Back to Journals » Infection and Drug Resistance » Volume 13
Candida auris: From Multidrug Resistance to Pan-Resistant Strains
Received 14 February 2020
Accepted for publication 19 April 2020
Published 5 May 2020 Volume 2020:13 Pages 1287—1294
DOI https://doi.org/10.2147/IDR.S249864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Muluneh Ademe,1 Friehiwot Girma2
1Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Pediatrics and Child Health Nursing, School of Health Sciences, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Muluneh Ademe
Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University, P.O. Box 9086, Addis Ababa, Ethiopia
Email [email protected]
Abstract: Candida auris is an emerging multidrug-resistant fungus that is rapidly spreading worldwide. Currently, C. auris cases have been reported globally from > 30 countries. Most reported infections involve critically ill patients in hospitals, mainly in intensive care unit settings. Infection with C. auris is associated with high mortality rates, and it is often resistant to multiple classes of antifungal drugs. Despite the rapid global spread, it is difficult to predict the actual burden of the infection as the standard laboratory methods fail to correctly identify the fungi. Longer stays in healthcare facilities, use of tracheostomies and percutaneous endoscopic gastrostomy tubes, ventilators in clinical care units and mobile equipment in healthcare settings are shown as major risk factors of C. auris infection. Due to its propensity to cause outbreaks and its antifungal resistance, C. auris poses a risk for patients in healthcare facilities. The emergence of pan-resistant C. auris strains in some areas is an alarming signal for the disease with limited treatment options, high mortality rates, and the ability of the pathogen to spread easily in healthcare settings. In this regard, susceptibility testing on clinical isolates, mainly for patients treated with echinocandins, is needed. Increasing awareness about C. auris infection and advancing the diagnostic methods are also essential for early detection and control of the deadly fungal infection.
Keywords: Candida auris, multidrug resistance, pandrug resistance, risk factors, challenges
Background
C. auris is one of the few species of the genus Candida which cause candidiasis in humans. It is one of the emerging fungus that can cause invasive infections. As it was detected in the external ear canal of the patient, it was named as Candida auris. Auris is the Latin word for ear. Currently, C. auris has emerged globally as a multidrug-resistant nosocomial pathogen and it is considered a major threat to healthcare settings. Worldwide reports of C. auris have considerably increased within a decade.1,2 The aim of this review is, therefore, to describe the current status of C. auris, mainly focusing on the current challenges in clinical and laboratory diagnosis, emergence of pan-drug resistance and possible ways of preventing and controlling the spread of the infection. For this purpose, a systematic search of bibliographical databases was done using the search engines: Google Scholar, Google search, Scopus and PubMed Central. The following key words (phrases) were used in the search engine including but not limited to C. auris, mechanisms of drug resistance, risk factors, diagnosis, treatment, prevention and control. Peer-reviewed research articles, reviews and short communications by international organizations were included. The search was restricted to English language and duplicates were removed.
