Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Frailty in Chronic Obstructive Pulmonary Disease and Risk of Exacerbations and Hospitalizations
Authors Yee N, Locke ER , Pike KC , Chen Z, Lee J, Huang JC, Nguyen HQ, Fan VS
Received 14 January 2020
Accepted for publication 24 May 2020
Published 11 August 2020 Volume 2020:15 Pages 1967—1976
DOI https://doi.org/10.2147/COPD.S245505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Nathan Yee,1 Emily R Locke,2 Kenneth C Pike,3 Zijing Chen,3 Jungeun Lee,4 Joe C Huang,5 Huong Q Nguyen,6 Vincent S Fan2,7
1Department of Medicine, University of Washington, Seattle, WA, USA; 2Department of Health Services Research and Development, Veterans Affairs Puget Sound Health Care System, Seattle, WA, USA; 3Department of Child, Family, and Population Health Nursing, School of Nursing, University of Washington, Seattle, WA, USA; 4College of Nursing, University of Rhode Island, Kingston, RI, USA; 5Division of Gerontology & Geriatric Medicine, University of Washington, Seattle, WA, USA; 6Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, CA, USA; 7Division of Pulmonary and Critical Care Medicine, University of Washington, Seattle, WA, USA
Correspondence: Vincent S Fan
Department of Health Services Research and Development, Veterans Affairs Puget Sound Health Care System, Pulmonary Section, S-111-Pulm, 1660 S. Columbian Way, Seattle, WA 98108, USA
Tel +1 206 764-2504
Fax +1 206 764-2659
Email [email protected]
Background: Frailty is a complex clinical syndrome associated with vulnerability to adverse health outcomes. While frailty is thought to be common in chronic obstructive pulmonary disease (COPD), the relationship between frailty and COPD-related outcomes such as risk of acute exacerbations of COPD (AE-COPD) and hospitalizations is unclear.
Purpose: To examine the association between physical frailty and risk of acute exacerbations, hospitalizations, and mortality in patients with COPD.
Methods: A longitudinal analysis of data from a cohort of 280 participants was performed. Baseline frailty measures included exhaustion, weakness, low activity, slowness, and undernutrition. Outcome measures included AE-COPD, hospitalizations, and mortality over 2 years. Negative binomial regression and Cox proportional hazard modeling were used.
Results: Sixty-two percent of the study population met criteria for pre-frail and 23% were frail. In adjusted analyses, the frailty syndrome was not associated with COPD exacerbations. However, among the individual components of the frailty syndrome, weakness measured by handgrip strength was associated with increased risk of COPD exacerbations (IRR 1.46, 95% CI 1.09– 1.97). The frailty phenotype was not associated with all-cause hospitalizations but was associated with increased risk of non-COPD-related hospitalizations.
Conclusion: This longitudinal cohort study shows that a high proportion of patients with COPD are pre-frail or frail. The frailty phenotype was associated with an increased risk of non-COPD hospitalizations but not with all-cause hospitalizations or COPD exacerbations. Among the individual frailty components, low handgrip strength was associated with increased risk of COPD exacerbations over a 2-year period. Measuring handgrip strength may identify COPD patients who could benefit from programs to reduce COPD exacerbations.
