Back to Journals » Journal of Pain Research » Volume 12
Determinants Of Patient Experience With Low Back Pain Interdisciplinary Care: A Pre-Post Interventional Study
Authors Gogovor A , Visca R, Ware MA , Valois MF, Bartlett G, Hunt M, Ahmed S
Received 8 March 2019
Accepted for publication 4 September 2019
Published 27 November 2019 Volume 2019:12 Pages 3203—3213
DOI https://doi.org/10.2147/JPR.S207989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Erica Wegrzyn
Amédé Gogovor,1,2 Regina Visca,3,4 Mark A Ware,4,5 Marie-France Valois,6 Gillian Bartlett,4 Matthew Hunt,7 Sara Ahmed2,4,7
1Division of Experimental Medicine, McGill University, Montreal, Quebec, Canada; 2Centre for Outcomes Research and Evaluation, McGill University Health Centre, Montreal, Quebec, Canada; 3RUIS McGill Centre of Expertise in Chronic Pain, Montreal, Quebec, Canada; 4Department of Family Medicine, McGill University, Montreal, Quebec, Canada; 5Alan Edwards Pain Management Unit, McGill University Health Centre, Montreal, Quebec, Canada; 6Department of Medicine, McGill University, Montreal, Quebec, Canada; 7School of Physical and Occupational Therapy, Faculty of Medicine, McGill University, Montreal, Quebec, Canada
Correspondence: Sara Ahmed
School of Physical and Occupational Therapy, Faculty of Medicine, McGill University, 3654 Prom Sir-William-Osler, Montreal, QC H3G 1Y5, Canada
Tel +1 514-398-4400 Ext 00531
Fax +1 514-398-6360
Email [email protected]
Background and purpose: Measuring patients’ experiences of health services has become an essential part of quality of care reporting and a means for identifying opportunities for improvement. This study aimed to evaluate change in patient experience in an interdisciplinary primary care program and to estimate the impact on patient experience of sociodemographic, function, pain and general health status, resource utilization, and process variables.
Patients and methods: A 6-month interdisciplinary care program for individuals with low back pain (LBP) was implemented at four primary care settings and evaluated using an observational pre/post study design. The change in patient experience was evaluated using the Patient Assessment of Chronic Illness Care questionnaire (PACIC) completed at baseline and 6 months post-intervention (n=132). Descriptive and multivariable analyses were performed using SAS version 9.3.
Results: The average patient age was 57 (SD: 14) years of age and the majority were female (53%). The mean overall PACIC score was 2.6 (SD: 1.1) at baseline and 3.6 (SD: 0.9) at 6 months. The experience of care improved for 62% of the participants based on the minimal clinically important difference (MCID). No significant determinants of overall PACIC change score were identified in the multivariable regression models.
Conclusion: The lack of association of hypothesized determinants requires further examination of the properties of the PACIC and with a larger sample. Future investigation is needed on the relationship between improved patient experience and outcomes.
Keywords: patient care team, low back pain, patient experience, PACIC, primary health care
Introduction
Low back pain (LBP) is one of the most common non-specific chronic pain conditions, and up to 85% of the cases cannot be attributed to any specific underlying pathology.1 LBP is associated with high economic burden for individuals and societies, increased incidence of disability with low levels of physical activity, impaired quality of life and the highest consultation rate in general practice.2–4 Clinical guidelines for LBP now recommend non-pharmacological approaches as first-line treatment options.5–8 These approaches include advice and education supported by self-management, cognitive behavioural approach, as well as some forms of complementary and alternative medicine. Procedures, imaging and surgery are not recommended for patients with non-specific LBP. Pharmacological treatments are recommended for selected patients and for limited use, and include nonsteroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, antidepressants, anticonvulsants, opioids; most for limited use and in selected patients.6–10
Given the complexity and suboptimal management, one of the main recommendations to improve the management of LBP is the use of multidisciplinary and interdisciplinary teams in the delivery of care. The expectation behind this recommendation is that teams will enhance integration of care and improve provider, patient and managerial satisfaction, thereby improving administrative and clinical processes and patient outcomes.11–13 Considerable attention has been focused on the effectiveness of multidisciplinary and interdisciplinary teams, and studies have linked team performance to positive patient outcomes.14,15 For chronic pain management, improved outcomes across a range of domains including pain severity and interference, and functioning have been reported.16–19
Interdisciplinary care can be distinguished from multidisciplinary treatment in that not only do health professionals with different backgrounds work in concert with the patient, but they also organize meetings to discuss cases, make collective therapeutic decisions, use one record system and, most importantly, often employ a uniform approach to patient management.20,21 There is a growing body of evidence suggesting health care teams can have beneficial impacts on clinical and health resource endpoints, and on patient and provider experience.17
