Back to Journals » Research Reports in Clinical Cardiology » Volume 11
A Review of Neuromodulation in the Treatment of Cardiovascular Disease
Authors Yang A , Chakravarthy KV, Pope JE, Deer TR
Received 26 October 2019
Accepted for publication 3 December 2019
Published 18 February 2020 Volume 2020:11 Pages 7—18
DOI https://doi.org/10.2147/RRCC.S210146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Kones
Video abstract presented by Timothy R Deer.
Views: 838
Ajax Yang,1 Krishnan V Chakravarthy,2 Jason E Pope,3 Timothy R Deer4
1Department of Anesthesiology, New York-Presbyterian Hospital/Weill Cornell Medicine, New York, NY, USA; 2Department of Anesthesiology and Pain Medicine, University of California San Diego Health Sciences, La Jolla, CA; VA San Diego Healthcare System, San Diego, CA, USA; 3Evolve Restorative Center, Santa Rosa, CA, USA; 4Center for Pain Relief, Charleston, WV, USA
Correspondence: Timothy R Deer
Center for Pain Relief, 400 Court Street, Suite 100, Charleston, WV 25301, USA
Tel +1304 347 6120
Email [email protected]
Introduction: The algorithmic use of neurostimulation to treat chronic pain is routine. However, it is underutilized in managing pain and other symptoms relating to cardiovascular dysfunctions. The goal of this article is to focus on the clinical results from using spinal cord stimulation (SCS) in the realm of cardiovascular medicine.
Material and Methods: The current literature was reviewed, summarized and tabulated. This manuscript contains results from systematic reviews, randomized clinical trials and observational study search results on PubMed spanning the last 30 years. The official positioning statement from the International Neuromodulation Society Neuromodulation Appropriateness Consensus Committee (NACC) was also highlighted.
Results: Evidence supports that SCS is asafe, reversible, minimally-invasive and efficacious modality to mitigate chronic symptoms of refractory angina pectoris and critical limb ischemia.
Discussion: Spinal cord stimulation is effective in providing relief, improve quality of life and functional mobility in patients living with ischemic pain of systemic arterial occlusive disease.
Conclusion: Spinal cord stimulation should be considered early in the treatment algorithm among individuals with inoperable ischemic pain.
Keywords: angina pectoris, critical limb ischemia, ischemic pain, congestive heart failure, Reynaud’s syndrome, spinal cord stimulation
Introduction
The International Neuromodulation Society defined neuromodulation as, “the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body.” The concept of the therapeutic use of electricity in cardiology and pain medicine is not new. The first cardiac pacer was invented by John Hopps in the 1940s where a bipolar lead was threaded through the venous conduits to reach the atria to exert rate control.1 Mirowski and colleagues installed the first implantable cardioverter defibrillator in 1980.2 Paralleling the cardio-electrical medicine discovery, the first spinal cord stimulation (SCS) was introduced in 1967 for the treatment of intractable neuropathic pain in an oncological patient.3 Nearly a decade later, Cook and colleagues successfully demonstrated a novel SCS application in cardiovascular medicine.4,5 The authors showed that SCS improved microcirculation and ulcer healing among patients at risk for limb amputation where reconstruction surgery was impossible or failed.4,5 Significant technological advances have been made in the field of neuromodulation to pave the way for novel SCS indications that extend beyond pain management. In this review, we will present the current evidence of neuromodulation in managing cardiovascular diseases from the perspective of interventional pain medicine physicians. Recommendations from the Neuromodulation Appropriateness Consensus Committee (NACC) Guidelines will be discussed. We will selectively focus on neurostimulation use in managing ischemic cardiovascular pathologies and congestive heart failure (CHF). The purpose of this review is not only to summarize the effect of SCS in providing pain palliation, quality of life improvement among patients with cardiovascular pathologies, but also the disease progression modification and functional outcome through neuromodulation.