Keywords: chronic obstructive pulmonary disease, frailty, weakness, handgrip strength
Introduction
Chronic obstructive pulmonary disease (COPD) is a significant cause of morbidity and remains the third leading cause of death in the United States.1 Acute exacerbations of COPD (AE-COPD) frequently occur and place a significant utilization and cost burden on the US healthcare system.2,3 Additionally, AE-COPD have been shown to accelerate declines in lung function, health-related quality of life, and increase hospitalizations and mortality.4–7 Identifying patients at high risk may help target interventions to reduce AE-COPD.8–10 Risk factors for AE-COPD include age, sex, forced expiratory volume (FEV1), and frequent prior exacerbations.7–9,11,12
Frailty has been described as a state of decreased physiologic reserve causing vulnerability to adverse events,13,14 and identifies individuals who are at risk for disability, adverse health outcomes, and increased all-cause mortality.14–17 The frailty phenotype proposed by Fried et al is widely accepted and is defined as the presence of three or more of the following measures: exhaustion, weakness, slowness, low activity, and/or weight loss,14,18 with pre-frailty defined as having 1–2 frailty measures.19
COPD patients are twice as likely to be frail than those without COPD, and a review of 27 studies found that an estimated 19% were frail, with 56% meeting criteria for pre-frailty.19,20 The prevalence of frailty varies by setting, ranging from 9% to 28% in the majority of studies, whereas pre-frailty prevalence varies from 48% to 64%.19 Few longitudinal studies have examined the association of frailty with COPD outcomes such as exacerbations,20 and have used heterogenous definitions of frailty.19 Most studies have used questionnaires to assess frailty, with few using objectively measured muscle strength or gait speed to assess frailty in people with COPD. A longitudinal study using the Fried criteria found that mortality among frail COPD patients was four times higher than non-frail patients.20 Longitudinal studies using questionnaires to measure frailty have found an increased risk of re-admissions and mortality in COPD.13,21 Also, among participants in the National Emphysema Treatment Trial, frail COPD participants had an increased risk of all-cause hospitalization and worse quality of life.22
Frailty is defined as a syndrome; however, one of the individual frailty components is muscle weakness measured by handgrip strength (HGS), which has been associated with increased risk of acute COPD exacerbations in a cross-sectional study.23 Limb muscle dysfunction is common in COPD and contributes to poor clinical outcomes including exercise tolerance and mortality.24,25 Importantly, limb muscle strength can be improved with exercise training and is a modifiable risk factor for adverse outcomes.
This study’s primary aim is to examine the relationship between the frailty syndrome with the risk of COPD exacerbations in a longitudinal outpatient cohort of patients with COPD utilizing objectively measured muscle strength and physical activity. We examined the impact of grip strength and other individual components of frailty on risk of adverse COPD outcomes. Additional aims are to evaluate the associations between frailty and all-cause hospitalizations, non-COPD hospitalizations, and mortality.
Methods
Study Design
This study analyzed data collected as part of the COPD Activity: Serotonin Transporter, Cytokine, and Depression (CASCADE) study.26 Approvals were obtained by institutional review boards, in compliance with the Declaration of Helsinki, at the three clinical sites: University of Washington, VA Puget Sound Health Care System, and University of Texas Health Science Center-San Antonio. All participants provided written informed consent.
Inclusion criteria included a diagnosis of COPD, post-bronchodilator FEV1/FVC <0.70% and FEV1% predicted <80%, age ≥40 years, current or past cigarette use (>10 pack-years), and stable disease in the past 4 weeks. Exclusion criteria were: non-COPD lung disease (eg, asthma, diffuse parenchymal lung disease), chronic inflammatory diseases, lung cancer or metastatic cancer, severe chronic kidney disease, uncompensated heart failure, advanced liver disease, HIV/AIDS, chronic antibiotic or oral prednisone use, bipolar disease, psychotic disorders, or dementia.
Measures
Disease Severity
Measures of disease severity included: forced expiratory volume in 1 second, body mass index (BMI), home oxygen use, Charlson co-morbidity index,27,28 COPD hospitalizations in the prior year, and dyspnea with Modified Medical Research Council scale (mMRC).29 Individual comorbidities were also examined including myocardial infarction, angina, heart failure, peripheral vascular disease, stroke, hemiplegia, neurologic disease (multiple sclerosis or Parkinson’s disease), pneumonia, ARDS, drug abuse, peptic ulcer disease (history of endoscopy), visual impairment, hearing impairment, degenerative disc disease, diabetes, diabetes treatment (diet, oral hypoglycemics or insulin), diabetes complications (eye, kidney), post-traumatic stress disorder, chronic kidney disease, rheumatoid arthritis, osteoarthritis, osteoporosis, cirrhosis, leukemia, lymphoma, and non-metastatic cancer.
