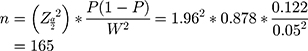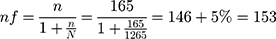Back to Journals » Infection and Drug Resistance » Volume 13
The Association Between Incorrect Use of Antibiotic Prophylaxis and in-Hospital Surgical Site Infections – A Prospective Observational Study
Authors Tefera GM , Feyisa BB , Taye GM, Tesfaye Umeta G , Negash Bereded F , Dinsa Ayeno H , Alemayehu Gadisa D , Melaku Kebede T
Received 5 May 2020
Accepted for publication 28 August 2020
Published 7 September 2020 Volume 2020:13 Pages 3063—3072
DOI https://doi.org/10.2147/IDR.S260238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sahil Khanna
Gosaye Mekonen Tefera,1 Beshadu Bedada Feyisa,2 Getu Melesie Taye,3 Gurmu Tesfaye Umeta,1 Fekadu Negash Bereded,4 Hunduma Dinsa Ayeno,1 Diriba Alemayehu Gadisa,3 Tsegaye Melaku Kebede5
1Department of Pharmacy, Clinical Pharmacy Course Unit, Ambo University, Ambo, Ethiopia; 2Department of Public Health, Human Nutrition Course Unit, Ambo University, Ambo, Ethiopia; 3Department of Pharmacy, Pharmacology Course Unit, Ambo University, Ambo, Ethiopia; 4School of Medicine, Department of Surgery, Wachemo University, Hossana, Ethiopia; 5School of Pharmacy, Department of Clinical Pharmacy, Jimma University, Jimma, Ethiopia
Correspondence: Gosaye Mekonen Tefera Email [email protected]
Background: Surgical site infection (SSI) is the most prevalent in developing countries where 61– 90% of cases develop in-hospital. The study aimed to assess the correctness of antibiotic prophylaxis (AP) use, the incidence of in-hospital SSI, and its determinants.
Patients and Methods: A 3-month hospital-based prospective observational study design was used on general surgery patients. The criteria for identification of SSI were performed based on the Center for Disease Control and Prevention’s (CDC’s) definition of SSI. The correctness of AP was performed based on the American Society of Health System Pharmacist 2013 guideline (ASHP). Multiple stepwise backward logistic regression analysis was used at p-value < 0.05 to predict SSI.
Results: Of 269 adult patients, the type of admission was almost equal between emergency and elective surgery. The mean (± SD) age of the study participants was 41.95± 17.764. Only 19.7% of the study participants used AP correctly. The incidence rate of in-hospital SSI was 16.7% (45/269), which corresponds to 45/4736 or 9.5/1000 person-days. Independent predictors for SSI were American Society of Anesthesiology (ASA) class III–IV (p-value < 0.0001), patients with age-adjusted Charlson co-morbidity index (CCI) of ≥ 1 score (p value=0.008), and incorrect use of AP (p-value =0.025).
Conclusion: Incorrect antibiotic prophylaxis use contributed to an increased risk of SSI, which needs urgent attention in the present study area.