Patient experience of health and health care is defined as any combination of satisfaction, expectations, and experience.22 Measuring patients’ experiences of health services has become an essential part in reporting on the quality of care and defining health policy and an important component of performance assessment and service improvement.23–29 This measurement can have different purposes: (i) describing health care from the patient’s point of view; (ii) measuring the process of care, thereby both identifying problem areas and evaluating improvement efforts; and (iii) evaluating the outcome of care.30–33
Studies that have examined the relationship between patient experience and health outcomes are very scarce and have yielded mixed results:22,34–37 some studies found positive association38–40 while others found null or negative association.41–44 In a recent systematic review of 55 studies, Doyle et al concluded that patient experience is positively associated with clinical effectiveness and patient safety but did not assess the strengths of positive associations in different studies.45 Building on Doyle et al's review and focusing on Consumer Assessments of Healthcare Providers and Systems (CAHPS) surveys to measure patient experience, Anhang Price et al showed associations between positive patient experiences and clinical processes, patient safety, and unnecessary utilization of health services.34 Yet, these studies were based on the acute care model, which differs from a long-term care approach such as the chronic care model (CCM).46 CCM defines six elements that are important for improving outcomes for individuals with chronic conditions: organizational support, delivery system redesign, decision support, self-management support, clinical information systems, and linkages to community services. Tan et al showed a significant association between patients’ ratings of services and outcome measures for chronic pain in a multidisciplinary outpatient clinic at a tertiary teaching hospital but they used a non-validated satisfaction measure.47 Thus, previous studies examining the determinants of patient experience were conducted in the acute care model, were cross-sectional in design, and focused on conditions other than LBP.
This study aimed to investigate the association between change in patient experience of care of individuals with LBP participating in an interdisciplinary care program, and patient and process variables. Specific objectives were to evaluate change in patient experience after a 6-month period of participating in a primary interdisciplinary care program, and to estimate the relationship of sociodemographic, functioning, pain and general health status, resource utilization, and process variables with change in patient experience. Our hypothesis was that a positive change in patient experience would be associated with a better functioning outcome.
Materials And Methods
Study Design
This paper is based on a larger pre/post multiple time series study design.48 Individuals attended the program for 6 months and completed questionnaires at baseline, 6 weeks, 3 months and 6 months post program initiation. The type of data and instruments used varied across these time points.
Participants And The Interdisciplinary Program
Based on the framework of CCM, a primary care interdisciplinary program was developed by the Centre of Expertise in Chronic Pain (Quebec, Canada). Program development integrated needs assessment and evidence-based guidelines. The composition of the interdisciplinary team was determined by the assessment of the needs for individuals suffering from LBP and included a nurse, a physician, a physiotherapist, and a psychologist. We identified relevant assessment tools through literature review and validated them by clinician experts and health system decision-makers. The model included referral criteria; a treatment algorithm; standardized clinical process and assessment tools for the interdisciplinary team; provision of self-management support for patients; and defined administrative and clinical indicators supported by an electronic data collection and management system for the clinicians and for evaluation. Primary care physicians referred individuals with subacute (4 to 12 weeks) and chronic (>12 weeks) LBP to the program which was implemented at four Health and Social Service Centres (CSSS). Participants received an interdisciplinary evaluation at the start of the program and individualized evidence-based treatments including pharmacological, physiotherapy and psychological therapies, and structured self-management support. We collected data on socio-demographic status, impact of pain, physical and mental health, function, and quality of life using self-report and standardized questionnaires. The inclusion criteria for this study were i) individuals aged 18 years or older; ii) suffering from sub-acute and chronic LBP ≤ 1 year; and iii) that answered at least 10 questions of the Patient Assessment of Chronic Illness Care (PACIC) at baseline and 6 months.