Cardiovascular Epidemiology/Pathophysiology
Ischemia is caused by a disruption to the blood flow to a region of the body or an organ resulting in metabolic demand and supply asymmetry. Devoid of adequate blood supply and oxygenation, the tissues distal to the vessel occlusion may become hypoxic, damaged and painful.6 At the cellular level, hypoxia causes a reduction in intracellular cAMP availability and adenylate cyclase function resulting in an increase in the endothelial permeability and lysosomal extracellular migration leading to apoptosis and necrosis.7,8 The microcirculatory derangement in the muscular arterioles and capillaries occurs within minutes following ischemia. Microthrombosis forms within 3–4 hrs.9 In order to abolish an ischemic event, proper metabolic homeostasis and circulation must be restored. Paradoxically, even after the reopening of the vessels, the ischemic area may not regain its perfusion; this is also known as the no-reflow phenomenon. The no‐reflow phenomenon perpetuates the ischemic insult by impeding flow from the surrounding tributaries.10 Additionally, reperfusion injury frequently occurs as a consequence of the reintroduction of blood flow. This often results in further tissue affliction and an intense inflammatory cascade.11–13 Reperfusion injury is closely associated with the no-reflow phenomenon.14
The morbidity and mortality of cardiovascular diseases are high. Approximately 6.5 million Americans are living with congestive heart failure. The survival rate is roughly 50% within 5 years of diagnosis. The healthcare-associated cost is estimated to reach 69.7 billion by the year 2030.15 Similarly, there are roughly 8.5 to 10 million Americans live with PAD and angina due to coronary artery disease (CAD) (half of a million new cases annually).16 Of those, only 12-20% are older than 60 years of age.17 Therefore, PAD also poses a significant negative socioeconomic burden from both unemployment and disability among the workforce.18,19 The typical symptoms of PAD in the lower extremities are achy pain, cramping in the buttocks, thighs, legs or toes that are aggravated by ambulation (claudication) and relieved by rest. The affected limb will have the appearance of hair loss, smooth shiny skin, numbness, decreased or absent pulses, muscle atrophy and reduced temperature upon palpation.20 In more severe and chronic cases, pain may persist while at rest with non-healing ulcers that can develop.21,22 The stages of limb PAD are described by the Fontaine classification (Table 1) where critical limb ischemia (CLI) is considered the end stage of PAD as untreated CLI leads to limb loss and potential subsequent postamputation pain development.22,23 Of the 8.5 million Americans living with PAD, approximately 2 million have CLI (500–1000 new cases per million people per year) and the risks are associated with age, male, diabetes, and African American race.24 The number of individuals living with CLI is projected to reach 2.8 million by 2020 or as high as 3.5 million after factoring in population prevalence of diabetes.25 Additionally, CLI has a 50-60% and 70% mortality rate at 5 years and 10 years respectively because CLI is a mere clinical manifestation of lethal systemic PAD i.e. CAD.26–28 It has been reported that 7% of patients with intractable and inoperable CAD have significantly elevated mortality after 3 years.29
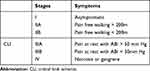 |
Table 1 Fontaine Classification of Limb Peripheral Arterial Disease Severity |
Neuromodulation Mechanisms Pertain to Cardiovascular Disease Treatment
Although SCS has been in the repertoire for treating chronic pain syndromes in modern medicine, the exact mechanism by which SCS provides pain relief is yet to be elucidated. The earliest thoughts on the mechanism of SCS was largely based on the gate control theory, proposed by Melzack and Wall, who suggested that by selectively stimulating the large diameter A-beta fibers one could inhibit pain transmission via the small A-delta and C fibers in the superficial dorsal horn.30 However, clinical observation and animal investigations indicated that there were other significant mechanisms involving neurotransmitters, such as GABA, substance-P, and serotonin release due to SCS.31 Additionally, SCS was shown to trigger an upregulation of calcitonin gene-related peptide (CGRP), a potent endogenous vasodilator. The production of CGRP subsequently leads to downstream nitric oxide release and vascular smooth muscle relaxation, vasodilation and reduced vasospasms.32–34 Through the use of laser doppler flowmetry and neurotransmitter antagonists to reverse the effect of SCS in the rodent model, neuroscientists have been able to gain insight into SCS generated vascular resistance and ischemic pain treatments. SCS was observed to increase cutaneous blood flow by down-modulating the sympathetic nervous tone.35,36 This vasodilatory effect is confirmed by a mechanical shift mediated through sympathectomy or by pharmacologically diminishing or reversing the improved circulation brought on by SCS. It was observed that hexamethonium and chlorisondamine, both ganglioplegic agents, inhibited pre- and postganglionic transmissions.35,36 These findings suggested that SCS caused a vasodilatory effect at the level of postganglionic receptors by inhibiting alpha-1 adrenergic receptor activation. SCS was observed to promote GABA release, protein kinase B (AKT) and kinase (ERK) and phosphorylation in the superficial dorsal horn resulting in extremity vasodilation.37 Similar to the above-mentioned study methodology, GABA-A, ERK and AKT antagonists were introduced to attenuate SCS induced vasodilation.32,38
Neuromodulation Types and Complications