Keywords: antibiotic prophylaxis, surgical site infection, predictors, JUMC
Introduction
Surgical site infections (SSIs) are a major contributing factor to morbidity and mortality among patients undergoing surgery.1 While advances have been made in infection control practices, including improved operating room ventilation, sterilization methods, barriers, surgical technique, and availability of antibiotic prophylaxis, SSIs remain a substantial cause of morbidity, prolonged hospitalization, extra cost, and death (death rate of 3% and 75% of deaths are directly attributable to the SSI).2,3 Furthermore, SSIs contribute to reoperation, readmission, and reduce patient quality of life.4,5
Antibiotic prophylaxis (AP) is well established for the prevention of SS,6 with a duration of fewer than 24 hours, while up to 48 hours is permissible for procedures in which infection would be catastrophic such as cardiac surgery, nephrectomy, and neurosurgery.6,7 World health organization (WHO) panel of 2016 recommends administration of AP before the surgical incision when indicated and within 120 minutes before incision for an antibiotic that needs an infusion.6 Moreover, some attention should be paid to excessive blood loss (>1500 mL) during surgery as well as prolonged surgery for considering re-dosing of AP.7,8
According to the Center for Disease Control and Prevention (CDC), SSIs develop within 30 days of operation.9 The prevalence of SSI varies based on the site and discipline of operation (general surgery versus gynecology and obstetrics) (0–36.6%).10 Even though it is possible to prevent and/or reduce the occurrence of SSI, the preventative measures were often poorly implemented. For instance, incorrect use of AP is common in the sub-Saharan hospital, Tanzania11 which deserves improvement in compliance with prevention measures.12 SSIs are frequent, and the incidence varies from country to country, even within a country as well as depending on the type of operation and wound class (5–40%).13 Studies showed that SSI is the second most common nosocomial infection worldwide, with the highest prevalence in low- and middle-income countries than that of high-income countries.14–17 It results in antibiotic resistance,11 prolonged hospitalization, risk of death, and a major source of worry to the patients, doctors, hospitals, and the community as a whole.9,18 For instance, the study done in an African hospital, Tanzania, found a 22% SSI rate,11 in Ethiopia Hawassa University Referral Hospital 19.1% of SSIs,19 Wachemo University referral Hospital 16.5% of SSIs.14 Furthermore, in Italia SSI was 5.2%, of which 61–90% developed in-hospitals.14,20 The risk for SSI is multi-factorial and it was reported by different studies with the heterogeneous result.10,13,14,19,21-23 Prevention of SSI relies on the identification and optimization of those modifiable factors to reduce the risk of SSI.21
There was a problem of sterility of operation room at the study area24 and non-adherence to American Society of Health System Pharmacist (ASHP) 2013 AP guideline in most of the Ethiopian surgical wards.25 Besides, there were indicators of the poor practice of AP use in the study area; despite having good knowledge regarding AP use26 which might contribute to SSI. Patients are suffering from SSIs because of incorrect AP use.2,27
In Ethiopia, there is a less functional antimicrobial stewardship program, national controlling system or policy on AP use, and SSI surveillance; neither do the hospitals including specialized hospitals have their own timely updated AP guidelines, which could help for consistent and standard antimicrobial prescribing practice for reducing the occurrence of SSIs. Because of the above reasons, even though the high incidence of SSI is suspected, few studies in Ethiopia assess the magnitude of the problem and the association of incorrect AP use with SSI, specifically for Jimma University Medical Center (JUMC). Therefore, the current study aimed to assess the correctness of AP use, the incidence of in-hospital SSIs, and its determinants in the hospitalized adult surgical patients.
Patients and Methods
Study Area, Period and Design
The study was conducted from April 24 to July 24/2017 on general surgery patients at JUMC. The detail was provided in28 since the study was carried out parallel. A hospital-based prospective observational study was used. The study populations were patients who were admitted to the surgical ward for surgery during the study period with inclusion criteria but, did not have a surgical procedure at the initial time of data collection and ages of ≥18 years were included. Those who were not willing to participate, patients only on a topical antibiotic for superficial wound care and infected or non-infected burn wound, a trauma-related wound which need only debridement or wound care were excluded.
Study Variables, Sample Size, and Sampling Technique
Independent variables: Age, sex, residence, types of surgery, type of wound classification, ASA class, co-morbid conditions, smoking status, the timing of prophylactic antimicrobial administration, duration of hospital stay before surgery, duration of operation, Charlson Co-morbidity index, the correctness of AP use, blood loss during operation. Dependent variable: incidence of in-hospital SSI.
Sample size (n) was calculated by using a single population proportion formula, to know the minimum sample size required for estimation of true proportion as follows:
P is the incidence of SSI 0.122 from reference29 Z is the level of confidence= 1.96 with 95% CI. N is the size of the population that the sample is to represent = 1265 per 3 months. W is margin of error = 5%. Since N is less than 10,000 correction formula,
Surveillance by using a consecutive type of sampling technique was used to collect data from 269 patients available within 3months to increase its robustness.
