and cost-effectiveness of goal-directed versus outcome-based financial incentives.47 Goal-directed patients earned money for participating in lifestyle interventions, meeting physical activity targets, and monitoring weight and diet whereas the outcome-based patients earned money for simply losing weight.47 This trial is still ongoing, but could have important ramifications for HF patients where these factors play a key role in disease management.
Gamification
The concept of gamification has been utilized to stimulate users to complete otherwise routine tasks, and the ubiquity of mobile devices make this a fertile area to stimulate adherence driving behaviors. As an early exemplar the diabesties app. encouraged friendly competition and communication by pairing diabetes patients to compete in treatment adherence tasks such as checking blood glucose levels.32 Likewise, Hopelab introduced the Re-Mission video game, to draw parallels between combat marksmanship and real-life cancer treatment strategies.48 Cancer patients who played Re-Mission had significantly higher rates of cancer knowledge, self-efficacy, and oral medication adherence.49 A subsequent study revealed that actively playing the game stimulated neural circuits associated with incentive motivation,50 and increased information-seeking behavior.51 Though yet to be deployed for studies on HF patients and their care givers, clear and obvious opportunities exist.
Peer and Community Support
Social support has been shown to play a critical role in reducing patient stress associated with chronic diseases,41,52 and the effect of psychosocial factors noted in the progression of HF.41 Medicine and treatment adherence rates have been found to increase when social support is present for CHF and CVD patients,53,54 which can be categorized into three types: patient-to-patient, patient-to-family, and patient-to-provider.
Patient-to-Patient Interactions
In pioneering studies, Kamark et al highlighted the association between lower blood pressure and patients' proximity to a companion, reasoned to help alleviate psychological stressors.54 More recent research by Lauffenburger identified that patients with family members who were diagnosed with the same chronic diseases were more likely to adhere to their medication if their relatives also did so.55 This prompts the question of whether communities of HF patients might experience the benefits of such positive reinforcement and its effects on medicine adherence.
Patient-to-Family Interactions
Many caregivers and family members feel overwhelmed by their responsibility to aid and assist patients with chronic diseases. Alleviating this stress and improving communication between the two can lead to positive effects on both parties.41 In one study, incorporating family support and health information for family members and patients was shown to improve medicine adherence within three months.36 Research has also shown that adherence rates increase in up to 38% of patients when family members are prescribed the same drug regimen.55 These family-level associations offer potential inroads to the design of adherence systems, and very likely have deep cultural and ethnic components.
Patient-to-Provider Interactions
Personal connections and interventions by medical professionals have shown an increase in patient engagement with their health and medical literacy.56 Yang et al suggest using “app prescriptions” to open a platform between patients and medical professionals and enhance involvement. A review on the effect of healthcare professional interactions on the self-care of HF patients analyzed 24 separate studies during the period 1999–2012 and indicated a significant correlation between healthcare professional involvement and patient self-care.57 When providers were accessible, attentive, and collaborative with their patients, self-care improved. Conversely, when healthcare professionals appeared non empathetic, poor communicators, and inconsistent, they had a detrimental impact.57 Combining best practice in terms of demeanor, cadence, and consistency through engaging apps may offer opportunity to impact adherence rates at scale.
Connecting through Patient Personas
The use of personas (often referred to as patient segmentation or stratification) has the ability to aid designers when building platforms and solutions for adherence in e-Health.58 Widely utilized in technology outside of healthcare,59 patients who have utilized persona-tailored experiences have reported higher levels of satisfaction and increased awareness of their own health.60 Utilizing personas in potential adherence tools could combine the emotional and functional needs of patients to produce impactful results, and the increasingly available electronic data from patients help to build accurate models.61 In a recent study involving 24 chronic HF patients and 14 caregivers, participants were interviewed about their self-care decision making.62 The following three personas were identified; i) Rule Following: These patients find confidence in clinician experts and search for clear rules and understanding. They exercise caution in relation to the uncertainty in their medical affliction and treatment. ii) Researching: These patients gather their own information in addition to that provided to them by healthcare professionals. They create their own strategies for combating and mitigating their disease with a mixture of knowledge and support from external sources as well as clinicians. iii) Disengaging: These patients lack the drive to perform self-care behaviors. They do not actively seek information nor do they work towards reducing uncertainty related to their ailments. The hope and expectation is that these discrete personas can be used to design adherence drivers which are responsive to and mindful of these disparate mindsets.