Trends in C. auris Occurrence
C. auris was first described in Japan in 2009.2 Then, C. auris isolates have started to be identified and reported across five continents as agents of hospital-associated infections.3 To mention but a few reports, twelve isolates of C. auris were identified in India from 2009 to 2011.4 In 2011, three cases of C. auris fungemia were reported from South Korea of which two isolates were obtained during a 2009 study and a third one was discovered in a stored sample from 1996.5 The first outbreak of C. auris in Europe was reported in 2016 in Royal Brompton Hospital, a London cardio-thoracic hospital.6 C. auris began spreading in the United States (U.S) in 2015 and a total of 77 C. auris cases were reported in 2017.7 According to the 2019 CDC report, C. auris has been isolated and reported globally from >30 countries (Figure 1) from which multiple cases of C. auris have been reported from Australia, Bangladesh, Canada, China, Colombia, France, Germany, India, Israel, Japan, Kenya, Kuwait, Malaysia, the Netherlands, Oman, Pakistan, Panama, Russia, Saudi Arabia, Singapore, South Africa, South Korea, Spain, the United Kingdom (UK) and the United States.8 Indeed, the real prevalence and the epidemiology of C. auris still remain uncertain mainly due to the limited accuracy of available conventional diagnostic tools.9,10
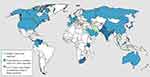 |
Figure 1 Countries from which Candida auris cases have been reported, as of December 31, 2019. Notes: Reproduced from CDC. Candida auris: A Drug-resistant Germ That Spreads in Healthcare Facilities. Available at: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html. Accessed on 15 January 2020.8 Content source: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED). Use of the material, including any links to the materials on the CDC, ATSDR or HHS websites, does not imply endorsement by CDC, ATSDR, HHS or the United States Government; reference to specific commercial products, manufacturers, companies, or trademarks does not constitute its endorsement or recommendation by the US Government, Department of Health and Human Services, or Centers for Disease Control and Prevention. The material is otherwise available on the agency website for no charge. |
The phylogenetics of C. auris suggests distinct genotypes exist in different geographical regions with substantial genomic diversity. Whole-genome sequencing and analyses of isolates from Pakistan, India, South Africa, Venezuela, Japan and previously sequenced C. auris genomes deposited in the National Center for Biotechnology Information’s (NCBI) sequence read archive identified a distinct geographic distribution of genotypes.11,12 In this regard, four distinct clades were identified, namely South American clade, African clade, South Asian clade and East Asian clade. These clades segregated geographically to South Asia (India and Pakistan), South Africa, Venezuela and Japan.12,13 Recently, however, an isolate representative of a potential fifth clade, separated from the other clades by >200,000 single-nucleotide polymorphisms, was identified in a 14-year-old patient in Iran who had never traveled outside the country.14,15 In some areas such as the US, isolated cases cluster to all four C. auris clades, likely introduced through international travel and subsequent spread in healthcare facilities.16
Risk Factors of C. auris Infection
Usually, longer stays in certain types of post-acute care facilities such as intensive care units (ICUs) is considered as a major risk factor of C. auris infection. For instance, the first and only C. auris candidemia case in Kuwait was a patient in the ICU with chronic renal failure.17 In India, more than two-thirds of ICUs (19 out of 27 ICUs) were found to have C. auris isolates.18 In Spain, an average length of stay of 25 days in healthcare institutions was associated with developing C. auris infection.19 This might be attributed to the nature of disease transmission which mostly involves exposure to contaminated facilities in healthcare institutions.7 C. auris colonizes both biotic (skin and other body sites) and abiotic surfaces. Notably, its ability to adhere to surfaces and plastic materials (eg, catheters), biofilm formation, and salt tolerance facilitate the acquisition of the fungus in healthcare settings such as ICUs.20 The majority of C. auris infected patients have had a recent exposure to an indwelling device or have undergone some invasive procedures.21 Infections have been observed several days to weeks after hospitalization in susceptible patients, suggesting exogenous sources of infection.
In agreement with this, patients with tracheostomies and percutaneous endoscopic gastrostomy (PEG) tubes are more likely to acquire C. auris infection.22 Ventilators in clinical care units were reported to spread C. auris infection. In this regard, the prevalence of C. auris in nursing home units with ventilator beds was 7.7% compared to regular nursing homes without ventilator (0.7%).23 Mobile equipment’s in healthcare settings has also been implicated in the transmission of C. auris infection.23 Furthermore, the incidence of C. auris increases among patients with primary or acquired altered immune response, therapeutic management of broad-spectrum antibiotics, transplantation, patients with different comorbidities, and other conditions requiring immunosuppressive agents.24,25
C. auris has been isolated from patients of both sexes and of all age groups. Though the reported C. auris isolates were mostly isolated from males,13 no reason has been provided yet for the sexual differences in terms of frequency of C. auris infections.