Regardless of the manufacturer software, a dorsal column SCS device consists of a lead(s), a pulse generator, and a wireless remote controller. The number of contacts ranges from 4 to 32. The power source is provided by an internally implanted or externally placed pulse generator (IPG) in most cases. Most commonly used leads are cylindrical and are placed into the epidural space percutaneous with real-time fluoroscopic visualization. On the other hand, paddle leads are flat and their placements are performed by a neurosurgeon as an open laminotomy or partial laminectomy as required. The lead location can be placed according to the anatomic or neurologic target. For the treatment of pain syndrome of cardiovascular origin, both transcutaneous electrical nerve stimulation and SCS leads placed subcutaneously demonstrated benefits.39–41 Irrespective of the type of SCS device used, there is evidence that SCS is cost-effective despite an initial higher cost.42 In terms of SCS related complications, it was reported around 30-40%.43 However, the complication rate is decreasing as surgical techniques and technology continue to mature.44
The Rationale and Evidence for Using Neuromodulation for Cardiovascular Conditions
The mainstay therapy for PAD consists of medical therapy (weight reduction, smoking cessation counseling, cholesterol-lowering agents, cardiac rehabilitation, beta-blockers, nitrates, calcium channel blockers) and revascularization techniques, such as endovascular coronary catheterization and coronary artery bypass surgeries. Regardless of anatomical structure location, the goal is to maximize blood flow and oxygenation.44 Although the first-line treatment options are efficacious, approximately 7% of the patients are non-responders.29,45 Following failed conventional and cardiac interventions, treatment options become limited as some patients may be excluded from undergoing more advanced surgeries due to their comorbidities. In cases where multiple small vessels are diseased, there are no clear endovascular procedural or surgical targets. In these cases, a historical umbrella term “cardiac syndrome X” was coined to describe potential causes of cardiac pain with all-encompassing pathophysiologies, including reduced coronary vasodilation, augmented sympathetic nervous activity, reduced pain threshold, abnormal endothelial function, esophageal dysmotility, and microvascular spasm.46 Neurostimulation was noted as a reasonable option to mitigate the clinical symptoms of treatment-resistant PAD.41 As early as 2002, the Joint Study Group of European Society of Cardiology (the ESBY study), lead by Mannheimer et al reported that neurostimulation techniques are beneficial and should be considered early in the chronic refractory angina treatment algorithm.47 SCS is particularly beneficial when the patient’s CLI severity is greater than Fontaine criterium III (pain at rest). More impressively, there is evidence that demonstrates SCS augments capillary blood flow and skin temperature in the diseased limb thus facilitating skin ulcers less than 3 cm in diameter among patients with an adequate collateral reserve and potentially to prevent amputation.5,48,49
Kinfe et al searched the PubMed database that pertained to SCS in the management of PAD and chronic refractory angina pectoris compared to conventional therapies. Their search was limited to prospective and randomized controlled trials studying the effect of SCS compared to medical therapy on PVD on pain control (Table 2). The authors specifically examined the number of patients that achieved minimal clinically important difference (MCID), defined as a reduction of 3 points on the visual analog pain scale (VAS). Their search yielded seven studies. The authors further stratified their findings into short- (1–2 months), medium- (3–11 months) and long-term (greater than 12 months) term follow up results.50 Totaling 7 studies (short-term: 148 patients, medium-term: 272 patients, and long-term: 239 patients), individuals with refractory angina pectoris who received SCS obtained a mean reduction in VAS of 1.6 (short-term), 3.2 (medium-term), 3.4 (long-term) versus 0.3 (short-term), 1.5 (medium-term) and 0.3 (long-term) among patients that were treated conservatively. Similarly, comparing SCS and conservative treatment cohorts with PAD, the VAS decrease was 1.1, 1.9 and 2.8 versus 1.4, 2.0 and 2.1. The author concluded that the effects of SCS on pain reduction increased over time as the amount of VAS decrease in both PAD and refractory angina pectoris groups either achieved or trended towards MCID as the SCS treatment duration lengthened.50 Deoganonkar and coworkers reviewed 11 studies examining the effect of SCS in treating clinical signs and symptoms of CLI from 1983 to 2013.51 The summary from that analysis is listed in Table 3A and B. Consistent with the other studies, Deoganonkar et al concluded that SCS is both safe and efficacious in alleviating the symptoms of CLI. SCS was also shown to improve limb survival, physical activity tolerance, and quality of life. The author also cautioned that patient selection is paramount and SCS should only be carefully considered as a limb salvage therapy after pharmacologic and revascularization interventions are first considered in reversing the underlying ischemic causes.51
 |
Table 2 Literature Search Results Performed by Kinfe et al50: Randomized Clinical Trials and rospective Studies |
 |
Table 3 (A) Randomized Clinical Trials and Prospective Studies reviewed by Deoganonkar et al51 (B) Literature Search Results Performed by Deogaonkar et al51: Retrospective Studies and Case Series |
NACC Guideline Recommendations
In the same year, Deer and colleagues from The neuromodulation Appropriateness Consensus Committee (NACC) from the International Neuromodulation Society published a consensus statement on the use of spinal cord and peripheral nervous system neuromodulation which is also known as the NACC guidelines among pain medicine practitioners.44 The NACC recommends that PAD should be first treated with conventional medical and revascularization therapies in the majority of the patients who are good surgical candidates. However, in patients that cannot tolerate medication side effects, angioplasty or coronary bypass surgeries, SCS is not only effective in mitigating the signs and symptoms of CLI but it also decreases amputation risks and improves outcomes. However, it was noted that using SCS as a limb salvage means incurring a significantly higher cost.68,69
Ischemic Pain Syndrome Recommendations