Frailty
Using baseline data, we adapted the 5 components of the frailty phenotype model defined by Fried using the following established measures:14 1) Exhaustion was defined as a score of ≤55 on the vitality scale of the SF-36 quality of life measure, an approach used in other studies of frailty;30,31 2) Weakness was assessed using a Jamar dynamometer (Fabrication Enterprises, White Plains, NY).32 While standing, participants were instructed to squeeze the grip handle as hard as they were able using their dominant hand with a 90-degree flexion at the elbow. The measurement was repeated 3 times and the best results used. Low HGS was defined using cutoffs by Fried which is commonly used and based on one cohort of older participants.14 Because our cohort included younger participants than in the Fried study, we also performed a sensitivity analysis using a statistically based cutoff of 2 standard deviations below the gender-specific peak mean from 12 large population studies by Dodd et al which included participants <65 years;33 3) Participants wore a StepWatch 3 Activity Monitor (OrthoCare Innovations, Washington, DC)34,35 during waking hours for 7 consecutive days. Low activity was defined as total daily step count <2500,36 4) Slowness was defined as a cadence <30 steps/minute,37 obtained using the StepWatch 3 Activity Monitor reflecting the average steps/minute during the top 30 one-minute periods each day; and, 5) Chronic undernutrition defined using the BMI cutoff of ≤21 as described in the BODE index.29
Other Covariates
Baseline demographic characteristics and smoking status were obtained by questionnaire. Peak performance, a measure of physical activity, was measured using the StepWatch Activity Monitor and defined as the highest number of steps per minute during the top 30 one-minute periods each day.
Outcomes
Moderate-to-severe acute COPD exacerbations during the 2-year follow-up were defined as exacerbations treated with prednisone and/or antibiotics, treated in the emergency department or requiring hospitalization. AE-COPDs were identified through standardized phone surveys every 3 to 4 months. All-cause mortality was reported to research coordinators throughout the study duration.
Statistical Analysis
We compared unadjusted participant characteristics by the frailty phenotype defined by Fried’s study: Non-frail (0 frailty measures), Pre-frail (1–2 frailty measures), and Frail (≥3 frailty measures) using Wald test with robust standard errors of linear regression models for continuous variables, and Wald chi-square test for categorical variables. For significant comparisons, we calculated p-values for each pairwise comparison of baseline characteristic by frailty status with adjustment for multiple testing with Bonferroni correction. Next, we modeled the risk of moderate-to-severe exacerbations, hospitalizations, and mortality over 2 years for each frailty measure. Regression models were adjusted for the following baseline covariates: age, sex, Charlson comorbidity index ≥1, supplemental home oxygen use, COPD hospitalizations in the prior year, FEV1% predicted, and mMRC score. The logarithm of the total number of days of follow-up was included in the model with the coefficient constrained to 1 to measure time at risk. Due to over-dispersion of AE-COPD and hospitalization data, negative binominal modeling was used producing incidence rates ratios. Cox proportional hazards modeling was used for survival analysis. To examine whether individual comorbidities might confound the relationship between frailty and COPD exacerbations, we examined the association between comorbid conditions and both frailty and COPD exacerbations. A sensitivity analysis was performed with variables associated with frailty and COPD exacerbations at p <0.10 level to assess whether the coefficient for frailty changed by >10%. Analyses were conducted using Stata 15.0 (StataCorp LP, College Station, TX).
Results
Participant Characteristics and Frailty Measures
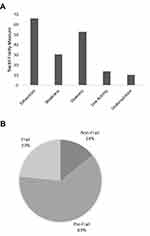
This analysis included 280 participants with complete measures of HGS, physical activity data, and longitudinal follow-up out of 302 enrolled in the CASCADE study. Overall, there was a high prevalence of frailty measures (Figure 1).