Data Collection Process and Statistical Analysis
Semi-structured questionnaires (English version) were used with a slight modification of tools used on the previously published research.28 This questionnaire contains five parts; part I (socio-demographic characteristics), part II (patient’s clinical information and Charlson co-morbidity index), part III (patient’s medication information), and Part IV (correctness of AP use) and part V (SSI). For those patients who had several surgical procedures during the study period, only the first procedure was eligible for inclusion. For instance, if the first procedure was done on Monday for acute abdomen and the second operation was done on Friday for goiter ahead of discharge; CDC wound class and ASA class were considered for acute abdomen. The source of data for patient‘s history of antibiotic use before the study was from the medical card if that patient has a follow-up at that hospital or from referral paper if the patient is new or if new with no referral paper, the patient was asked based on his profession and education level, if not possible to differentiate the antibiotic, it was recorded as unknown.
Case Finding
Surveillance of SSI was done through direct observation of patient chart (surgeon’s progress note, anesthesiologist note, operation note, and patient interview) by a member of the surveillance team/data collectors during daily routine examination alongside the treating surgical team. The first Surveillance was started on admission date and then perioperative, postoperative, and continued until the day of discharge. The criteria for identification of SSI were performed clinically based on the CDC’s definition of SSI.9 Due to the limited resources, routine wound cultures were not performed; hence, only a few cultures and gram stain were available as one part of patient care. Therefore, the diagnosis of SSI was primarily done clinical with attending physician and one 4th year surgery resident independently and the discrepancy between two was solved by communication until agreement reached. Correct use of antibiotic prophylaxis was considered if the given antibiotic was correct in terms of selection, dose, timing, duration and route of administration for the given surgical procedure; which conforms to the 2013 ASHP’s and 2016 WHO’s AP guideline recommendations. Thus, at least an error in one of the above criteria was considered as incorrect use of AP.7,12 The detail for this part was already provided in the previous publication.28
The model fitness for the variables was evaluated by the Hosmer-Lemeshow goodness of fit test and the p-value was found to be 0.245 in binary analysis and p> 0.05 for all variables in multivariate analysis using SPSS version 20.
Ethical Consideration and Operational Definition
The ethical clearance letter was sought from Jimma University, Institute of health under protocol number IHRPGQ/103/207. Confidentiality and written informed consent of the patients’ were secured. This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Operational and standard definitions for the following terminologies are similar to our previous study report (antibiotic prophylaxis, therapeutic antimicrobial use, poly-pharmacy, and co-morbid condition).28 In-hospital SSIs: if infection occurred in hospital after the operation that conforms to CDC’s definition of SSI. Nevertheless, the infected burn wound is not an SSI.9 American Society of Anesthesiology (ASA) patient’s physical status classification was provided as per the CDC’s definition.9 The definition for CDC wound class I/clean is an uninfected intact skin with no inflammation during operation. Class II wound or clean-contaminated wound is defined as an operative wound in whom the respiratory, alimentary, genital, or urinary tracts are entered under controlled conditions and without unusual contamination. Class III/Contaminated wound is a wound with no evidence of infection but, open, fresh, accidental wounds. Class IV/Dirty wound is old penetrating wounds that involve confirmed clinical infection or perforated viscera.30
Results
Socio-Demographic Characteristics of the Study Participants
There were 4736 person-days follow up after surgery to determine the outcome variable (SSI). A total of 300 patients’ charts were reviewed; of which 269 patients were included in the study. The mean (± SD) age of the participant was 41.95 ± 17.764 and most of the patients (56.1%) were in the age range of 18 to 34 years. The majority of the study participants were male (66.5%), live in a rural area (55.4%), and nonsmokers (88.1%) (Table 1).
 |
Table 1 Socio-Demographic Characteristics of the Study Participants |
Clinical Characteristics of the Study Participants
As per American Society of an anesthesiologist (ASA), patient’s physical status classification 43.1% of patients were class I and 40.9% of study participants had a co-morbid condition, while only 73.2% had a low risk (0–2) score per age-adjusted Charlson co-morbidity index (CCI) scoring. CDC wound class of IV (35.7%) followed by class II (33.5%) was the most common surgical wound type. The type of admission was almost equal between emergency and elective. Among 269 patients for whom surgery was done, in about 41% of study participants amount of blood loss was not recorded while 57.6% of patients’ blood loss during surgery is recorded as ≤1500 mL. The mean ± SD (minimum to maximum) time of operation in minutes was 90.21 ± 52.22 (10 to 368 minutes) (Table 2).