The European Peripheral Vascular Disease Outcome Study (SCS-EPOS), a multicentered prospective study, demonstrated in patients with non-reconstructable CLI and transcutaneous oxygenation (TcPO2) reserve of at least 20 mm Hg, but 10–30 mm Hg increase in response to SCS. SCS treatment of non-reconstructable critical leg ischemia was shown to provide a significantly better limb survival rate compared to conservative treatment.59 Ubbink et al conducted a systematic review and meta-analysis comparing SCS with conventional medical management (CMM) to CMM alone with patients with inoperable CLI. Their results demonstrated SCS coupled with CMM improved limb salvage, ischemic pain, and several clinical outcomes.68 SCS is particularly efficacious in patients with Fontain II classification without trophic changes in the foot.62 In patients without arterial hypertension where limb amputation is inevitable, SCS was shown to lower the amputation levels.52
Raynaud’s Syndrome (RS) Recommendations
Although RS is a rare diagnosis, RS can be debilitating as it primarily affects fingers. RS is characterized by cyanotic discoloration, burning, paresthesia, and allodynia (perceiving a non-noxious stimulus as painful). The symptoms typically worsen in response to cold or stress. RS can be a stand-alone condition or a part of larger systemic disorder, such as scleroderma, CREST syndrome or lupus. The underlying disease mechanism is thought to originate from increased vasospastic tone and activity in the distal extremities resulting in ischemic pain. Even though the current available literature on SCS for RS pain is limited to case series,70–76 the largest study consisted of 1048 patients followed longitudinally for a decade and 40 with severe symptoms treated with SCS.77 The results showed a responder rate of 60%(24/40) with 45%(18/40) and 15%(6/40) reporting excellent and good outcomes respectively. The patients that were treated with SCS within 5 years of RS diagnosis did better. The benefit of early SCS intervention was echoed by Devulder et al.78 The authors also noted the best results were obtained from placing the SCS lead at T1. Based on the current pooled evidence, the NACC recommends that SCS should be selectively and judiciously offered to patients with RS within the first 12 weeks of painful ischemic pain.44
Chronic Refractory Angina Recommendations
The evidence supporting the SCS in managing chronic refractory angina is well defined and comprehensive. The ESBY study (Electrical Stimulation versus Coronary Artery Bypass Surgery in Severe Angina Pectoris) randomized 104 patients with severe refractory angina pectoris to coronary artery bypass grafting or SCS. At five years, the survival rate or quality of life were equitable.79 SCS resulted in significantly fewer angina attacks, hospital admission, immediate-release nitrate consumption, improved exercise capacity and quality of life outcome measures. Several authors noted that the improved circulation may be due to the fact that the patients treated with SCS had enhanced exercise tolerance, thus facilitating new collateral vascular circuitry formation.49,80,81 Due to the rigor in refractory angina pectoris neurostimulation research and continually improved evidence level, the NACC was able to provide the highest recommendation level: “A” as defined by the US Preventive Service Task Force as “extremely recommendable (high-level evidence that the measure is effective and benefits outweigh the harms).” Concordantly, SCS as a method for treating refractory angina pectoris has been accepted by the European Society of Cardiology and American Heart Association/American College of Cardiology.82 In summary, SCS could be an option for patients with severe refractory angina pectoris and serious comorbidities who could not receive invasive bypass grafting technique because of unacceptable surgical complication risks and other contraindications. Since the NACC guideline publication, Pan et al performed a systematic review and meta-analysis consisting of only randomized clinical trials with the primary indication towards refractory angina pectoris, focusing on pain parameters, and long-term functional outcomes. The individual studies are summarized in Table 4. The study results showed that SCS was effective and safe in enhancing exercise capacity, quality of life, well-being and diminishing nitroglycerin needs. Additionally, Canadian Cardiovascular grading and pain reductions were also noted.83
 |
Table 4 Randomized Clinical Trials and Prospective Studies Included in the Systematic Review Conducted by Pan et al.83 |
Congestive Heart Failure Recommendations
The effect of SCS on the cardiovascular system is known among neuromodulators. Depending on the location of the spinal stimulation, corresponding vasodilatory effects were observed.44,93 The following lead locations and physiological effects are summarized from the evidence presented in the NACC guidelines: Increasing cerebral blood flow, vasomotor tone, neurochemical mediators and reducing sympathetic activities have been achieved with SCS leads placed at C1/C2. Increased upper extremity circulation is achieved with lower cervical cord (C3-6) stimulation. Similarly, lower extremity and pedal vasodilatation is attained with lower thoracic and upper lumbar spinal (T11-L3) stimulation. T1-T3 stimulation 24 hrs daily was successful in relieving chronic congestive heart failure,94 while T2-4 at 12 hrs/day was not efficacious in the DEFEAT-HF study lead by Zipes et al.95 Despite the outcome discrepancies, methodological differences, and respective study limitations, the authors cautioned drawing premature conclusions on negating the role of SCS in congestive heart failure management.95 The United States Food and Drug Administration (FDA) has just released premarket approval for the first neuromodulatory device for CHF treatment in the United States.96
Conclusion
Spinal cord stimulation is effective in providing relief, improved quality of life, and functional mobility in patients living with ischemic pain of systemic arterial occlusive disease. Spinal cord stimulation should be considered early in the treatment algorithm among individuals with inoperable ischemic pain. As researchers, engineers and physicians continue to challenge the current technological limitations, novel stimulation parameters with new anatomical targets may provide increased efficacy in the use of neuromodulation within the cardiovascular field.
Funding
There were no sponsors involved in this physician initiated work.