Exhaustion (SF 36-vitality scale score < 55) was present in 65.6% of participants, slowness (cadence < 30 steps/min) in 52.8%, weakness (low HGS) in 30.1%. Nearly two-thirds of participants fell into the pre-frail category with 1–2 frailty measures (62.4%). Approximately a quarter had 3 or more frailty measures (23.4%) consistent with the frailty phenotype. When using the criteria by Dodd et al to define weakness (low handgrip strength), there was a slightly larger proportion of patients classified as having weakness (37.2% vs 30.1%). The proportion of patients who were pre-frail and frail were similar using the Dodd HGS criteria (non-frail 13.1%, pre-frail 62.1%, frail 24.8%). The study cohort was mainly white non-Hispanic (88%), male (80%) with mean age of 68 years. Non-white participants were more likely to be pre-frail or frail (p = 0.04). Comparison of the baseline characteristics found that frail participants were more likely to use home supplemental oxygen (p < 0.001; Table 1).
 |
Table 1 Participant Characteristics (N=280) |
Frailty and Acute Exacerbations of COPD
The unadjusted rates of moderate-to-severe exacerbations did not differ significantly by frailty phenotype class (Table 2).
 |
Table 2 Mean Annual Exacerbation, Hospitalization Rates and Mortality by Frailty Category |
Results from adjusted negative binomial regression models examining the association between frailty and acute exacerbations are summarized in Table 3.
 |
Table 3 Associations Between Frailty Measures and Acute COPD Exacerbations and All-Cause Hospitalizations Using Handgrip Strength Based on Fried et al Criteria |
There was no association between the frailty phenotype (≥3 criteria) and increased risk of moderate-to-severe AE-COPD. Among the individual frailty components, exhaustion was associated with higher risk of moderate-to-severe AE-COPD (IRR 1.47, 95% CI 1.07–2.01) in unadjusted models but not after adjustment for co-variates (IRR 1.25, 95% CI 0.93–1.69). Only weakness, measured by low HGS defined using Fried criteria, was associated with increased risk of AE-COPD (IRR 1.46, 95% CI 1.09–1.97) in adjusted models. Sensitivity analysis using low HGS as defined by Dodd also showed an association with increased risk of AE-COPD (IRR 1.40, 95% CI 1.05–1.85). The frailty measures of slowness and undernutrition were not associated with increased risk of AE-COPD in unadjusted or adjusted models. Low physical activity was associated with a decreased risk of AE-COPD in adjusted models.
In sensitivity analyses, we examined whether individual comorbidities were associated with frailty and COPD exacerbations, and found that only neurologic disease (multiple sclerosis and Parkinson’s disease) were associated with both frailty and COPD exacerbations at a p<0.10. Adding neurologic disease to the final model did not change the results (data not shown).
Frailty and Hospitalizations
Those defined as frail (≥3 frailty measures) had an increased risk of all-cause hospitalization in the unadjusted analysis using the frailty measure of weakness as defined by Fried et al (IRR 2.26, 95% CI 1.09–4.71), but failed to have a significant association in adjusted analyses (IRR 1.96, 95% CI 0.92–4.19). In both unadjusted and adjusted analyses, individual frailty measures of exhaustion, weakness, slowness, low activity, and undernutrition were not significantly associated with risk of all-cause hospitalizations (Table 3).
In an analysis restricted to non-COPD-related hospitalizations, participants with the frailty phenotype were at increased risk of non-COPD-related hospitalizations (IRR 2.62, 95% CI 1.00–6.84, p = 0.05). Among the individual frailty measures, slowness was significantly associated with increased risk of non-COPD hospitalizations whereas there was a non-significant increased risk of non-COPD hospitalizations with exhaustion, weakness and low activity.