Current Challenges of C. auris Infection
Non-Specific Clinical Presentations
C. auris can cause severe invasive infections. However, clinical conditions associated with C. auris are non-specific and it is often difficult to differentiate between other types of systemic infections.10 Clinical conditions associated with most of the reported C. auris cases include bloodstream infections, urinary tract infection, otitis, surgical wound infections, skin abscesses, myocarditis, meningitis, bone infections and wound infections.18,26 According to the CDC report,8 fever and chills that do not improve after antibiotic treatment for a suspected bacterial infection are usually considered among the common symptoms of invasive C. auris infection. However, none of the aforementioned clinical conditions provide definitive diagnosis, and laboratory investigation should be considered for confirmation.
Misidentification by Conventional Diagnostic Tests
Early detection of C. auris infections has been shown to be beneficial as earlier initiation of appropriate antifungal therapy saved many lives.27 However, identification of C. auris remained challenging because most phenotypic methods misidentify C. auris. Until the sequence analysis correctly identified isolates as C. auris, they were initially misidentified as other Candida species.5 So far, culture and microscopy characteristics have been used to differentiate different Candida species. In view of this, C. auris cells are ellipsoid in shape, an ovoid to elongate budding yeast, which seldom forms rudimentary pseudohyphae and typically appears as pink. C. auris also grows as yeast and forms smooth, shiny, whitish-gray, viscous colonies on growth media and it has high tolerance for salinity and heat.10,28 However, these features may not provide definitive diagnosis for C. auris as there are other Candida species, such as C. haemulonii, with the most similar phenotypic characteristics.29
It was confirmed by molecular techniques that conventional diagnostic tests using biochemical typing often misidentify C. auris.30 C. auris isolates have been misidentified as a range of other Candida species with phenotypic and biochemical methods (Table 1), including API 20C, Vitek 2 (bioMérieux), Phoenix (BD), and MicroScan.28,31,32 As recommended by CDC (Table 1), correct identification of C. auris can be done using accepted diagnostic methods such as matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) platforms.33 Moreover, molecular identification techniques like polymerase chain reaction, sequencing, and amplified fragment length polymorphism fingerprinting will detect C. auris and differentiate it from closely related species such as C. haemulonii, C. duobushaemulonii, or C. lusitaniae.34
 |
Table 1 Identification of Candida auris by Different Diagnostic Methods |
It has to be noted that isolations from non-sterile body sites such as lungs, urinary tract, skin and soft tissue, and genital apparatus may represent colonization rather than infections.26,35 Hence, the presence of signs and symptoms of C. auris infections should be considered to differentiate simple colonization from infection.36 Certainly, it is important to identify C. auris even from a non-sterile body site because colonization poses the risk of transmission, which requires implementation of infection control precautions.10
Multidrug Resistance and Emergence of Pan-Resistant Strains
C. auris is characterized by high rate of antifungal resistance with reduced susceptibility to azoles, polyenes, and echinocandins.37 In vitro, more than 90% of C. auris isolates have shown resistance to fluconazole.38 C. auris resistance to voriconazole and amphotericin B was shown to be 3–73% and 13–35%, respectively.21,27 In the US, more than 99% of the C. auris isolates were shown to be resistant to fluconazole, nearly two-thirds were resistant to amphotericin B, and roughly 4% were resistant to echinocandins.39 A systematic review and meta-analysis from Sekyere et al also reported that most of C. auris isolates are resistant to fluconazole (44.29%), followed by amphotericin B (15.46%), voriconazole (12.67%), caspofungin (3.48%) and flucytosine (1.95%).13 Thus, higher resistance to fluconazole in a Candida non-albicans species has become one of the distinguishing characteristics indicative of a potential C. auris infection.40
Indeed, echinocandins are the first-line therapy for C. auris infection. As synergistic interactions have a better efficacy, a combination therapy of an echinocandin and liposomal amphotericin B is prescribed in cases of unresponsiveness to echinocandins.41–43 However, most recently in US, three cases of pan-resistant C. auris with resistance to all three classes of commonly prescribed antifungal drugs have been reported.39 In the same report, it was mentioned that a total of 801 patients with C. auris were identified in New York as of June 28, 2019. Among these patients, three of them developed resistance to all antifungal medications, including echinocandins.39 In fact, echinocandin-resistant C. auris isolates have been previously described44; however, the isolates were susceptible to azoles. Chowdhary et al also reported 14 (4%) pan-azole-resistant C. auris cases9; however, this data was limited to only micafungin and anidulafungin. Although the overall numbers of pan-resistant cases reported so far are few, it is an alarming signal for the disease with limited treatment options, high mortality rates, and the ability of the pathogen to spread easily in healthcare settings.