Outcome And Covariates Measures
PACIC
The main outcome variable of this study is patient experience with care, measured by the Patient Assessment of Chronic Illness Care (PACIC). The PACIC has been identified as the most appropriate instrument to measure patient experience with aspects of care associated with the CCM.22,49–51
Participants completed the 20-item PACIC at baseline and 6 months, and scored from 1 (none of the time) to 5 (almost always). It measures specific actions or qualities of care experienced by patients. Its test–retest reliability (0.58), internal consistency (0.93) and convergent validity (0.42–0.60) have been demonstrated in varied chronic condition patient populations including hypertension, depression, diabetes, asthma, and chronic pain.49,50,52 Only one study reported a responsiveness of 1.11 (standardized response mean).53 The PACIC is scored by averaging scores across all 20 items.52 The single score structure is recommended by recent research in order to obtain an overall picture of patients’ experiences.50,54–56
Patient Covariates
The selection of the predictor variables was based on the literature review and findings from a qualitative study on patient experience conducted by our team which identified themes related to the effect of interdisciplinary care including “togetherness of the clinician team members/varied professionals” and “meaning of recovery”.57 The construct of functional ability, the most important recovery “item” mentioned by the participants in the qualitative study was the main predictor variable and was measured using the Oswestry Disability Index (ODI), the most commonly used outcome measure for LBP. The ODI is divided into 10 sections of 6 statements, each section scored on a 0–5 scale (higher values represent greater disability), with a test–retest reliability of 0.83–99 and an internal consistency (Cronbach α) of 0.71–0.87.58,59
The association between pain outcomes and socio-demographic, depression, anxiety, and health-related quality of life has been shown in previous studies.20,60 Thus, other predictor variables we included in the study were: baseline socio-demographic variables of age, sex, marital status, level of education, employment status, social assistance, private insurance, and ethnicity; Start Back, a risk (low, medium, high) stratification tool to assess risk of delayed recovery;61 anxiety measured by the Hospital Anxiety and Depression scale (HADS) with score ranging from 0 (no distress) to 21 (highest distress);62 depression using the Patient Health Questionnaire (PHQ-9) with score ranging from 0 to 27 (the higher the more severe);63 general health status (physical and mental) measured by the SF-12 and ranging from 0 to 100 with higher scores meaning a better health-related quality of life;64 and pain severity measured by the Brief Pain Inventory (BPI) and ranging from 0 to 10 with higher scores meaning severe pain.65,66
Staff And Process Covariates
We assessed team functioning using the Team Climate Inventory (TCI) that was completed by the clinician and non-clinician staff members from the four sites. The 19-item TCI67 is grouped under 4 subscales: participative safety and support for innovation score on a 5-point Likert scale, and vision and task orientation that score on a 7-item Likert scale. Sub-scale scores are derived by averaging items within the sub-scale; to obtain the overall score for each team, individual scores are then summed up and divided by the number of team members. Higher scores indicate more desirable team climate.68,69
Other variables include the total number of visits with health professionals over the 6-month program (physician, nurse, physiotherapist, psychologist); number of months since implementation of the program; adherence to the program: given that the minimum number of visits (with the nurse/physiotherapist) required is 6, adherence is coded Yes, if the number of visits ≥6, and No otherwise; and interdisciplinary evaluation: Yes, if the date of assessment for the initial visit is the same for ≥3 health professionals.
Data Collection
We collected the study data manually and electronically and used an electronic data capture tool (Research Electronic Data Capture or REDCap)70 hosted at the Research Institute of the McGill University Health Centre (MUHC), for data management. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. For this multisite study, the use of the Data Access Group feature allowed the restriction of records to each site.
Sample Size
Because no minimal clinically important difference (MCID) for PACIC was found in the literature, we used an estimate of 0.5*SD, equivalent to a moderate effect to estimate sample size.71,72 Findings from studies on patients with chronic illness showed SD ranging from 0.8 to 1.1.49,73,74 Based on an MCID of 0.5 and SD = 1, using an alpha of 5% and a power of 80% the minimum required sample size is 63 subjects, increased with an additional 10 subjects for every additional variable that is included in the multivariable analysis.