Burden Relief for Polypharmacy Patients
HF patients often have numerous medications and complex treatment regimens to balance, which are only compounded by comorbidities. Polypills, which combine several medications into one pill, are a promising strategy to simplify dose administration and thus adherence. However, patients require different doses and combinations of medicines and a rigid formulation would only be suitable for a very limited number of patients.11 One proposed solution is to use 3D printing as a way to quickly and easily manufacture personalized polypills at pharmacy level. 3D printing has the capability to create highly modular capsules which allow for bespoke drug release patterns by controlling concentric shell thickness or shell porosity. A clinical study on the use of 3D printed polypills was supportive, with 86% adherence registered at 12 months, remarkable in that currently approximately 50% of patients become non-adherent with their cardiovascular medications within a year.63 However, although 3D printing approaches show promise in trials and proof-of-concept research studies, deployment at scale under FDA mandated regulatory guidelines remains to be demonstrated as numerous obstacles will need to be addressed.
Multi-Factor Interventions
We highlight eight studies and use cases which support multi-factor interventions organized by the methods employed. As with single-factor interventions, most also relate to indications with known relationships to HF.
Multi-Factor Components: (1) Adherence Tracker, (2) Reminders
Smart pill bottles generally aim to remind patients to take their medication and also track adherence. One of the earliest models, the Vitality Glowcap was a lid that attached to standard prescription bottles and required a home base to transmit the data.18,64 It provided visual and audio reminders by glowing or chiming, had options to send reports to caregivers or call the pharmacy, and measured adherence through bottle opening and closing.64 Smart bottles have since gained more features and have become more streamlined, automatically uploading data through the bottle itself or a smartphone in lieu of a wired home base. The AdhereTech device retains the lights and chimes and adds in additional data sources (a measure of bottle contents in addition to timestamp of bottle opening/closing events), personalized dosing schedules, and follow up for missed doses via automated and personal calls or texts.17,65 The Pillsy device builds upon these features with an engaging app with data analytics, customizable reminders, double dosing warnings, educational materials, live notifications to keep caregivers in the loop, and timely refill orders.66 Similarly, CleverCap offers a personal management app with educational materials, caregiver access for monitoring, personalizable alerts, and the ability to make prescriber-approved edits to dosing.67 It also has an analytics portal for providers and stakeholders. CleverCap offers two different products which both connect with the app and portal: the basic CleverCap LITE and the CleverCap PRO which has the additional feature of dispensing exact doses.67 Smart pill bottles are generally well received by patients. Subjects in a 2019 trial using CleverCap PRO had positive feedback for the system, ascribing it an average score of 9 out of 10.68 Several studies have been conducted regarding the efficacy of the AdhereTech system and affirmed its overall correlation with better adherence and therapy retention, more positive outcomes, and fewer gap days without medication between refills.69–72 However, there are still challenges with measuring adherence solely through bottle opening and closing as patients can simply open the bottle and not remove any of the medication. The CleverCap PRO device attempts to address this and prevent overdosing by only dispensing exact doses.67 The AdhereTech device also ensures that medication is removed from the bottle by measuring its contents through capacitance sensors embedded in its walls.65 While these features ensure that medication is actually removed from the bottle, smart bottles still suffer from the caveat that it is impossible to verify if patients actually administer their medication.
Multi-Factor Components: (1) Communication with Healthcare Professional, (2) Patient Education, (3) Collaborative Care, (4) Reminders
A study conducted by Ho et al examined the efficacy of a four component intervention on medication adherence in 253 patients with acute coronary syndrome over 12 months.73 After patients were initially discharged from the hospital with medication information, a pharmacist contacted them within 7–10 days to discuss their medication and any questions or concerns. The pharmacist also contacted each patient’s primary care clinician to inform them of the study and each step the patient took. Educational messages and contact with the pharmacist continued throughout the year to ensure retention of the information. Finally, phone call reminders were set for medication reminders and medication refills the first 6 months and tapered out to only medication refill calls in the last 6 months. Results showed patients receiving the intervention method had higher medicine adherence rates but no statistically significant changes were recorded in LDL-C or BP levels.73
Multi-Factor Components: (1) Health Records, (2) Clinical Decision Support, (3) Educational Materials, (4) Self-Care Features, (5) Follow-Up Component
The mAFA Trial was a prospective randomized trial that tested the efficacy of a mobile app designed for atrial fibrillation patients (mAF App) against usual care.74 The app included multiple components: (1) a personal health record which tracks medical history, lab tests, and more; (2) clinical decision support through app-calculated stroke and bleeding risks; (3) educational materials to improve disease background and management knowledge; (4) self-care suggestions to monitor vitals; and (5) a structured follow-up component. Over 90% of patients reported favorable feedback and reported the app was easy to use and helpful. In addition, patients who used the app had significant improvements in knowledge, self-care, and adherence as compared to the standard care patients.74
Multi-Factor Components: (1) Financial Incentives, (2) Intrinsic Motivators
A study performed by Shapiro et al hypothesized that incorporating personalized motivators with financial incentives would improve blood pressure control, especially in low-income patients.24 Control group patients received funds at each study visit and for completing questionnaires. Intervention group patients received fixed payments, contingent payments, and lotteries for returning to the study. In addition, intervention patients received a personalized calendar with “images of loved ones” and patient-identified “activities or life goals associated with being healthy” to record medication use. Additionally, staff met with intervention participants to discuss how improved blood pressure control would impact goals and relationships and to also link lottery or contingent payments to motivational goals (eg, “so that you can dance at your daughter’s wedding”). The results showed that intervention patients were significantly more likely to achieve the study goal (systolic blood pressure <140 mm Hg) compared with the control patients. Improved control was primarily seen in intervention patients whose regimens were intensified (eg, increased dosage, changed medication, or added medication), but was not seen in control patients. This suggests incorporating intrinsic motivators with regimen changes may be effective at improving control.