Data regarding the molecular mechanisms of resistance of C. auris to antifungal agents are scarce, and the precise mechanism of resistance in isolates is not well known. As shown by some studies (Table 2), C. auris escapes from the microbicidal effect of all the classes of anti-fungal agents through different mechanisms, including but not limited to mutations in ERG3 and ERG11 genes, up-regulation of efflux pump genes and single-nucleotide polymorphisms (SNPs) in different genome loci.13 The South Asia clade is shown to exhibit increased antifungal resistance compared to other clades of C. auris.9 Likewise, the reported U.S. pan-resistant cases emerged in New York where the South Asia clade (clade 1) predominates.45
 |
Table 2 Mechanism of Antifungal Resistance by C. auris |
Increased Risk of Mortality
Invasive infections of C. auris are fatal unless early detection and treatment is initiated. The ability of C. auris to develop resistance to commonly used antifungal agents is responsible for its high rates of mortality.37 In fact, the reported mortality rates due to C. auris vary. The 30-day mortality rate of C. auris in Colombia was 35.2%.46 Mortality rates in Asia, Far East, and the United States is estimated to be more than 50% for those with invasive infections.5,47,48 In Spain, a thirty-day mortality rate was reported to be 41.4%.19 In general, the crude in-hospital mortality rate for C. auris candidemia is estimated to range from 30% to 72%.10
Awareness About the Infection
C. auris is a newly emerging fungus and has subsequently been associated with invasive infections and outbreaks in healthcare settings worldwide. Lack of awareness about this drug-resistant Candida could lead to unnoticed transmission and outbreaks in healthcare settings.40 In this regard, the healthcare workers should be aware of this fatal invasive infection and need to adapt the laboratory testing strategies and implement enhanced control measures early enough to prevent healthcare outbreaks.49 So far, there is no concrete evidence on the current state of knowledge of the public and healthcare workers regarding C. auris infection. CDC itself claimed that CDC fungal experts had never heard and received a report describing a Candida infection resistant to all antifungal medications before 2016. It was after hearing the news that CDC sounded the alarm in the US about C. auris.50 Currently, only few countries worldwide (about 30 countries) have reported C. auris,8 suggesting that the infection is either undetected or unreported in the majority of countries. However, there is no much information whether the disease does not actually exist in those countries where C. auris is not reported yet. In this regard, the awareness of health workers about the whole picture of C. auris infection including the diagnostic challenges could not be underestimated as most of the routine diagnostic methods miss the fungus and the infection may continue to be unreported.
Prevention and Control
C. auris strains are characterized by their ability to form a biofilm structure on biotic and abiotic surfaces.20 Patients are often indefinitely colonized by C. auris primarily on skin, nares and other body sites. Indeed, there is no currently known decolonization strategy and colonization may persist for months leading to invasive infection and transmission to others.51 C. auris also persists in the environment for months, and persistent environmental contamination, contaminated medical equipment and other fomites are believed to play a role in nosocomial C. auris transmission.52 In view of this, rigorous attention to environmental cleaning is important for preventing transmission within a healthcare facility. Indeed, common disinfectants such as quaternary ammonia compounds do not work.8 But, surface disinfectants such as chlorine and hydrogen peroxide were shown to have good efficacy against C. auris.53,54
C. auris was made nationally notifiable at the 2018 Council for State and Territorial Epidemiologists (CSTE) Annual Conference.8 Hence, detected cases have to be reported to local or state health departments. Patients with confirmed infections or suspected to have C. auris infections should be kept in separate wards under strict contact precautions.8 Patients or healthcare workers coming in close contact with infected persons should also be placed under strict contact precautions until they are proven to be negative for confirmatory diagnostic tests. Moreover, it is recommended to thoroughly disinfect the wards of patients found to be colonized or infected with C. auris. Cleaning and disinfection of shared medical equipment is encouraged.6
Conclusion
Infections by C. auris are progressively emerging in hospitals and ICU settings. C. auris with high mortality rates, multi-drug resistance, environmental resilience, difficulty in microbiological identification and horizontal transmission has become an issue in clinical practice. Most reported infections of C. auris involved critically ill patients. The emergence of pan resistance is an alarming signal and controlling the spread of the resistance strain is needed. Because of the potential for the development of resistance, patients on antifungal treatment for C. auris should be monitored closely for clinical improvement. Susceptibility testing should also be conducted, especially in patients treated with echinocandins. Moreover, increasing awareness about the infection and advancing the diagnostic methods is essential to control C. auris infection.