Disclosure
Dr. Deer is a consultant for Axonics, Abbott, Nalu, Saluda, Stimgenics, Flowonix, Cornerloc, SPR Therapeutics, Vertos, Vertiflex, Spinethera, Jazz. He is a minor equity holder for Saluda, Nalu, Spinethera, Stimgenics, Vertiflex, Vertos, and Bioness, and received research funding from Mainstay. In addition, Dr. Deer has a patent Abbott pending. Dr. Jason Pope serves as a paid consultant for Abbott, Flowonix, Jazz Pharmaceuticals, Stimgenics, SPR Therapeutics, Medtronic, Nevro, Tersera, Vertos, and Saluda. Dr. Krishnan Chakravarthy is a consultant to Abbott, Bioness, Medincell, SPR Therapeutics, Nalu, Oska Wellness, Medtronic. He is a minor equity holder in Nalu and Oska Wellness. Dr. Yang has no conflict of interest to declare. The authors report no other conflicts of interest in this work.
References
1. Aquilina O. A brief history of cardiac pacing. Images Paediatr Cardiol. 2006;8(2):17–81.
2. Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303(6):322–324. doi:10.1056/nejm198008073030607
3. Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46(4):489–491. doi:10.1213/00000539-196707000-00025
4. Cook AW. Epidural stimulation for vascular disease of extremities. J Neurosurg. 1981;55(4):664. doi:10.3171/jns.1981.55.2.0303
5. Cook AW, Oygar A, Baggenstos P, Pacheco S, Kleriga E. Vascular disease of extremities. Electric stimulation of spinal cord and posterior roots. N Y State J Med. 1976;76(3):366–368.
6. Ogawa S, Gerlach H, Esposito C, Pasagian-Macaulay A, Brett J, Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990;85(4):1090–1098. doi:10.1172/jci114540
7. Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell Death. N Engl J Med. 2009;361(16):1570–1583. doi:10.1056/nejmra0901217
8. Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi:10.1056/nejmra0910283
9. Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10(6):620–630. doi:10.1016/S0967-2109(02)00070-4
10. Gillani S, Cao J, Suzuki T, Hak DJ. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43(6):670–675. doi:10.1016/j.injury.2011.03.008
11. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi:10.1056/NEJMra071667
12. Carroll MC, Michael Holers V. Innate autoimmunity. Adv Immunol. 2005;137–157. doi:10.1016/s0065-2776(04)86004-8
13. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi:10.1038/nri2873
14. Cowled PA, Khanna A, Laws PE, Field JBF, Varelias A, Fitridge RA. Statins inhibit neutrophil infiltration in skeletal muscle reperfusion injury. J Surg Res. 2007;141(2):267–276.
15. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492.
16. Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi:10.1161/CIRCULATIONAHA.108.191259
17. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209.
18. Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114(7):688–699. doi:10.1161/CIRCULATIONAHA.105.593442
19. Peters EJ, Childs MR, Wunderlich RP, Harkless LB, Armstrong DG, Lavery LA. Functional status of persons with diabetes-related lower-extremity amputations. Diabetes Care. 2001;24(10):1799–1804. doi:10.2337/diacare.24.10.1799
20. Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine.
21. Hernando S, Francisco J, Martín Conejero A. Peripheral artery disease: pathophysiology, diagnosis and treatment. Revista Española Cardiología. 2007;60(9):969–982. doi:10.1157/13109651
22. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–S67. doi:10.1016/j.jvs.2006.12.037
23. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, AAVS/SVS when guideline initiated, now merged into SVSSociety for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease). J Am Coll Cardiol. 2006;47(6):1239–1312. doi:10.1016/j.jacc.2005.10.009
24. Verpillat P, Baser O, Wang L. Prevalence, incidence, and outcomes of critical limb ischemia in the US medicare population. Rev ’épidémiol Santé Publique. 2011;59:S73. doi:10.1016/j.respe.2011.08.018
25. Davies MG. Criticial limb ischemia: epidemiology. Methodist Debakey Cardiovasc J. 2012;8(4):10–14. doi:10.14797/mdcj-8-4-10
26. Seeger JM. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Yearbook Surg. 2007;2007:350–351. doi:10.1016/s0090-3671(08)70252-0
27. Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a survival prediction model to facilitate clinical decision making. J Vasc Surg. 2010;51(5):52S–68S. doi:10.1016/j.jvs.2010.01.077
28. Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):
29. Williams B, Menon M, Satran D, et al. Patients with coronary artery disease not amenable to traditional revascularization: prevalence and 3-year mortality. Catheter Cardiovasc Interv. 2010;75(6):886–891. doi:10.1002/ccd.22431
30. Guan Y, Wacnik PW, Yang F, et al. Spinal cord stimulation-induced analgesiaelectrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010;113(6):1392–1405. doi:10.1097/ALN.0b013e3181fcd95c
31. Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31(2):
32. Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Extracellular signal-regulated kinase (ERK) and protein kinase B (AKT) pathways involved in spinal cord stimulation (SCS)-induced vasodilation. Brain Res. 2008;1207:73–83. doi:10.1016/j.brainres.2007.12.072
33. Tanaka S, Komori N, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Mechanisms of sustained cutaneous vasodilation induced by spinal cord stimulation. Auton Neurosci. 2004;114(1–2):55–60. doi:10.1016/j.autneu.2004.07.004
34. Gherardini G, Linderoth B, Jernbeck J, Lundeberg T Calcitonin gene-related peptide and electrical spinal cord stimulation reduce ischemia in a model of mechanically induced vasospasm.