Multi-Factor Components: (1) Wearable m-Health Device, (2) Medication Adherence Tool, (3) Daily Personalized Text Messages
A study initiated in 2019 is designed to test the efficiency of m-Health technologies to improve drug adherence and physical activity levels for patients with HF and diabetes mellitus.24 It is one of the first studies to test a completely digital strategy for improving medication adherence. A wearable device is utilized to track physical activity which affects the frequency and type of feedback reminders. An online adherence tool is used to supplement health education for patients. The study will be evaluating 200 patients over a longer period than previous studies (6 months) and is currently active.
Multi-Factor Components: (1) Wireless Technology, (2) Personalized Lifestyle Text Messages
This pilot study tested the effects of a combined intervention on 12 patients with type 2 diabetes and/or hypertension.75 Patients were provided with wireless home blood pressure (BP) monitors and motivational text messages and advice for seven weeks. Mean blood pressure, medication adherence, and satisfaction with the program all increased at the end of the study. The combined intervention was also found to have a greater reduction in BP than was seen in other studies which utilized a single intervention method (text message reminders or BP monitoring). The study was limited by the small sample size but offers promise in multi-factor solutions having larger and more sustainable impacts on medication and treatment adherence.
Multi-Factor Components: (1) Wearable Device, (2) Mobile Application, (3) Communication with Healthcare Professional
A study reported by Angellotti et al worked with acute myocardial infarction (AMI) survivors for a 12-week tele-rehabilitation program.76 Eighteen patients received a Philips Healthwatch to monitor heart rate, access to an app which displayed progress and goals, and weekly phone sessions with a cardiac rehabilitation nurse. After twelve weeks, patients reported higher adherence to exercise regimens and greater angina stability. This study established the feasibility of a technical multi-factor approach in treating patients and paves the way for more research with larger sample sizes.
Multi-Factor Components: (1) Wireless Device, (2) Mobile Application, (3) Electronic Medication Tray, (4) SMS Reminders
This 2019 study focused on 56 Hispanic patients with uncontrolled hypertension and poor medication adherence to test medicine adherence with solutions tailored to cultural norms and needs.77 Patients received a Bluetooth-enabled BP device which connected to the SMASH app. The app provided auditory and text instructions as well as a cumulative table of blood pressure readings in a daily/weekly/monthly scale. SMS notifications were sent to patients to remind them to take their BP at required times. The frequency of these notifications was tailored to each patient based on their previous adherence to measurements. A provided electronic medication tray, associated with the pill bottles, also offered a series of notifications in increasing intensity as time passed to alert patients (blinking light, chiming, SMS/phone call). The results of this study included statistically significant BP reductions as well as sustained medication adherence and BP self-monitoring behaviors. This study highlighted the significance of culturally tailored solutions to adherence and the need to personalize solutions based on demographics.
These eight examples not withstanding, there are also reports of multi-factor solutions which were not effective in improving adherence rates. For example, a study of 1509 patients with AMI involved medicine adherence (recorded by electronic pill bottle) over a 12-month period.78 Patients were given daily lottery incentives if they adhered to medication, had a friend/family member notified if they had not taken medication for over two days, had access to social work resources, and were monitored by a medical professional. No significant effect was seen on medication adherence, hospitalizations or medical costs between patients in the study versus control arm. One theory advanced to explain was the extended time lapse between patient discharge from the hospital and commencement of the intervention.78 Another study sought to assess the impact of mailed information and interactive phone calls on adherence to medication and cardiac rehabilitation programs after an AMI.79 None of the interventions resulted in significant increases in medication adherence, possibly pointing to the diminished relevance of printed materials.