In a sensitivity analysis using the Dodd et al criteria for weakness based on low handgrip strength, similar results were seen (Table 4).
 |
Table 4 Sensitivity Analysis: Associations Between Frailty Measures and Acute COPD Exacerbations and All-Cause Hospitalizations Using Handgrip Strength Based on Dodd et al Criteria |
Frailty and Mortality
There was a low number of deaths in our study population with most deaths occurring in those who were frail (Table 2). In unadjusted analysis, there was an association between weakness as defined by Dodd and increased risk of mortality (HR 2.36, 95% CI 1.03–5.38). In all other unadjusted and adjusted analysis, there was no significant association between frailty measures and death (Table 5).
 |
Table 5 Associations Between Frailty Measures and Mortality |
Discussion
In this prospective cohort study of COPD patients, the prevalence of pre-frailty and frailty was high. The frailty phenotype was associated with an increased risk of non-COPD hospitalizations, but not with the risk of moderate-to-severe exacerbations in adjusted analyses. Of the five standard measures of frailty, only handgrip strength was independently associated with increased risk of moderate-to-severe AE-COPD.
Our findings add to the existing evidence that pre-frailty and frailty are common among persons with COPD. The prevalence of the frailty phenotype using the criteria of Fried et al in this outpatient cohort of COPD patients was similar to the pooled prevalence described in a systematic review by Marengoni (23% vs 19%, respectively).19 Similarly, the prevalence of pre-frailty in this study was 64%, compared to 56% described by Marengoni et al.19
Like other studies we found that frailty is associated with non-COPD hospitalizations, however, the frailty syndrome as a whole was not associated with the key COPD outcome of exacerbations. When the individual components of the frailty syndrome were examined separately, however, handgrip strength was predictive of COPD exacerbations. To the best of our knowledge, this is the first study that describes an association between low HGS and increased risk of AE-COPD in a prospective analysis, and the results are consistent with a cross-sectional study that found that HGS was associated with risk of COPD exacerbations in the past year.23,38 HGS is also associated with quality of life measured with the EuroQOL EQ-5D in COPD,39 and another measure of limb muscle strength, quadriceps strength, predicted mortality in COPD.25 Limb muscle dysfunction is common in COPD, and is related to poor exercise tolerance.24 The exact mechanism in which low HGS contributes to increased risk of AE-COPD has not been well understood, however. The relationship between HGS and exacerbations was similar whether we used the same cut-offs for low HGS from Fried’s original study, or using the reference range by Dodd et al that defined low HGS as 2 standard deviations below the predicted peak handgrip strength as an adult.14,33
Frailty is a broader syndrome than low handgrip strength and is characterized by a physiologic state of decreased reserve causing vulnerability to adverse events.40,42 Overall the relationship between frailty and COPD outcomes is unclear.19,42 We used Fried’s framework to define frailty based on the presence of ≥3 out of 5 frailty measures.14 Since we did not have identical measures used to define the Fried criteria, we used externally validated criteria. We defined frailty measures of slowness and low activity with objectively measured data obtained through a validated accelerometer, undernutrition by baseline BMI, and exhaustion by the SF 36-vitality scale score.
Interestingly, we found that except for handgrip strength, the other 4 measures of physical frailty were not associated with a significantly increased risk of AE-COPD. Exhaustion showed a trend towards increased risk of exacerbations, but slowness, low activity and undernutrition were not associated with exacerbations. Low physical activity, defined as total daily steps <2500,36 was associated with a decreased exacerbation risk (IRR 0.55, 95% CI 0.35–0.88), but there was no association between low physical activity and risk of non-COPD hospitalizations. This result was unexpected as prior studies have shown that decreasing step count is associated with an increased risk of acute exacerbations and COPD hospitalizations.43–45 The reason why low physical activity might reduce exacerbation risk is unclear, although patients with low step counts may theoretically be limiting potential environmental exposures triggering exacerbations.