35. Augustinsson LE, Carlsson CA, Holm J, Jivegård L. Epidural electrical stimulation in severe limb ischemia. Pain relief, increased blood flow, and a possible limb-saving effect. Ann Surg. 1985;202(1):104–110. doi:10.1097/00000658-198507000-00017
36. Horsch S, Schulte S, Hess S. Spinal cord stimulation in the treatment of peripheral vascular disease: results of a single-center study of 258 patients. Angiology. 2004;55(2):111–118. doi:10.1177/000331970405500201
37. Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138(1–2):9–23. doi:10.1016/j.autneu.2007.11.001
38. Barron KW, Croom JE, Ray CA, Chandler MJ, Foreman RD. Spinal integration of antidromic mediated cutaneous vasodilation during dorsal spinal cord stimulation in the rat. Neurosci Lett. 1999;260(3):173–176. doi:10.1016/S0304-3940(98)00972-0
39. Buiten MS, DeJongste MJL, Beese U, Kliphuis C, Durenkamp A, Staal MJ. Subcutaneous electrical nerve stimulation: a feasible and new method for the treatment of patients with refractory angina. Neuromodulation. 2011;14(3):
40. Moore R, Chester M. Neuromodulation for chronic refractory angina. Br Med Bull. 2001;59:269–278. doi:10.1093/bmb/59.1.269
41. Jessurun GAJ, Hautvast RWM, Tio RA, DeJongste MJL. Electrical neuromodulation improves myocardial perfusion and ameliorates refractory angina pectoris in patients with syndrome X: fad or future?. Eur J Pain. 2003;7(6):507–512. doi:10.1016/S1090-3801(03)00022-3
42. Manca A, Kumar K, Taylor RS, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial). Eur J Pain. 2008;12(8):1047–1058. doi:10.1016/j.ejpain.2008.01.014
43. Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108(1):137–147. doi:10.1016/j.pain.2003.12.016
44. Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the neuromodulation appropriateness consensus committee. Neuromodulation. 2014;17(6):515–550. doi:10.1111/ner.12208
45. Deer TR, Raso LJ. Spinal cord stimulation for refractory angina pectoris and peripheral vascular disease. Pain Physician. 2006;9(4):347–352.
46. Cannon RO, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation. 1992;85(3):883–892. doi:10.1161/01.CIR.85.3.883
47. Mannheimer C, Camici P, Chester MR, et al. The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J. 2002;23(5):355–370. doi:10.1053/euhj.2001.2706
48. Horsch S, Claeys L. Epidural spinal cord stimulation in the treatment of severe peripheral arterial occlusive disease. Ann Vasc Surg. 1994;8(5):468–474. doi:10.1007/BF02133067
49. Mekhail NA, Cheng J, Narouze S, Kapural L, Mekhail MN, Deer T. Clinical applications of neurostimulation: forty years later. Pain Pract. 2010;10(2):103–112. doi:10.1111/papr.2010.10.issue-2
50. Kinfe TM, Pintea B, Vatter H. Is spinal cord stimulation useful and safe for the treatment of chronic pain of ischemic origin? A review. Clin J Pain. 2016;32(1):7–13. doi:10.1097/AJP.0000000000000229
51. Deogaonkar M, Zibly Z, Slavin KV. Spinal cord stimulation for the treatment of vascular pathology. Neurosurg Clin N Am. 2014;25(1):25–31. doi:10.1016/j.nec.2013.08.013
52. Jivegård LE, Augustinsson LE, Holm J, Risberg B, Ortenwall P. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischaemia: a prospective randomised controlled study. Eur J Vasc Endovasc Surg. 1995;9(4):421–425. doi:10.1016/S1078-5884(05)80010-3
53. Hautvast RW, DeJongste MJ, Staal MJ, et al. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study. Am Heart J. 1998;136(6):1114–1120.