Discussion and Future Directions
Patient nonadherence can generally be categorized into unintentional and intentional behaviors. Unintentional nonadherence is defined as “passively inconsistent medication-taking behavior (forgetfulness or carelessness)”.80 This passive nonadherence has been linked largely to patients’ memory and complexity of medication regimens.81 Interventions which encourage reminders, improve communication with healthcare professionals about health education, simplify medication regimens, and introduce community/support options can help address the needs of these patients. A 2016 study with patients suffering from hypertension showed that patients who displayed unintentional nonadherence were younger in age.82 This makes unintentional nonadherence an important topic for further studies, as if uncorrected could compromise behaviors throughout lifespan. On the other hand, a patient is intentionally nonadherent if they deliberately do not follow their treatment plan. Intentional nonadherence is associated with patient motivation and beliefs, for example, fear of medication or perceived social stigmas.10 Studies have revealed that intentionally nonadherent patients question the utility of prescribed medications far more than unintentionally nonadherent patients.83 Due to the personal nature of motivation and belief structures, interventions for active nonadherers need to be customizable and specific to each patient. Consequently, multi-factor solutions may be especially beneficial. To target intentional nonadherers features such as financial incentives, community building, and gamification may help inspire patients to follow treatment plans by targeting intrinsic motivators beyond improved health.84 For this reason, in ideating potential solutions, we believe a multi-factor solution should be responsive to both intentional and unintentional non-adherers. Equally important are the values placed on personas in approaching these solutions.59 Building in personas, especially understanding a patient’s changes between them, is an integral portion of medication adherence and allows interventions to be designed that are specific to their needs.
Potential Solutions
Guided by the published studies we postulate some potential approaches as logical avenues for future research:
Wrist-Worn Smart Health Monitoring Devices
These could allow groups of patients to establish new peer connections and share relevant data among the group (and their care providers) to incentivize adherent behaviors.
Personalized Apps
Such could customize the patient experience through segmentation and personas and build in social media and community. Gamification and financial incentives could be introduced to encourage adherence, as could automated reminders, eg, prescription auto-refill functions.
Conclusions
Medication adherence is a challenge across the healthcare industry. Roughly 50% of patients with chronic medication treatments are non-adherent.85 Heart failure patients with poor adherence have increased mortality, hospitalization needs, and healthcare costs. A variety of single-factor and multi-factor adherence methods have been advanced and subjected to field-based trials. Real-world evidence from these studies, coupled to evolving understanding surrounding the psychology of nonadherence will allow design and optimization of effective adherence drivers for this vulnerable group of patients. These strategies would also be applicable to clinical trial design, where even minor perturbations in drug adherence rates can have an impact on statistical analysis of outcome measures as evidenced by recent studies.86,87 The widespread availability of broadband networks coupled with increasing miniaturization and technological capabilities of mobile devices and sensors promise to play a key role in developing patient-centered adherence solutions and we look forward to future developments. Additionally, such considerations should inform guidelines for the treatment of heart failure, which are continually evolving.88
Acknowledgments
We warmly thank the TRD Innovation Program for support of this work.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
JMW and GBJ are employees of Novartis Pharmaceuticals, where both EM and LH conducted 2020 summer internships for Novartis Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke Statistics—2019 update: a report from the American Heart Association. Circulation (New York, N.Y. 2019;139(10):e56–e66. doi:10.1161/cir.0000000000000659.
2. Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions: results from the medical outcomes study. JAMA. 1989;262(7):907–913. doi:10.1001/jama.1989.03430070055030.
3. McGrath R, Al Snih S, Markides K, Hall O, Peterson M. The burden of health conditions for middle-aged and older adults in the United States: disability-adjusted life years. BMC Geriatrics. 2019;19(1):100. doi:10.1186/s12877-019-1110-6.
4. Vigen R, Maddox TM, Allen LA. Aging of the United States population: impact on heart failure. Curr Heart Fail Rep. 2012;9(4):369–374. doi:10.1007/s11897-012-0114-8.
5. Gastelurrutia P, Lupón J, Moliner P, et al. Comorbidities, fragility, and quality of life in heart failure patients with midrange ejection fraction. Mayo Clin Proc. 2018;2(2):176–185. doi:10.1016/j.mayocpiqo.2018.02.004.
6. Medications used to treat heart failure. American Heart Association. 2017. Available from: https://www.heart.org/en/health-topics/heart-failure/treatment-options-for-heart-failure/medications-used-to-treat-heart-failure.
7. Schwartz JB, Schmader KE, Hanlon JT, et al. Pharmacotherapy in older adults with cardiovascular disease: report from an American College of Cardiology, American Geriatrics Society, and National Institute on Aging Workshop. J Am Geriatrics Soc. 2019;67(2):371–380. doi:10.1111/jgs.15634.