We had few participants with undernutrition (10%) and were not able to assess weight loss prior to baseline visit, limiting the utility of this frailty measure. Therefore, in COPD, physical measures of frailty such as slowness, low activity, and undernutrition appear to be less predictive of AE-COPD than weakness or exhaustion. The participants in this study had moderate-to-severe COPD and therefore it is possible that some of the frailty components such as slowness and low activity are already captured in the COPD severity measures of FEV1, dyspnea (mMRC) and home oxygen use. We found that frailty was associated with increased risk of non-COPD-related hospitalizations. This suggests that the frailty phenotype is still an important risk factor for non-COPD-related hospitalizations.
Although the frailty phenotype was associated with increased risk of mortality, this were not statistically significant. We suspect this was largely driven by a lack of statistical power given the low number of deaths (21 deaths) over the two-year course of the study. This is supported by the fact that two factors that commonly associated with mortality in COPD (low BMI29 and weakness46) had increased point estimates for morality but were not statistically significant.
Our finding that HGS is the main frailty measure predictive of AE-COPD supports other studies that have suggested that HGS alone may be a useful marker of frailty and those with decreased physiologic reserve.47,48 Assessing weakness by measuring handgrip strength can be easily done in clinical practice for COPD patients and could provide important prognostic information and identify patients who may benefit from targeted interventions.18,19 Prior studies have shown reversibility in frailty and sarcopenia status with pulmonary rehabilitation.19,40,41,49 More detailed longitudinal studies on frailty, weakness, and COPD-related outcomes are warranted in addition to further study on the potential impact of pulmonary rehabilitation on COPD outcomes in frail patients.
Several limitations are worth noting. The majority of our sample were men and frailty is more common in older women.50 Additionally, due to the primary aim of the main study, participants were excluded if they were chronically using oral corticosteroids or had chronic inflammatory states other than COPD. Although these exclusion criteria reduced the confounding because of steroids or other inflammatory states on the relationship between frailty and COPD outcomes, they may limit the generalizability of our findings and may have selected for a heathier baseline study population. Another potential limitation is the use of frailty measure definitions that did not exactly match the original Fried definitions. However, similar to others,22 we were careful to use externally developed criteria to represent the measures of exhaustion, slowness, low physical activity, and undernutrition/weight loss.
The use of accelerometer data from a 7-day period to define slowness and low physical activity may be biased by the Hawthorne effect, in which participants behave differently initially due to being observed but over time return to their usual physical activity not captured by the accelerometer. In the final model to predict non-COPD hospitalizations, we were not able to adjust for prior non-COPD hospitalizations which may have influenced the results. As previously discussed, the low number of deaths over the study duration likely resulted in an underpowered analysis for evaluation between frailty and mortality. Finally, exacerbation and hospitalization data were obtained through regular surveys, but ultimately reliant on patient self-report.
Conclusion
The results of this longitudinal cohort study show a high proportion of patients with COPD are either frail or pre-frailty of frailty, and that the individual frailty measure of weakness, as defined by low handgrip strength, was significantly associated with increased risk of moderate-to-severe acute exacerbations of COPD. The frailty phenotype was not associated with COPD exacerbations but was associated with an increased risk of non-COPD-related hospitalizations. Evaluating weakness as measured by low handgrip strength in patients with COPD identifies a high-risk population that could potentially benefit from targeted interventions to prevent exacerbations and for interventions that can improve muscle weakness in COPD such as pulmonary rehabilitation. Future studies should examine whether interventions for frailty and weakness may reduce adverse outcomes in COPD.
Abbreviations
AE-COPD, acute exacerbations of chronic obstructive pulmonary disease; BMI, body mass index; CASCADE Study, COPD Activity: Serotonin Transporter, Cytokine, and Depression Study; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume; FVC, forced vital capacity; HGS, handgrip strength; IRR, incident rate ratio; SD, standard deviation.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. N.Y. contributed data to analysis and interpretation, preparation of the manuscript, and served as principal author. E.R.L. contributed to the study design, data acquisition, data analysis and interpretation, and preparation of the manuscript. K.C.P, J.L., and Z.C. contributed to the data analysis and interpretation. J.C.H contributed to the preparation of the manuscript. H.Q.N. contributed to the study design and preparation of the manuscript. V.S.F. contributed to the study design, data analysis and interpretation, and preparation of the manuscript.