54. Spincemaille GH, Klomp HM, Steyerberg EW, Habbema JD. Pain and quality of life in patients with critical limb ischaemia: results of a randomized controlled multicentre study on the effect of spinal cord stimulation. Eur J Pain. 2000;4(2):173–184 doi:10.1053/eujp.2000.0170
55. Lanza GA, Sestito A, Sgueglia GA, et al. Effect of spinal cord stimulation on spontaneous and stress-induced angina and 'ischemia-like' ST-segment depression in patients with cardiac syndrome X. Eur Heart J. 2005;26(10):983–989 doi:10.1093/eurheartj/ehi089
56. Sgueglia GA, Sestito A, Spinelli A,et al. Long-term follow-up of patients with cardiac syndrome X treated by spinal cord stimulation. Heart. 2007;93(5):591–597. doi:10.1136/hrt.2006.102194
57. Eddicks S, Maier-Hauff K, Schenk M, Muller A, Baumann G, Theres H. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomised study. Heart. 2007;93(5): 585–590. doi:10.1136/hrt.2006.100784
58. Lanza GA, Grimaldi R, Greco S, et al. Spinal cord stimulation for the treatment of refractory angina pectoris: A multicenter randomized single-blind study (the SCS-ITA trial). Pain. 2011;152(1):45–52. doi:10.1016/j.pain.2010.08.044
59. Amann W, Berg P, Gersbach P, Gamain J, Raphael JH, Ubbink DT. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur J Vasc Endovasc Surg. 2003;26(3):280–286. doi:10.1053/ejvs.2002.1876
60. Ubbink DT, Spincemaille Geert HJJ, Prins MH, Reneman RS, Jacobs Michael JHM. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: The Dutch Multicenter Randomized Controlled Trial. J Vasc Surg. 1999;30(2):236–244. doi:10.1016/S0741-5214(99)70133-3
61. Petrakis E, Sciacca V. Prospective study of transcutaneous oxygen tension (TcPO2) measurement in the testing period of Spinal Cord Stimulation in Diabetic patients with critical Lower Limb Ischaemia. Int Angiol. 2000;19(1):18-25.
62. Kumar K, Toth C, Nath RK, Verma AK, Burgess JJ. Improvement of limb circulation in peripheral vascular disease using epidural spinal cord stimulation: a prospective study. J Neurosurg. 1997;86(4):662–669. doi:10.3171/jns.1997.86.4.0662
63. Claeys LG, Horsch S. Transcutaneous oxygen pressure as a predictive parameter for ulcer healing in endstage vascular patients treated with spinal cord stimulation. Int Angiol. 1996;15(4):344–349.
64. Tallis RC, Illis LS, Sedgwick EM, Hardwidge C, Garfield JS. Spinal cord stimulation in peripheral vascular disease. J Neurol Neurosurg Psychiatry. 1983;46(6):478–484. doi:10.1136/jnnp.46.6.478
65. Brümmer U, Condini V, Cappelli P, Di Liberato L, Scesi M, Bonomini M, Costantini A. Spinal cord stimulation in hemodialysis patients with critical lower-limb ischemia. Am J Kidney Dis. 2006;47(5): 842–847. doi:10.1053/j.ajkd.2006.02.172
66. Reig E, Abejon D, del Pozo C, Wojcikiewicz R. Spinal Cord Stimulation in Peripheral Vascular Disease: A Retrospective Analysis of 95 Cases. Pain Pract. 2001;.1:324–331. doi:10.1046/j.1533-2500.2001.01033.x
67. Ubbink DT, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2013;(2):CD004001. doi:10.1002/14651858.CD004001.pub3
68. Ubbink DT, Vermeulen H, Gersbach PA, Berg P, Amann W. Systematic review and meta-analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischaemia. Br J Surg. 2004;91(8):948–955. doi:10.1002/bjs.4629
69. Klomp HM, Steyerberg EW, van Urk H, Habbema JDF, ESES Study Group. Spinal cord stimulation is not cost-effective for non-surgical management of critical limb ischaemia. Eur J Vasc Endovasc Surg. 2006;31(5):500–508. doi:10.1016/j.ejvs.2005.11.013
70. Fiume D. Spinal cord stimulation in peripheral vascular pain. Appl Neurophysiol. 1983;46(5–6):290–294. doi:10.1159/000101276
71. Neuhauser B, Perkmann R, Klingler PJ, Giacomuzzi S, Kofler A, Fraedrich G. Clinical and objective data on spinal cord stimulation for the treatment of severe Raynaud’s phenomenon. Am Surg. 2001;67(11):1096–1097.
72. Provenzano DA, Nicholson L, Jarzabek G, Lutton E, Catalane DB, Mackin E. Spinal cord stimulation utilization to treat the microcirculatory vascular insufficiency and ulcers associated with scleroderma: a case report and review of the literature. Pain Med. 2011;12(9):1331–1335. doi:10.1111/j.1526-4637.2011.01214.x
73. Benyamin R, Kramer J, Vallejo R. A case of spinal cord stimulation in Raynaud’s Phenomenon: can subthreshold sensory stimulation have an effect? Pain Physician. 2007;10(3):473–478.
74. Münster T, Tiebel N, Seyer H, Maihöfner C. Modulation of somatosensory profiles by spinal cord stimulation in primary Raynaud′ s syndrome. Pain Pract. 2012;12(6):469–475. doi:10.1111/ppr.2012.12.issue-6
75. Issa MA, Kim CH. Cervical spinal cord stimulation with 5-column paddle lead in Raynaud’s disease. Pain Physician. 2012;15(4):303–309.
76. Sibell DM, Colantonio AJ, Stacey BR. Successful use of spinal cord stimulation in the treatment of severe Raynaud’s disease of the hands. Anesthesiology. 2005;102(1):225–227. doi:10.1097/00000542-200501000-00032
77. Raso AM. Results of electrostimulation of the spinal cord in Raynaud’s disease and syndrome. J Mal Vasc. 1989;14(1):52–54.