8. Zhang KM, Dindoff K, Arnold JMO, Lane J, Swartzman LC. What matters to patients with heart failure? The influence of non-health-related goals on patient adherence to self-care management. Patient Educ Counseling. 2015;98(8):927–934. doi:10.1016/j.pec.2015.04.011.
9. Gao Y, Peterson E, Pagidipati N. Opportunities for improving use of evidence‐based therapy in patients with type 2 diabetes and cardiovascular disease. Clinical Cardiol (Mahwah, N.J. 2019;42(11):1063–1070. doi:10.1002/clc.23252.
10. Herrera PA, Moncada L, Defey D. Understanding non-adherence from the inside: hypertensive patients’ motivations for adhering and not adhering. Qual Health Res. 2016;27(7):1023–1034. doi:10.1177/1049732316652529.
11. Pereira BC, Isreb A, Isreb M, Forbes RT, Oga EF, Alhnan MA. Additive manufacturing of a Point‐of‐Care “Polypill:” Fabrication of concept capsules of complex geometry with bespoke release against cardiovascular disease. Advan Healthcare Mater. 2020;9(13):2000236. doi:10.1002/adhm.202000236
12. Eidam A, Roth A, Lacroix A, et al. Methods to assess patient preferences in old age pharmacotherapy – a systematic review. Patient Pref Adher. 2020;14:467–497. doi:10.2147/PPA.S236964
13. Marcum ZA, Gellad WF. Medication adherence to multidrug regimens. Clin Geriatric Med. 2012;28(2):287–300. doi:10.1016/j.cger.2012.01.008
14. Morawski K, Ghazinouri R, Krumme A, et al. Association of a smartphone application with medication adherence and blood pressure control: the MedISAFE-BP randomized clinical trial. JAMA Internal Med. 2018;178(6):802–809. doi:10.1001/jamainternmed.2018.0447
15. Diemert S, Richardson K, Hunter P, Weber J, Price M. SmartMed: a medication management system to improve adherence. Studi Health Technol Informatics. 2015;208:125.
16. Kalantarian H, Alshurafa N, Tuan L, Sarrafzadeh M. Non-invasive detection of medication adherence using a digital smart necklace. PERCOMW. 2015;348–353.
17. DeMeo D, Morena M. Medication adherence using a smart pill bottle. CEWIT. 2014;1–4.
18. Kalantarian H, Alshurafa N, Nemati E, Tuan L, Sarrafzadeh M. A smartwatch-based medication adherence system. BSN. 2015;1–6.
19. Chen C, Kehtarnavaz N, Jafari R. A medication adherence monitoring system for pill bottles based on a wearable inertial sensor. EMBC. 2014;2014:4983–4986.
20. Aldeer M, Javanmard M, Martin R. A review of medication adherence monitoring technologies. Appl Syst Innovation. 2018;1(2):14. doi:10.3390/asi1020014
21. Chai P, Castillo-Mancilla J, Buffkin E, et al. Utilizing an ingestible biosensor to assess real-time medication adherence. J Med Toxicol. 2015;11(4):439–444. doi:10.1007/s13181-015-0494-8
22. Ding EY, Dickson E, Albuquerque D, Saczynski JS, Chon K, McManus David D. Provider Perspectives on Smartwatch Monitoring for Atrial Fibrillation. American Heart Association Scientific Sessions; 2019.