 |
Table 2 Clinical Characteristics of the Study Participants |
Use and Incorrect Use of AP Among the Study Participants
All parameters evaluated for AP, only 19.7% of the study participants used AP correctly as recommended by the ASHP 2013 guideline. Around 20% of patients had a history of antibiotic exposure within the past 3 months before admission. Of 269 patients, for 14 patients AP re-dosing were required, though in reality it was provided only for 14.29%. Most of the patients were used ceftriaxone (52.8%) followed by ceftriaxone + metronidazole (24.2%) as AP. The majority of study participants used a single antibiotic for AP (60.4%) followed by two antibiotics (32.1%), the route of AP administration was through an intravenous (IV) in most of the study participants (89.22%). The study participants had a history of pre-admission medication for acute and/or chronic conditions in about 19.0%. The prevalence of poly-pharmacy in the study participants accounts for 15.6%. A total number of antibiotic exposures (for both the AP and therapeutic purposes) during their hospital stay were ≤2 antibiotics in most of the study participants (76.6%) (Table 3).
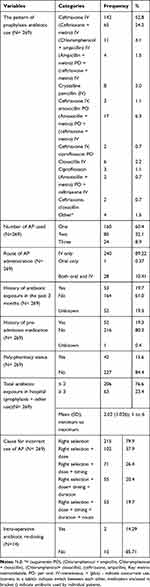 |
Table 3 Medication History and Incorrect Use of Antibiotic Prophylaxis |
Incidence and Predictors of in-Hospital SSIs
The incidence rate of in-hospital SSI was 16.7% (45/269); which corresponds to 45/4736 or 9.5/1000 person-days.
Based on the bivariate analysis results the following factors were considered as a candidate (p-value <0.25) for multivariate analysis: ASA class of III–IV (p value <0.0001), Charlson CI (p-value = 0.157), co-morbid condition (p value= 0.194), poly-pharmacy (p value= 0.158), overall length of hospital stay (p value =0.028), duration of surgery >1 hour (p-value = 0.16), and correctness of AP (selection +dose+ timing + duration + route) (p-value = 0.013) (Table 4).
 |
Table 4 SSI and Associated Factor for Selecting Candidate Variables for Multivariate Analysis |
A stepwise backward multivariate logistic regression analysis showed that patients with ASA class III–IV were about 5 times more likely to have SSI compared to ASA class of < III [AOR= 4.658 (2.159–10.048) at 95% CI; p-value <0.0001]. Patients with CCI of ≥1 score were less likely to have SSI relative to those with zero CCI score [AOR= 0.366 (0.174–0.769) at 95% CI; p value= 0.008, AOR= 0.17 (0.045–0.66) at 95% CI; p-value = 0.011]. Similarly, study participants with incorrect use of AP (incorrect selection +dose+ timing + duration + route) were about 3 times more likely to have SSI compared to those individuals who used AP correctly [AOR= 3.218 (1.160–8.926) at 95% CI; p-value <0.025] (Table 5).
 |
Table 5 Independent Predictors of in-Hospital SSIs |
Discussion
This was a 3-month hospital-based prospective observational study on 269 patients with the primary objective of the correctness of AP use, incidence, and predictor of in-hospital SSI at the surgery ward of JUMC, was found a high rate of incorrect AP use and SSI.
The practice of AP use in JUMC did not conform to the recombination by the ASHP 2013 guideline; which indicates AP use-related poor practice at JUMC. Similar to this study, in a different area of the world, incorrect use of AP was reported with different prevalence. For instance, in Ayder referral hospital Mekelle31 (80.6%), Singapore32 (66.3%), a mean of 1.4 AP errors per surgery,33 in sub-Saharan hospital (88%); which resulted in 22% of SSI and (60%) of antimicrobial-resistant.11 However, the problem was less when compared with the study done in Iran only 0.65%,34 and in the Nekemte referral hospital, only 10.6%25 used AP correctly. Similar to this another study also reported the problems in AP use in Malaysia.32 These problems contributed to worse clinical outcomes, especially a high rate of in-hospital SSI, in the study area, which could prolong the hospital stay and cost to the individual patients and the health care system, as a result of treatment of this infection. Therefore, the hospital and department of surgery of JUMC should take evidence-based action to reduce the incorrect use of AP and through this reduce the rate of SSI, death, and prolonged hospitalization, as well as a hospital-acquired infection (HAI).