Disclosure
Some of these results were previously presented as an abstract. Yee N, Locke ER, Nguyen HQ, Fan VS Frailty in Patients with Chronic Obstructive Pulmonary Disease and Risk of Acute Exacerbations. D104 phenotypes and the multiple dimensions of COPD. p. A7497-A7497.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Vincent Fan reports grants from VA BLR&D, VA HSR&D, VA Cooperative Studies Program, Firland Foundation, VA RR&D, and PCORI, during the conduct of the study. The authors report no other possible conflicts of interest in this work.
References
1. Heron M. Leading Causes for 2015. Hyattsville, MD: National Center for Health Statistics; 2017.
2. Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012;9(2):131–141. doi:10.3109/15412555.2011.650239
3. Dhamane AD, Moretz C, Zhou Y, et al. COPD exacerbation frequency and its association with health care resource utilization and costs. Int J Chron Obstruct Pulmon Dis. 2015;10:2609–2618. doi:10.2147/COPD.S90148
4. Rennard SI, Farmer SG. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(2):88–92. doi:10.1513/pats.2306026
5. Halpin DM, Decramer M, Celli B, Kesten S, Liu D, Tashkin DP. Exacerbation frequency and course of COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:653–661. doi:10.2147/COPD.S34186
6. Connors AF
7. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi:10.1164/ajrccm.157.5.9709032
8. Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation–systematic review. Int J Chron Obstruct Pulmon Dis. 2007;2(3):241–251.
9. Garcia-Aymerich J, Monso E, Marrades RM, et al. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J Respir Crit Care Med. 2001;164(6):1002–1007. doi:10.1164/ajrccm.164.6.2006012
10. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
11. Cao Z, Ong KC, Eng P, Tan WC, Ng TP. Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188–195. doi:10.1111/j.1440-1843.2006.00819.x
12. Lau AC, Yam LY, Poon E. Hospital re-admission in patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2001;95(11):876–884. doi:10.1053/rmed.2001.1180
13. Bernabeu-Mora R, Garcia-Guillamon G, Valera-Novella E, Gimenez-Gimenez LM, Escolar-Reina P, Medina-Mirapeix F. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Ther Adv Respir Dis. 2017;11(10):383–392. doi:10.1177/1753465817726314
14. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.M146
15. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10–12. doi:10.1093/ageing/aft160
16. Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55(8):1216–1223. doi:10.1111/j.1532-5415.2007.01259.x
17. Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the cardiovascular health study. J Am Geriatr Soc. 2008;56(5):898–903. doi:10.1111/j.1532-5415.2008.01656.x
18. Kennedy CC. Handgrip strength in chronic obstructive pulmonary disease: ready for prime time or frailty research tool? Ann Am Thorac Soc. 2017;14(11):1630–1631. doi:10.1513/AnnalsATS.201706-487ED
19. Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. 2018;154(1):21–40. doi:10.1016/j.chest.2018.02.014
20. Lahousse L, Ziere G, Verlinden VJ, et al. Risk of frailty in elderly with COPD: a population-based study. J Gerontol a Biol Sci Med Sci. 2016;71(5):689–695. doi:10.1093/gerona/glv154
21. Galizia G, Cacciatore F, Testa G, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res. 2011;23(2):118–125. doi:10.1007/BF03351076
22. Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2019;16(2):217–224. doi:10.1513/AnnalsATS.201803-175OC
23. Martinez CH, Diaz AA, Meldrum CA, et al. Handgrip strength in chronic obstructive pulmonary disease. associations with acute exacerbations and body composition. Ann Am Thorac Soc. 2017;14(11):1638–1645. doi:10.1513/AnnalsATS.201610-821OC
24. Bui KL, Nyberg A, Rabinovich R, Saey D, Maltais F. The relevance of limb muscle dysfunction in chronic obstructive pulmonary disease: a review for clinicians. Clin Chest Med. 2019;40(2):367–383. doi:10.1016/j.ccm.2019.02.013
25. Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62(2):115–120. doi:10.1136/thx.2006.062026
26. Nguyen HQ, Herting JR, Pike KC, et al. Symptom profiles and inflammatory markers in moderate to severe COPD. BMC Pulm Med. 2016;16(1):173. doi:10.1186/s12890-016-0330-1
27. Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi:10.1097/00005650-199601000-00006
28. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
29. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
30. Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi:10.1111/j.1532-5415.2005.53405.x
31. Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi:10.1681/ASN.2007020221
32. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222–226. doi:10.1016/S0363-5023(84)80146-X
33. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12):e113637. doi:10.1371/journal.pone.0113637
34. Cindy NLW, Jenkins S, Hill K. Accuracy and responsiveness of the stepwatch activity monitor and ActivPAL in patients with COPD when walking with and without a rollator. Disabil Rehabil. 2012;34(15):1317–1322. doi:10.3109/09638288.2011.641666
35. Nguyen HQ, Burr RL, Gill DP, Coleman K. Validation of the StepWatch device for measurement of free-living ambulatory activity in patients with chronic obstructive pulmonary disease. J Nurs Meas. 2011;19(2):76–90. doi:10.1891/1061-3749.19.2.76
36. Tudor-Locke C, Brashear MM, Johnson WD, Katzmarzyk PT. Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese U.S. men and women. Int J Behav Nutr Phys Act. 2010;7(1):60. doi:10.1186/1479-5868-7-60
37. Tudor-Locke C, Rowe DA. Using cadence to study free-living ambulatory behaviour. Sports Med. 2012;42(5):381–398. doi:10.2165/11599170-000000000-00000
38. Ansari K, Keaney N, Taylor I, Burns G, Farrow M. Muscle weakness, health status and frequency of exacerbations in chronic obstructive pulmonary disease. Postgrad Med J. 2012;88(1041):372–376. doi:10.1136/postgradmedj-2011-130293
39. Jeong M, Kang HK, Song P, et al. Hand grip strength in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2385–2390. doi:10.2147/COPD.S140915
40. Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017;14(1):85–99. doi:10.1177/1479972316679664
41. Jaitovich A, Barreiro E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. What we know and can do for our patients. Am J Respir Crit Care Med. 2018;198(2):175–186. doi:10.1164/rccm.201710-2140CI
42. Chainani V, Shaharyar S, Dave K, et al. Objective measures of the frailty syndrome (hand grip strength and gait speed) and cardiovascular mortality: A systematic review. Int J Cardiol. 2016;215:487–493. doi:10.1016/j.ijcard.2016.04.068
43. Moy ML, Teylan M, Weston NA, Gagnon DR, Garshick E. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS One. 2013;8(4):e60400. doi:10.1371/journal.pone.0060400
44. Garcia-Rio F, Rojo B, Casitas R, et al. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest. 2012;142(2):338–346. doi:10.1378/chest.11-2014
45. Gimeno-Santos E, Frei A, Steurer-Stey C, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014;69(8):731–739. doi:10.1136/thoraxjnl-2013-204763
46. Burtin C, Ter Riet G, Puhan MA, et al. Handgrip weakness and mortality risk in COPD: a multicentre analysis. Thorax. 2016;71(1):86–87. doi:10.1136/thoraxjnl-2015-207451
47. Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32(6):650–656. doi:10.1093/ageing/afg111
48. Velghe A, De Buyser S, Noens L, Demuynck R, Petrovic M. Hand grip strength as a screening tool for frailty in older patients with haematological malignancies. Acta Clin Belg. 2016;71(4):227–230. doi:10.1080/17843286.2016.1162381
49. Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–995. doi:10.1136/thoraxjnl-2016-208460
50. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–687. doi:10.1111/j.1532-5415.2010.02764.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.