23. Lucas GM, Gratch J, King A, Morency L. It’s only a computer: virtual humans increase willingness to disclose. Comp Human Behav. 2014;37:94–100. doi:10.1016/j.chb.2014.04.043
24. Sharma A, Mentz RJ, Granger BB, et al. Utilizing mobile technologies to improve physical activity and medication adherence in patients with heart failure and diabetes mellitus: rationale and design of the TARGET-HF-DM trial. Am Heart J. 2019;211:22–33. doi:10.1016/j.ahj.2019.01.007
25. Cajita MI, Hodgson NA, Lam KW, Yoo S, Han H. Facilitators of and barriers to mHealth adoption in older adults with heart failure. Comp Informatics Nurs. 2018;36(8):1–382. doi:10.1097/cin.0000000000000442
26. Ware P, Dorai M, Ross HJ, et al. Patient adherence to a mobile Phone–Based heart failure telemonitoring program: a longitudinal mixed-methods study (preprint). JMIR mHealth uHealth. 2018;7(2):e13259. doi:10.2196/13259
27. Badawy SM, Cronin RM, Hankins J, et al. Patient-centered eHealth interventions for children, adolescents, and adults with sickle cell disease: systematic review. J Med Internet Res. 2018;20(7):e10940. doi:10.2196/10940
28. Matthew-Maich N, Harris L, Ploeg J, et al. Designing, implementing, and evaluating mobile health technologies for managing chronic conditions in older adults: a scoping review. JMIR mHealth uHealth. 2016;4(2):e29. doi:10.2196/mhealth.5127
29. Widmer RJ, Allison TG, Lennon R, Lopez-Jimenez F, Lerman LO, Lerman A. Digital health intervention during cardiac rehabilitation: a randomized controlled trial. Am Heart J. 2017;188:65–72. doi:10.1016/j.ahj.2017.02.016
30. Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. Journal of Medical Internet Research. 2015;17(2):e52. doi:10.2196/jmir.3951
31. Fishbein JN, Nisotel LE, MacDonald JJ, et al. Mobile application to promote adherence to oral chemotherapy and symptom management: a protocol for design and development. JMIR Res Protoc. 2017;6(4):e62. doi:10.2196/resprot.6198
32. Constantinescu G, Rieger J, Mummery K, Hodgetts W. Flow and grit by design: exploring gamification in facilitating adherence to swallowing therapy. American Journal of Speech-Language Pathology. 2017;26(4):1296–1303. doi:10.1044/2017_AJSLP-17-0040
33. Thakkar J, Kurup R, Laba T, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Internal Medicine. 2016;176(3):340–349. doi:10.1001/jamainternmed.2015.7667
34. Lee Y, Yu HY, You M, Son Y. Impact of health literacy on medication adherence in older people with chronic diseases. Collegian (Royal College of Nursing, Australia). 2017;24(1):11–18. doi:10.1016/j.colegn.2015.08.003
35. Talmor G, Nguyen B, Keibel A, Temelkovska T, Saxon L. Use of software applications to improve medication adherence and achieve more integrated disease management in heart failure. Trends Cardiovasc Med. 2018;28(7):483–488.
36. Wu J, Mark B, Knafl GJ, Dunbar SB, Chang PP, DeWalt DA. A multi-component, family-focused and literacy-sensitive intervention to improve medication adherence in patients with heart failure – a randomized controlled trial. Heart & Lung. 2019;48(6):507–514.
37. Zeng W, Chia SY, Chan YH, Tan SC, Low EJH, Fong MK. Factors impacting heart failure patients’ knowledge of heart disease and self-care management. Proc Singapore Healthcare. 2017;26(1):26–34. doi:10.1177/2010105816664537
38. Foroumandi E, Kheirouri S, Alizadeh M. The potency of education programs for management of blood pressure through increasing self-efficacy of hypertensive patients: a systematic review and meta-analysis. Pat Educ Counseling. 2019;103(3):451–461. doi:10.1016/j.pec.2019.09.018
39. Lynggaard V, Nielsen CV, Zwisler A, Taylor RS, May O. The patient education – learning and coping strategies – improves adherence in cardiac rehabilitation (LC-REHAB): a randomised controlled trial. Int J Cardiol. 2016;236:65–70. doi:10.1016/j.ijcard.2017.02.051
40. Unverzagt S, Meyer G, Mittmann S, Samos F, Unverzagt M, Prondzinsky R. Improving treatment adherence in heart failure: a systematic review and meta-analysis of pharmacological and lifestyle interventions. Deutsches Ärzteblatt Int. 2016;113(25):423–430.
41. Toukhsati SR, Hare DL. Towards optimal heart failure care: couples-oriented strategies to improve patient adherence and health outcomes. Current Cardiol Rev. 2016;12(3):243–248.
42. Wongvibulsin S, Martin SS, Steinhubl SR, Muse ED. Connected health technology for cardiovascular disease prevention and management. Curr Treat Options Cardio Med. 2019;21(6):1–15. doi:10.1007/s11936-019-0729-0
43. Asch DA, Troxel AB, Stewart WF, et al. Effect of financial incentives to physicians, patients, or both on lipid levels: a randomized clinical trial. JAMA. 2015;314(18):1926–1935. doi:10.1001/jama.2015.14850
44. Shea JA, Adejare A, Volpp KG, et al. Patients’ views of a behavioral intervention including financial incentives. Am J Manage Care. 2017;23(6):366–371.
45. Williams DM, Lee HH, Connell L, et al. Small sustainable monetary incentives versus charitable donations to promote exercise: rationale, design, and baseline data from a randomized pilot study. Contemporary Clin Trials. 2018;66:80–85. doi:10.1016/j.cct.2018.01.005
46. Harkins KA, Kullgren JT, Bellamy SL, Karlawish J, Glanz K. A trial of financial and social incentives to increase older adults’ walking. Am J Prev Med. 2017;52(5):e123–e130. doi:10.1016/j.amepre.2016.11.011
47. Jay M, Orstad SL, Wali S, et al. Goal-directed versus outcome-based financial incentives for weight loss among low-income patients with obesity: rationale and design of the financial incentives foR weight reduction (FIReWoRk) randomised controlled trial. BMJ Open. 2019;9(4):e025278. doi:10.1136/bmjopen-2018-025278
48. Tate R Can video games be designed to promote health. 2014. Available from: https://hopelab.org/blog/can-video-games-be-designed-to-promote-health-re-mission/.