Most of the time SSIs occur in-hospital in 61.4% to 90.0% of the cases as revealed by different studies.14,20 The incidence of in-hospital SSI in JUMC was higher than that of Singapore33 8.3%, but, fewer than the rural sub-Saharan hospital, Tanzania11 22%, and in JUMC surgery ward 5 years back,29 the rate of SSI was 12.2% which was lower than the current study. This indicates that there is a raise in SSI in the study area. Similarly, it was reported in Ethiopia, Hawassa 19.1%,19 and Hossain 16.5%.14 On top of this in another part of the world there were indicators that SSI has been increasing; in Tanzania 26.0%,35 in Vietnam, 10.9%,36 in Uganda 16.4%.37 The incidence of SSI varied between countries with high-income 9.4%, middle-income 14.0%, and low-income 23.2%.17
WHO 2016, reported that SSI was the most surveyed and frequent type (2nd) of HAI in low- and middle-income countries and affects up to 1/3 of patients who have undergone a surgical procedure while it was lowered in high-income countries.6 This could be explained by an increase in resistant microorganism38 to antibiotics that have been in use for prophylaxis because of an increase in the time. Because ceftriaxone was the most commonly used antibiotic for prophylaxis; 5 years back and still today in the study area, it was the most common antibiotic used in surgery for almost all types of surgery, alone or with another antibiotic.
The contributing factor for high in-hospital SSI at JUMC might be the incorrect use of AP. Incorrect AP use may result in SSI and antimicrobial resistance.39 Sterility of operation room might also contribute to SSI, as claimed by individual surgeons and one study at JUMC.24 There was a report of non-adherence to ASHP 2013 AP guideline in most of the Ethiopian surgical wards25,29 and at JUMC.40 Moreover, despite having good knowledge regarding AP use, there was an indicator of the poor practice of AP use in the study area.26 Despite the availability of AP, patients are suffering from SSIs and antimicrobial resistance because of incorrect AP use2,27 added by another study. Despite SSIs are preventable, but preventative measures are often poorly implemented because of different reasons. Therefore, the ministry of health of Ethiopia and other stakeholders should substantially improve compliance with preventative methods of SSI.12
ASA class III–IV, CCI of ≥1 score, and incorrect use of AP (incorrect selection +dose+ timing + duration + route of AP) were found to be independent predictors of in-hospital SSI for this study. Similarly, it was reported that the ASA score and Charlson co-morbidity index (CCI) were strongly linked with SSI.41 The risk for SSI is multi-factorial and it was reported by different studies with heterogeneous results. This includes a host of microbial, patient-related (male sex, age, BMI > 25 kg/m2, smoking, poor nutritional state, alcoholism), medical condition-related (wound class and ASA class 3 or more, presence of foreign body or prosthesis, hematoma, immune-suppressive illness, intraoperative blood loss >75 mL, and perioperative transfusion), and procedure-related factors (mechanical stress, acute generalized peritonitis, open surgery, emergency surgery, preoperative hospital stay >7 days, duration of operation >1 hour, and incorrect use of AP). The other factors are related to procedures in the operating room, personal factors, and patient preparation techniques that determine SSI development.10,13,14,19,21-23
Even though over-reporting and under-reporting will balance each other, there might be observer bias in identifying SSI; leaving the report to be valid. The inability to perform a culture of organisms could affect the interpretation of the type of micro-organism and susceptibility testing which can guide both prophylactic and therapeutic antibiotic selection.
Conclusion
There was a high prevalence of incorrect use of AP and this has had a contribution to the high incidence of in-hospital SSI in the study area. Therefore, in the study area, hospital-acquired infections are highly prevalent and need great attention or urgent review of infection control policies of the hospital and active antimicrobial stewardship program is expected from stakeholders. Therefore, the ministry of health of Ethiopia and other stakeholders should substantially improve compliance with preventative methods of SSI.