Abbreviations
AFLP, amplified fragment length polymorphism; ICU, intensive care unit; MALDI-TOF, matrix-assisted laser desorption/ionization–time of flight mass spectrometry; RUO, research use only.
Acknowledgments
First and foremost, we would like to thank God for giving us the courage and strength. Our sincere gratitude also goes to our families and friends for their support.
Author Contributions
Both authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests.
References
1. Spivak ES, Hanson KE. Candida auris: an emerging fungal pathogen. J Clin Microbiol. 2018;56(2):1–10.
2. Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53:41–44. doi:10.1111/j.1348-0421.2008.00083.x
3. Chowdhary A, Sharma C, Meis JF. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017;13:e1006290. doi:10.1371/journal.ppat.1006290
4. Chowdhary A, Sharma C, Duggal S, et al. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis. 2013;19(10):1670–1673.
5. Lee WG, Shin JH, Uh Y, et al. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol. 2011;49(9):3139–3142. doi:10.1128/JCM.00319-11
6. Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5(35):1–7. doi:10.1186/s13756-016-0132-5
7. Tsay S, Welsh RM, Adams EH, et al. Notes from the field: ongoing transmission of Candida auris in health care facilities- United States, June 2016-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:514–515. doi:10.15585/mmwr.mm6619a7
8. CDC. Candida auris: a drug-resistant germ that spreads in healthcare facilities. Available from: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html.
9. Chowdhary A, Prakash A, Sharma C, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73:891–899. doi:10.1093/jac/dkx480
10. Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 2018;6(69):1–13. doi:10.1186/s40560-018-0342-4
11. Sharma C, Kumar N, Pandey R, Meis JF, Chowdhary A. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect. 2016;13:77–82. doi:10.1016/j.nmni.2016.07.003
12. Lockhart SR, Berkow EL, Chow N, Welsh RM. Candida auris for the clinical microbiology laboratory: not your grandfather’s Candida species. Clin Microbiol Newsl. 2017a;39:99–103. doi:10.1016/j.clinmicnews.2017.06.003
13. Sekyere JO. Candida auris: A systematic review and meta‐analysis of current updates on an emerging multidrug‐resistant pathogen. Microbiologyopen. 2018;7(4):1–29.
14. Chow NA, de Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. Potential Fifth Clade of Candida auris, Iran, 2018. Emerg Infect Dis. 2019;25(9):1780–1781. doi:10.3201/eid2509.190686
15. Abastabar M, Haghani I, Ahangarkani F, Rezai MS, Armaki MT. Roodgari, et al. Candida auris otomycosis in Iran and review of recent literature. Mycoses. 2019;62:101–105.