49. Kato PM, Cole SW, Bradlyn AS, Pollock BH. A video game improves behavioral outcomes in adolescents and young adults with cancer: a randomized trial. Pediatrics (Evanston). 2008;122(2):e305–e317. doi:10.1542/peds.2007-3134
50. Cole SW, Yoo DJ, Knutson B. Interactivity and reward-related neural activation during a serious videogame. PLoS One. 2012;7(3):e33909. doi:10.1371/journal.pone.0033909
51. Khalil GE, Beale IL, Chen M, Prokhorov AV. A video game promoting cancer risk perception and information seeking behavior among young-adult college students: a randomized controlled trial. JMIR Serious Games. 2016;4(2):e13. doi:10.2196/games.5793
52. Martin KE Diabesties: how diabetic support on campus can alleviate diabetic burnout. Available from: http://scholarworks.umt.edu/etd/4479.
53. Happ MB, Naylor MD, Roe-Prior P. Factors contributing to rehospitalization of elderly patients with heart failure. J Cardiovasc Nurs. 1997;11(4):75–84. doi:10.1097/00005082-199707000-00008
54. Sayers SL, Riegel B, Pawlowski S, Coyne JC, Samaha FF. Social support and self-care of patients with heart failure. Ann Behav Med. 2008;35(1):70–79. doi:10.1007/s12160-007-9003-x
55. Lauffenburger J, Lauffenburger J, Khan N, et al. Quantifying social reinforcement among family members on adherence to medications for chronic conditions: a US-based retrospective cohort study. J Gen Intern Med. 2019;34(6):855–861.
56. Yang WE, Shah LM, Spaulding EM, et al. The role of a clinician amid the rise of mobile health technology. J Am Med Inf Assoc JAMIA. 2019;26(11):1385–1388. doi:10.1093/jamia/ocz131
57. Currie K, Strachan PH, Spaling M, Harkness K, Barber D, Clark AM. The importance of interactions between patients and healthcare professionals for heart failure self-care: a systematic review of qualitative research into patient perspectives. Eur J Cardiovasc Nurs. 2015;14(6):525–535.
58. Bhattacharyya O, Mossman K, Gustafsson L, Schneider EC. Using human-centered design to build a digital health advisor for patients with complex needs: persona and prototype development. J Med Internet Res. 2019;21(5):e10318. doi:10.2196/10318
59. LeRouge C, Ma J, Sneha S, Tolle K. User profiles and personas in the design and development of consumer health technologies. Int J Med Informatics. 2013;82(11):e251–e268. doi:10.1016/j.ijmedinf.2011.03.006
60. Serio CD, Hessing J, Reed B, Hess C, Reis J. The effect of online chronic disease personas on activation: within-subjects and between-groups analyses. JMIR Res Protoc. 2015;4(1):e20. doi:10.2196/resprot.3392
61. Holden RJ, Kulanthaivel A, Purkayastha S, Goggins KM, Kripalani S. Know thy eHealth user: development of biopsychosocial personas from a study of older adults with heart failure. Int J Med Informatics. 2017;108:158–167. doi:10.1016/j.ijmedinf.2017.10.006
62. Holden RJ, Daley CN, Mickelson RS, et al. Patient decision-making personas: an application of a patient-centered cognitive task analysis (P-CTA). Appl Ergonomics. 2020;87:103107. doi:10.1016/j.apergo.2020.103107
63. Muñoz D, Uzoije P, Reynolds C, et al. Polypill for cardiovascular disease prevention in an underserved population. The New England Journal of Medicine. 2019;381(12):1114–1123. doi:10.1056/nejmoa1815359
64. Vitality GlowCap review. Engadget [Engadget - BLOG] Web site. 2011. Available from: https://search.proquest.com/docview/839009831.
65. How it works. AdhereTech. https://www.adheretech.com/how-it-works.
66. Smart pill bottle and app. Pillsy Web site. Available from: https://www.pillsy.com/smart-pill-bottle-and-app.
67. Solutions. Compliance Meds Technologies Web site. Available from: https://cmtcares.com/solutions/.
68. Dockendorf MF, Murthy G, Bateman KP, et al. Leveraging digital health technologies and outpatient sampling in clinical drug development: a Phase I exploratory study. Clin Pharmacol Ther. 2019;105(1):168–176. doi:10.1002/cpt.1142
69. Avella’s use of AdhereTech improves adherence and generates one to two additional fills per patient per year, across multiple specialty medications and diseases. Avella Web site. 2018. Available from: https://www.avella.com/news/avellas-use-of-adheretech-improves-adherence-and-generates-one-to-two-additional-fills-per-patient-per-year-across-multiple-specialty-medications-and-diseases.