Acknowledgments
Our heartfelt gratitude goes to Jimma University for funding this study, data collectors, all surgery department staff and the study participants for their endless cooperation.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Astagneau P, Rioux C, Golliot F, Brücker G. Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J Hosp Infect. 2001;48(4):267–274. doi:10.1053/jhin.2001.1003
2. Awad SS. Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect (Larchmt). 2012;13(4):234–237. doi:10.1089/sur.2012.131
3. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–397. doi:10.1016/j.ajic.2008.12.010
4. Sullivan E, Gupta A, Cook CH. Cost and consequences of surgical site infections: a call to arms. Surg Infect. 2017;18(4):451–454. doi:10.1089/sur.2017.072
5. Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1–15. doi:10.1016/j.jhin.2017.03.004
6. Global guidelines for the prevention of surgical site infection [Internet]; 2016. Available from: http://www.who.int.
7. Bratzler DW, Patchen Dellinger E, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi:10.2146/ajhp120568
8. Golembiewski J. Antimicrobial prophylaxis for surgery–2014 update. J PeriAnesth Nurs. 2014;29(2):155–158. doi:10.1016/j.jopan.2014.01.001
9. Surgical site infection (SSI) event [Internet]; 2019. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
10. Ma S. Prevalence and risk factors associated with post operative infections in the limbe regional hospital of Cameroon. Open J Surg. 2014;8(1).
11. Fehr J, Hatz C, Soka I, et al. Antimicrobial prophylaxis to prevent surgical site infections in a rural sub-Saharan hospital. Clin Microbiol Infect. 2006;12(12):1224–1227. doi:10.1111/j.1469-0691.2006.01551.x
12. Allegranzi B, Aiken AM, Kubilay NZ, et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before–after, cohort study. Lancet Infect Dis. 2018;18(5):507–515. doi:10.1016/S1473-3099(18)30107-5
13. Bagnall NM, Vig STP. Surgical site infection. Surgery. 2009;27(10):426–430.
14. Billoro BB, Nunemo MH, Gelan SE. Evaluation of antimicrobial prophylaxis use and rate of surgical site infection in surgical ward of Wachemo University Nigist Eleni Mohammed Memorial Hospital, Southern Ethiopia: prospective cohort study. BMC Infect Dis. 2019;19(1):298. doi:10.1186/s12879-019-3895-5
15. Mukagendaneza MJ, Munyaneza E, Muhawenayo E, et al. Incidence, root causes, and outcomes of surgical site infections in a tertiary care hospital in Rwanda: a prospective observational cohort study. Patient Saf Surg. 2019;13(1):10. doi:10.1186/s13037-019-0190-8
16. Lubega A, Joel B, Justina Lucy N. Incidence and etiology of surgical site infections among emergency postoperative patients in mbarara regional referral hospital, South Western Uganda. Surg Res Pract. 2017;2017.
17. Bhangu A, Ademuyiwa AO, Aguilera ML, et al. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis. 2018;18(5):516–525.
18. Khan HA, Baig FK, Mehboob R. Nosocomial infections: epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7(5):478–482. doi:10.1016/j.apjtb.2017.01.019
19. Laloto TL, Gemeda DH, Abdella SH. Incidence and predictors of surgical site infection in Ethiopia: prospective cohort. BMC Infect Dis. 2017;17(1):119. doi:10.1186/s12879-016-2167-x
20. Petrosillo N, Drapeau CM, Nicastri E, Martini L, Ippolito G, Moro ML. Surgical site infections in Italian hospitals: a prospective multicenter study. BMC Infect Dis. 2008;8(1):34. doi:10.1186/1471-2334-8-34
21. Young PY, Khadaroo RG. Surgical site infections. Surg Clin North Am. 2014;94(6):1245–1264. doi:10.1016/j.suc.2014.08.008
22. Olowo-Okere A, Ibrahim Y, Sani A, Atata R, Olayinka B. Prevalence of surgical site infection in a Nigerian University Teaching Hospital. J Pharm Allied Sci. 2017;14:2430–2438.
23. Surveillance of surgical site infections and prevention indicators in European hospitals HAI-Net SSI protocol, version 2.2; 2017. Available from: https://ecdc.europa.eu/sites/portal/files/documents/HAI-Net-SSI-protocol-v2.2.pdf.