16. Chow NA, Gade L, Tsay SV, Forsberg K, Greenko JA, Southwick KL. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis. 2018;18(12):1377–1384. doi:10.1016/S1473-3099(18)30597-8
17. Emara M, Ahmad S, Khan Z, et al. Candida auris candidemia in Kuwait, 2014. Emerg Infect Dis. 2015;21:1091–1092. doi:10.3201/eid2106.150270
18. Rudramurthy SM, Chakrabarti A, Paul RA, et al. Candida auris candidaemia in Indian ICUs: analysis of risk factors. J Antimicrob Chemother. 2017;72:1794–1801. doi:10.1093/jac/dkx034
19. Ruiz-Gaitán A, Moret AM, Tasias-Pitarch M, Aleixandre-López AI, Martínez-Morel H, Calabuig E. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses. 2018;61(7):498–505. doi:10.1111/myc.12781
20. Larkin E, Hager C, Chandra J, et al. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother. 2017;61(5):1–13. doi:10.1128/AAC.02396-16
21. Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017b;64(2):134–140. doi:10.1093/cid/ciw691
22. Forsberg K, Woodworth K, Walters M, et al. Brendan Jackson1, Tom Chiller Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol. 2019;57:1–12. doi:10.1093/mmy/myy054
23. Adams E, Quinn M, Tsay S, et al. Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg Infect Dis. 2018;24(10):1816–1824. doi:10.3201/eid2410.180649
24. Ben-Ami R, Berman J, Novikov A. Multidrug-resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg Infect Dis. 2017;23(1):195–203. doi:10.3201/eid2302.161486
25. Azar MM, Turbett SE, Fishman JA, Pierce VM. Donor-derived transmission of Candida auris during lung transplantation. Clin Infect Dis. 2017;65:1040–1042. doi:10.1093/cid/cix460
26. Chowdhary A, Voss A, Meis JF. Multidrug-resistant Candida auris: “new kid on the block” in hospital-associated infections? J Hosp Infect. 2016;94:209–212. doi:10.1016/j.jhin.2016.08.004
27. Chowdhary A, Anil Kumar V, Sharma C, et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis. 2014;33:919–926. doi:10.1007/s10096-013-2027-1
28. Kathuria S, Singh PK, Sharma C, et al. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization-time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol. 2015;53:1823–1830. doi:10.1128/JCM.00367-15
29. Borman AM, Szekely A, Johnson EM. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere. 2016;1(4):1–8. doi:10.1128/mSphere.00189-16
30. Jeffery-Smith A, Taori SK, Schelenz S, et al. Candida auris: a review of the literature. Clin Microbiol Rev. 2018;31:1–18.
31. Wattal C, Oberoi JK, Goel N, Raveendran R, Khanna S. Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) for rapid identification of micro-organisms in the routine clinical microbiology laboratory. Eur J Clin Microbiol Infect Dis. 2017;36:807–812. doi:10.1007/s10096-016-2864-9
32. Kim TH, Kweon OJ, Kim HR, Lee MK. Identification of uncommon Candida species using commercial identification system. J Microbiol Biotechnol. 2016;26:2206–2213. doi:10.4014/jmb.1609.09012
33. CDC. Algorithm to identify Candida auris based on phenotypic laboratory method and initial species identification. Available from: https://www.cdc.gov/fungal/.../candidiasis/pdf/Testing-algorithm-by-Method-temp.pdf.
34. Kordalewska M, Zhao Y, Lockhart SR, Chowdhary A, Berrio I, Perlin DS. Rapid and accurate molecular identification of the emerging multidrug-resistant pathogen Candida auris. J Clin Microbiol. 2017;55:2445–2452. doi:10.1128/JCM.00630-17
35. Kumar D, Banerjee T, Pratap CB, Tilak R. Itraconazole-resistant Candida auris with phospholipase, proteinase and hemolysin activity from a case of vulvovaginitis. J Infect Dev Ctries. 2015;9:435–437. doi:10.3855/jidc.4582
36. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50. doi:10.1093/cid/civ1194
37. Navalkele BD, Revankar S, Chandrasekar P. Candida auris: a worrisome, globally emerging pathogen. Expert Rev Anti Infect Ther. 2017;15:819–827. doi:10.1080/14787210.2017.1364992
38. Alfouzan W, Dhar R, Albarrag A, Al-Abdely H. The emerging pathogen Candida auris: a focus on the Middle-Eastern countries. J Infect Public Heal. 2019;12(4):451–459. doi:10.1016/j.jiph.2019.03.009
39. Ostrowsky B, Greenko J, Adams E, et al. Candida auris isolates resistant to three classes of antifungal medications-New York, 2019. A CDC report, Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6901a2.htm.