78. Devulder J, van Suijlekom H, van Dongen R, et al. 25. Ischemic pain in the extremities and Raynaud’s phenomenon. Pain Pract. 2011;11(5):483–491. doi:10.1111/j.1533-2500.2011.00460.x
79. Ekre O, Eliasson T, Norrsell H, Währborg P, Mannheimer C, Electrical Stimulation versus Coronary Artery Bypass Surgery in Severe Angina Pectoris. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J. 2002;23(24):1938–1945. doi:10.1053/euhj.2002.3286
80. Mannheimer C, Eliasson T, Andersson B, et al. Effects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. BMJ. 1993;307(6902):477–480. doi:10.1136/bmj.307.6902.477
81. Murray S, Carson KG, Ewings PD, Collins PD, James MA. Spinal cord stimulation significantly decreases the need for acute hospital admission for chest pain in patients with refractory angina pectoris. Heart. 1999;82(1):89–92. doi:10.1136/hrt.82.1.89
82. Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina–summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the Management of Patients with Chronic Stable Angina). Circulation. 2003;107:149–158. doi:10.1161/01.CIR.47041:29
83. Pan X, Bao H, Si Y, et al. Spinal cord stimulation for refractory angina pectoris: a systematic review and meta-analysis. Clin J Pain. 2017;33(6):543–551. doi:10.1097/AJP.0000000000000435
84. Mannheimer C, Augustinsson LE, Carlsson CA, Manhem K, Wilhelmsson C. Epidural spinal electrical stimulation in severe angina pectoris. Br Heart J. 1988;59(1):56–61. doi:10.1136/hrt.59.1.56
85. Greco S, Auriti A, Fiume D, et al. Spinal cord stimulation for the treatment of refractory angina pectoris: a two-year follow- up. Pacing Clin Electrophysiol. 1999;22(1):26–32
86. Jessurun G, Dejongste M, Crijns H, et al. Clinical follow-up after cessation of chronic electrical neuromodulation in patients with severe coronary artery disease: a prospective randomized controlled study on putative involvement of sympathetic activity. Pacing Clin Electrophysiol. 1999;22(10):1432–1439.
87. Vulink NCC, Overgaauw DM, Jessurun GAJ, et al. The effects of spinal cord stimulation on quality of life in patients with therapeutically chronic refractory angina pectoris. Neuromodulation. 1999;2(1):33-40.
88. de Jongste MJ, Hautvast RW, Hillege HL, Lie KI. Efficacy of spinal cord stimulation as adjuvant therapy for intractable angina pectoris: A prospective, randomized clinical study. Working Group on Neurocardiology. J Am Coll Cardiol. 1994 Jun;23(7):1592-1597. doi:10.1016/0735-1097(94)90661-0
89. McNab D, Khan SN, Sharples LD, et al. An open label, single- centre, randomized trial of spinal cord stimulation vs. percutaneous myocardial laser revascularization in patients with refractory angina pectoris: the SPiRiT trial. Eur Heart J. 2006;27(9):1048–1053.
90. Bondesson S, Pettersson T, Erdling A, Hallberg IR, Wackenfors A, Edvinsson L. Comparison of patients undergoing enhanced external counterpulsation and spinal cord stimulation for refractory angina pectoris. Coron Artery Dis. 2008;19(8):627–634. doi:10.1097/MCA.0b013e3283162489
91. Dyer MT, Goldsmith K, Khan S, et al. Clinical and cost- effectiveness analysis of an open label, single-centre, randomised trial of spinal cord stimulation (SCS) versus percutaneous myocardial laser revascularisation (PMR) in patients with refractory angina pectoris: the SPiRiT trial. Trials. 2008;9:40.
92. Zipes DP, Svorkdal N, Berman D, et al. Spinal cord stimulation therapy for patients with refractory angina who are not candidates for revascularization. Neuromodulation. 2012;15(6):550–558
93. Kujacic V, Eliasson T, Mannheimer C, Jablonskiene D, Augustinsson LE, Emanuelsson H. Assessment of the influence of spinal cord stimulation on left ventricular function in patients with severe angina pectoris: an echocardiographic study. Eur Heart J. 1993;14(9):1238–1244. doi:10.1093/eurheartj/14.9.1238
94. Tse H-F, Turner S, Sanders P, et al. Thoracic spinal cord stimulation for heart failure as a restorative treatment (SCS HEART study): first-in-man experience. Heart Rhythm. 2015;12(3):588–595. doi:10.1016/j.hrthm.2014.12.014
95. Zipes DP, Neuzil P, Theres H, et al. Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure. JACC. 2016;4(2):129–136. doi:10.1016/j.jchf.2015.10.006
96. Zile MR, Abraham WT, Lindenfeld J, et al. First granted example of novel FDA trial design under Expedited Access Pathway for premarket approval: beAT-HF. Am Heart J. 2018;204:139–150. doi:10.1016/j.ahj.2018.07.011
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