Analyses
For all variables, we calculated mean values and standard deviations for the continuous variables and frequencies and percentages for categorical variables. Pearson (continuous) and Spearman (categorical) correlation matrices were calculated to investigate collinearity. We calculated the mean overall PACIC score for all the patients who completed at least 10 items at baseline and 6 months; the PACIC score representing the score of all completed questions. To determine the proportion of patients whose experience has improved, we categorized PACIC variable as improved, stable and worsened based on MCID (= 0.5*SD) and calculated the proportion for each category; improved if the difference (6 months - Baseline) is >0.5; stable if the difference is comprised between -0.5 and 0.5; and worsened if the difference is <-0.5. Responder analysis is an approach that permits to assess clinical relevance of the effect of interest, particularly while using PROM or PREM instruments.75 We conducted multivariable regression to evaluate the relationship between patient and staff and process covariates and the change score of PACIC as the outcome. We first estimated univariate models, and all significant covariates (95% confidence interval does not include the null value) in the unadjusted models were included in the multivariate models. We conducted the hierarchical multiple regression by adding more predictors to each successive model. Patient socio-demographic covariates were entered in the model first, followed by patient health status, and staff and process variables. The analyses were performed using SAS ver. 9.3. (SAS Institute, Cary, NC, USA) and R ver. 3.3.
Ethical Considerations
Ethical approval was obtained from the Research Ethics Board of the McGill University Health Centre (#MP-CUSM-12-220 GEN) and the study was conducted in accordance with the Declaration of Helsinki. We obtained written informed consent from the participants at their referral to the program. Electronic data are kept on institutional secured and password protected servers (at McGill University and McGill University Health Centre). All paper-based clinical data collection forms are kept at each participating pain clinic at the Health and Social Services Centres.
Results
Sociodemographic And Health Characteristic Of The Study Population
The sample, based on patients who were recruited from December 2012 until November 2016, completed the 6-month visit (by June 2017), and answered at least 10 PACIC questions at baseline and 6 months, was 132. The average patient age was 57 (SD: 14) years of age and the majority were female (53%). Men were slightly older on average (58 (SD: 14)) compared to women (55 (SD: 15)). Thirty percent of the participants were categorized as high risk while 34% and 29% were categorized as medium and low, respectively, according to the Start Back Tool. The anxiety score ranged from 1 to 18 (8.4 (SD: 3.7)) out of 21, and the depression from 0 to 25 (7.4 (SD: 6.0)) out of 27 at baseline. Characteristics of the participants (this study (n=132) versus the larger study (n=314)) are shown in Table 1. Only sex and pain severity variables were statistically significant.
 |
Table 1 Baseline Characteristics Of The Study Population |
Distribution Of PACIC Completion And Mean Change Scores
Of the 132 patients who completed at least 10 items at baseline and 6 months, 84% and 78% answered all 20 questions at baseline and 6 months, respectively (Table 2). The average overall PACIC was 2.6 (SD: 1.1) at baseline and 3.6 (SD: 0.9) at 6 months; men and women had similar scores at baseline (2.6 vs 2.7) and 6 months (3.4 vs 3.7). The distribution of PACIC score by category of risk (measured by the StarT Back tool) was similar even though the mean PACIC score appeared to increase with the level of risk at baseline (2.4, 2.7, 2.7) and at 6 months (3.4, 3.6, 3.6) for low, medium, and high risk, respectively (Table 3). At the end of the 6-month program, the experience of care for 62% of the patients improved (Table 4).
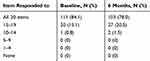 |
Table 2 Distribution Of PACIC Completion At Baseline And 6 Months |
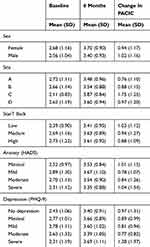 |
Table 3 Distribution Of PACIC Mean Change Scores |
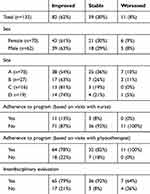 |
Table 4 Distribution Of PACIC Change Scores Based On MCID |
Potential Determinants Of PACIC Change Score
Sociodemographic variables such as age and employment (retired) and environmental variables (site and number of months of implementation) had a significant regression coefficient in the univariate models; statistically nonsignificant variables included sex, level of education, social assistance, private insurance, ethnicity, StarT Back risk category, anxiety, depression, functional status, health status, pain severity, team climate inventory, and interdisciplinary evaluation (Supplementary Table A1). None of the variables included in the multivariable regression models remained significant; age was of borderline statistical significance with an average change in PACIC score of −0.021 (−0.046, 0.004) for 1-year increase (Table 5, and Supplementary Table A2).