40. ECDC (The European Centre for Disease Prevention and Control). Rapid risk assessment: candida auris in healthcare settings – europe. Available from: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-candida-auris-healthcare-settings-europe.
41. Guarner J. Buruli ulcer. Review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56(4):1–8.
42. Singhala T, Kumara A, Boradea P, Shaha S, Soman R. Successful treatment of C. auris shunt infection with intraventricular caspofungin. Med Mycol Case Rep. 2018;22:35–37. doi:10.1016/j.mmcr.2018.08.005
43. Fakhim H, Vaezi A, Dannaoui E, et al. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses. 2018;61:377–382. doi:10.1111/myc.12754
44. Biagi MJ, Wiederhold NP, Gibas C, et al. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis. 2019;6:1–5. doi:10.1093/ofid/ofz262
45. Leach L, Zhu Y, Chaturvedi S. Development and validation of a real-time PCR assay for rapid detection of Candida auris from surveillance samples. J Clin Microbiol. 2018;56(2):1–7.
46. Morales-Lopez SE, Parra-Giraldo CM, Ceballos-Garzon A, et al. Invasive infections with multidrug-resistant yeast Candida auris, Colombia. Emerg Infect Dis. 2017;23:162–164. doi:10.3201/eid2301.161497
47. Vallabhaneni S, Kallen A, Tsay S, et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013-August 2016. Am J Transplant. 2016;17(1):296–299. doi:10.1111/ajt.14121
48. Sarma S, Kumar N, Sharma S, et al. Candidemia caused by amphotericin B and fluconazole resistant Candida auris. Indian J Med Microbiol. 2013;31:90–91. doi:10.4103/0255-0857.108746
49. Public Health Ontario (Ontario Agency for Health Protection and Promotion), Provincial Infectious Diseases Advisory Committee. Interim Guide for Infection Prevention and Control of Candida Auris. Toronto, ON: Queen’s Printer for Ontario; 2019:1–16.
50. CDC. Antibiotic resistance threats report. Available from: www.cdc.gov/DrugResistance/Biggest-Threats.html.
51. Caceres DH, Forsberg K, Welsh RM, et al. Candida auris: a review of recommendations for detection and control in healthcare settings. J Fungi. 2019;5(111):1–11. doi:10.3390/jof5040111
52. Shackleton J, Schelenz S, Rochon M, Hall A, Ryan L, Cervera-Jackson R. The impact of environmental decontamination in a Candida auris outbreak. J Hosp Infect. 2016;94(1):S88–9.
53. Abdolrasouli A, Armstrong-James D, Ryan L, Schelenz S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses. 2017;60:758–763. doi:10.1111/myc.12699
54. Cadnum JL, Shaikh AA, Piedrahita CT, et al. Effectiveness of disinfectants against Candida auris and other Candida species. Infect Control Hosp Epidemiol. 2017;38:1240–1243. doi:10.1017/ice.2017.162
55. Lamoth F, Kontoyiannis DP. The Candida auris alert: facts and perspectives. J Infect Dis. 2018;217:516–520. doi:10.1093/infdis/jix597
56. Escandón P, Chow NA, Caceres DH, et al. Molecular epidemiology of Candida auris in colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin Infect Dis. 2019;68:15–21. doi:10.1093/cid/ciy411
57. Prakash A, Sharma C, Singh A, Kumar Singh P, Kumar A, Hagen F. Evidence of genotypic diversity among Candida auris Isolates by multilocus sequence typing, matrix-assisted laser desorption ionization time-of-flight mass spectrometry and amplified fragment length polymorphism. Clin Microbiol Infect. 2016;22(3):
58. Rhodes J, Abdolrasouli A, Farrer RA, et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg Microbes Infect. 2018;7(43):1–12. doi:10.1038/s41426-017-0002-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