24. Genet C, Kibru G, Tsegaye W. Indoor air bacterial load and antibiotic susceptibility pattern of isolates in operating rooms and surgical wards at Jimma University specialized hospital, Southwest Ethiopia. Ethiop J Health Sci. 2011;21(1):9–18. doi:10.4314/ejhs.v21i1.69039
25. Alemkere G, Hawryluk GWJ. Antibiotic usage in surgical prophylaxis: a prospective observational study in the surgical ward of Nekemte referral hospital. PLoS One. 2018;13(9):e0203523. doi:10.1371/journal.pone.0203523
26. Tefera GM, Kebede TM, Feyisa BB. Knowledge, attitude and practice towards surgical antimicrobial prophylaxis among medical staffs in surgery department of Jimma university medical center: Ethiopia. J Bioanal Biomed. 2019;11(2):149–154.
27. Mohamoud SA, Yesuf TA, Sisay EA. Utilization assessment of surgical antibiotic prophylaxis at Ayder referral hospital, Northern Ethiopia. J Appl Pharm. 2016;8(2):1–5. doi:10.4172/1920-4159.1000220
28. Tefera GM, Feyisa BB, Kebede TM. Antimicrobial use–related problems and their costs in surgery ward of Jimma university medical center: prospective observational study. PLoS One. 2019;14(5):1–15. doi:10.1371/journal.pone.0216770
29. Yesuf BY. Prospective evaluation of antimicrobial prophylaxis and other risk factors for surgical site infection at surgical ward. Clin Pharmacol Biopharm. 2014;3(2):113.
30. Surgical wounds 101 [Internet]; 2018. Available from: https://www.woundsource.com/blog/surgical-wounds-101.
31. Abrha S, Assefa R, Fantahun Molla WM, et al. Antibiotics utilization and their cost in Ayder referral hospital, Mekelle, Ethiopia. Glob J Med Res. 2015;15(1).
32. Lim MK, Lai PSM, Ponnampalavanar SSLS, et al. Antibiotics in surgical wards: use or misuse? A newly industrialized country’s perspective. J Infect Dev Countr. 2015;9(11):1264–1271. doi:10.3855/jidc.6731
33. Young B, Ng TM, Teng C, Ang B, Tai HY, Lye DC. Nonconcordance with surgical site infection prevention guidelines and rates of surgical site infections for general surgical, neurological, and orthopedic procedures. Antimicrob Agents Chemother. 2011;55(10):4659–4663. doi:10.1128/AAC.00562-11
34. Vessal G, Namazi S, Davarpanah M, Foroughinia F. Evaluation of prophylactic antibiotic administration at the surgical ward of a major referral hospital, Islamic Republic of Iran. East Mediterr Health J. 2011;17(08):663–668. doi:10.26719/2011.17.8.663
35. Mawalla B, Mshana SE, Chalya PL, Imirzalioglu C, Mahalu W. Predictors of surgical site infections among patients undergoing major surgery at Bugando medical centre in Northwestern Tanzania. BMC Surg. 2011;11(1):21. doi:10.1186/1471-2482-11-21
36. Nguyen D, MacLeod WB, Phung DC, Cong QT, Nguyen VH, Hamer DH. Incidence and predictors of surgical-site infections in Vietnam. Infect Control Hosp Epidemiol. 2001;22(8):485–492. doi:10.1086/501938
37. Lubega A, Joel B, Justina Lucy N. Incidence and etiology of surgical site infections among emergency postoperative patients in Mbarara regional referral hospital, South Western Uganda. Surg Res Pract. 2017;6365172(10):12.
38. Mulu W, Kibru G, Beyene G, Damtie M. Postoperative nosocomial infections and antimicrobial resistance pattern of bacteria isolates among patients admitted at Felege Hiwot referral hospital, Bahirdar, Ethiopia. Ethiop J Health Sci. 2012;22(1):7–18.
39. Howe LM, Boothe HW. Antimicrobial use in the surgical patient. Vet Clin North Am Small Anim Pract. 2006;36(5):1049–1060. doi:10.1016/j.cvsm.2006.05.001
40. Hunduma J. Timing of prophylactic antibiotic administration in elective surgical patients at Jimma university teaching hospital: South West Ethiopia. J Anesth Clin Res. 2016;7(4):1–7.
41. Khan M, Rooh-ul-Muqim ZM, Khalil J, Salman M. Influence of ASA score and Charlson comorbidity index on the surgical site infection rates. J Coll Physicians Surg Pak. 2010;20(8):506–509. doi:08.2010/JCPSP.506509
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.