 |
Table 5 Multivariable Association Of Potential Determinants With Change PACIC Score |
Discussion
In this study, we used PACIC as a measure of patient experience with a primary interdisciplinary care program for the management of LBP, implemented at four Health and Social Services Centres in the province of Quebec. We found that the experience of care improved for the majority of the participants based on the MCID. However, no significant determinants of overall PACIC change score were identified in the multivariable regression models. Some variables selected as predictors of patient experience of care in this study were based on constructs identified as important by patients who participated in a qualitative study previously conducted by our team57 such as functional ability or interdisciplinary evaluation. None of these features were found to be significantly associated with change in patient experience. Given the fact that there is always some implementation variations,76 the absence of significant association of the overall PACIC change score with sites may indicate a negligible impact of implementation variation on the PACIC across program sites.
Our mean overall PACIC score at 6 months was higher than the numbers reported in other studies regardless of the type of chronic condition, setting, or design (cross-sectional, longitudinal).31,56,73,77–83 The higher PACIC scores obtained in our study suggest better patient experience. This may be due to the unique characteristics of the IDT program implemented and/or the study population (LBP). Additional data obtained from the implementation of similar IDT programs within LBP population will be needed to support these hypotheses.
Similar to some previous findings,77,80,84,85 no significant association with potential determinants of PACIC was found. However, other authors reported some significant associations. For example, having a degree/diploma, being retired, or having a greater duration of disease had negative effects on the total PACIC in a type 2 diabetes population from a cross-sectional design.83 In our study, only being retired was negatively associated with overall PACIC in univariable regression models. Other variables such as Interdisciplinarity and team functioning were not significant, even in univariable models while Houle et al reported a significant association with IDT care; however, in their study IDT care was assessed as “the number of visits with non-physician professionals at the clinic during the previous 2 years, as abstracted from the medical chart”.78 This is an indicator but not a comprehensive or direct measure of effective implementation of IDT care.
To our best knowledge, this is the first longitudinal study using PACIC as a measure of patient experience of interdisciplinary care for individuals living with LBP. Thus, the association of PACIC with some variables such as StarT Back risk category and team climate inventory has not been explored therefore comparisons are not possible. The rate of completion of all 20 PACIC questions in our sample was relatively high (84% and 78% at baseline and 6 months, respectively) compared with an average of 75% in studies in multiple chronic condition populations.73,83 This finding may be indicative of a better adaptability of PACIC questions for the LBP population.
The absence of significant association between PACIC and potential determinants is common in the literature and puts into perspective the notion that it is the best instrument to assess patient experience, particularly in the context of primary interdisciplinary care. The PACIC was developed for individuals with chronic illness to measure specific actions or qualities of care congruent with the CCM,52 and most recent analyses of PACIC supported the use of the overall summary score.50,54–56,86,87 However, the fact that we did not find strong associations with hypothesized predictors raises potential questions regarding the five-dimension structure of the PACIC. It may be that in the context of team-based chronic illness care, a modified and improved version of PACIC would be needed to capture aspects of interdisciplinarity patient-centred care including the role of other professionals (or the role of professionals other than doctors and nurses), and to reflect on team–patient relationship, the quality of communication and listening, the use of technology in decision-making and care coordination. Further evaluation of the factor structure of the PACIC, for example, using rash analyses, will help evaluate the domain structure of the PACIC and whether there are sufficient items to measure each domain.55,88 Qualitative data can enhance understanding of complex interventions when coupled with quantitative data in a mixed-methods approach. This study is part of a doctoral dissertation that extensively discussed this approach.89
It is worth noting that the results, based on data from patients who completed the PACIC questionnaire at baseline and 6 months (two time points) who form a subsample of the interdisciplinary program, should be interpreted with caution given the small sample size and the absence of control that may positively affect patient experience results. Existing studies on the natural history of LBP focused on pain and function outcomes so do not offer comparison for patient experience outcomes.90–92
In conclusion, the IDT program appears to have improved the experience for the majority of individuals living with LBP. The lack of association of hypothesized determinants requires further examination of the properties of the PACIC and with a larger sample. Future investigation is needed on the relationship between improved patient experience and outcomes, and to determine whether patient experience plays a mediation role in the relationship between team-based/patient-centred care and improved outcomes.