70. Diplomat expands AdhereTech relationship as program shows increased adherence and persistence. Apr 10, 2019. Available from: https://diplomat.is/news-and-media/press-releases/diplomat-expands-adheretech-relationship-program-shows-increased-adherence-persistence/.
71. US bioservices & AdhereTech collaborate to improve patient adherence to therapy. Business Wire. Jun 4, 2019. Available from: https://search.proquest.com/docview/2234433289.
72. Mauro J, Mathews KB, Sredzinski ES. Effect of a smart pill bottle and pharmacist intervention on medication adherence in patients with multiple myeloma new to lenalidomide therapy. JMCP. 2019;25(11):1244–1254. doi:10.18553/jmcp.2019.25.11.1244
73. Ho PM, Lambert-Kerzner A, Carey EP, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Internal Med. 2014;174(2):186–193. doi:10.1001/jamainternmed.2013.12944
74. Guo Y, Chen Y, Lane DA, Liu L, Wang Y, Lip GYH. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF app trial. Am J Med. 2017;130(12):1388–1396.e6. doi:10.1016/j.amjmed.2017.07.003
75. Angellotti E, Wong JB, Pierce A, Hescott B, Pittas AG. Combining wireless technology and behavioral economics to engage patients (WiBEEP) with cardiometabolic disease: a pilot study. Pilot Feasibility Stud. 2019;5(1):7. doi:10.1186/s40814-019-0395-8
76. Ensom E, Albuquerque D, Erskine N, et al. Feasibility Of, and Adherence To, a Novel, Home-Based Cardiac Tele-Rehabilitation Program for Heart Attack Survivors: The MI-PACE Study. American Heart Association Scientific Sessions; 2019.
77. Chandler J, Sox L, Kellam K, Feder L, Nemeth L, Treiber F. Impact of a culturally tailored mHealth medication regimen self-management program upon blood pressure among hypertensive hispanic adults. Int J Environ Res Public Health. 2019;16(7):1226. doi:10.3390/ijerph16071226
78. Levy AE, Huang C, Huang A, Michael HP. Recent approaches to improve medication adherence in patients with coronary heart disease: progress towards a learning healthcare system. Curr Atheroscler Rep. 2018;20(1):1–9. doi:10.1007/s11883-018-0707-0
79. Ivers NM, Schwalm J, Bouck Z, et al. Interventions supporting long term adherence and decreasing cardiovascular events after myocardial infarction (ISLAND): pragmatic randomised controlled trial. BMJ. 2020;369:m1731. doi:10.1136/bmj.m1731
80. Gadkari AS, McHorney CA. Unintentional non-adherence to chronic prescription medications: how unintentional is it really? BMC Health Serv Res. 2012;12(1):98. doi:10.1186/1472-6963-12-98
81. Hugtenburg J, Vervloet M, van Dijk L, Timmers L. Elders. Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions. Patient Pref Adher. 2013;7:675–682. doi:10.2147/ppa.s29549
82. Náfrádi L, Galimberti E, Nakamoto K, Schulz PJ. Intentional and unintentional medication non-adherence in hypertension: the role of health literacy, empowerment and medication beliefs. J Public Health Res. 2016;5(3):762.
83. Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity–Concerns framework. J Psychosomatic Res. 2008;64(1):41–46. doi:10.1016/j.jpsychores.2007.05.004
84. Roseleur J, Harvey G, Stocks N, Karnon J. Behavioral economic insights to improve medication adherence in adults with chronic conditions: a scoping review. Patient. 2019;12(6):571–592. doi:10.1007/s40271-019-00377-8
85. Ruppar TM, Delgado JM, Temple J. Medication adherence interventions for heart failure patients: a meta-analysis. Eur J Cardiovasc Nurs. 2015;14(5):395–404. doi:10.1177/1474515115571213
86. Vrijens B, Urquhart J. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther. 2014;95(6):617–626. doi:10.1038/clpt.2014.59
87. Redfern J, Coorey G, Mulley J, et al. A digital health intervention for cardiovascular disease management in primary care (CONNECT) randomized controlled trial. Npj Digit. Med. 2020;3:117. doi:10.1038/s41746-020-00325-z
88. Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure – web Addenda. Eur Heart J. 2016. doi:10.1093/eurheartj/ehw128
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