Acknowledgments
AG is supported by a FRQS doctoral awards. The LBP program was supported by the Pfizer-FRQS-MSSS Fund.
Author Contributions
AG, RV, MAW, and SA contributed to the conception of the larger study. AG designed this study, managed the data collection and drafted the manuscript. GB and MH revised the protocol. MFV performed the analyses. AG and SA performed initial data interpretation. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Mark Ware reports grants from Pfizer during the conduct of the study, and personal fees from Canopy Growth outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760–765. doi:10.1001/jama.1992.03490060092030
2. Croft P, Blyth FM, van der Windt D. Chronic Pain Epidemiology: From Aetiology to Public Health. Oxford: Oxford University Press; 2010.
3. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–2367. doi:10.1016/S0140-6736(18)30480-X
4. Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(6):968–974. doi:10.1136/annrheumdis-2013-204428
5. Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391(10137):2368–2383. doi:10.1016/S0140-6736(18)30489-6
6. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College Of Physicians. Ann Intern Med. 2017;166(7):514–530. doi:10.7326/M16-2367
7. Almeida M, Saragiotto B, Richards B, Maher CG. Primary care management of non-specific low back pain: key messages from recent clinical guidelines. Med J Aust. 2018;208(6):272–275. doi:10.5694/mja2.2018.208.issue-6
8. Stochkendahl MJ, Kjaer P, Hartvigsen J, et al. National clinical guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J. 2018;27(1):60–75. doi:10.1007/s00586-017-5099-2
9. Chou R, Deyo R, Friedly J, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166(7):480–492. doi:10.7326/M16-2458
10. Koes BW, Backes D, Bindels PJE. Pharmacotherapy for chronic non-specific low back pain: current and future options. Expert Opin Pharmacother. 2018;19(6):537–545. doi:10.1080/14656566.2018.1454430
11. Allen NJ, Hecht TD. The ‘romance of teams’: toward an understanding of its psychological underpinnings and implications. J Occup Organ Psychol. 2004;77(439):461. doi:10.1348/0963179042596469
12. Mickan SM. Evaluating the effectiveness of health care teams. Aust Health Rev. 2005;29(2):211–217. doi:10.1071/AH050211
13. Schofield RF, Amodeo M. Interdisciplinary teams in health care and human services settings: are they effective? Health Soc Work. 1999;24(3):210–219. doi:10.1093/hsw/24.3.210
14. Bower P, Campbell S, Bojke C, Sibbald B. Team structure, team climate and the quality of care in primary care: an observational study. Qual Saf Health Care. 2003;12(4):273–279. doi:10.1136/qhc.12.4.273
15. Temkin-Greener H, Bajorska A, Peterson DR, et al. Social support and risk-adjusted mortality in a frail older population. Med Care. 2004;42(8):779–788. doi:10.1097/01.mlr.0000132397.49094.b3
16. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119–130. doi:10.1037/a0035514
17. Gogovor A, Burnand B, Ahmed S, Montague T, Peytremann-Bridevaux I. Team effectiveness in patient health management: an overview of reviews. Int J Clin Med. 2012;3:614–627. doi:10.4236/ijcm.2012.37111
18. Oslund S, Robinson RC, Clark TC, et al. Long-term effectiveness of a comprehensive pain management program: strengthening the case for interdisciplinary care. Proc (Bayl Univ Med Cent). 2009;22(3):211–214. doi:10.1080/08998280.2009.11928516
19. Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep. 2012;16(2):147–152. doi:10.1007/s11916-012-0252-4
20. Agence d’évaluation des technologies et des modes d’intervention en santé. Management of chronic (non-cancer) pain: organization of health services. Montréal: AETMIS; 2006.
21. Stanos S, Houle TT. Multidisciplinary and interdisciplinary management of chronic pain. Phys Med Rehabil Clin N Am. 2006;17(2):435–50, vii. doi:10.1016/j.pmr.2005.12.004
22. de Silva D. Measuring Patient Experience. London: The Health Foundation; 2013.
23. Patient reported outcome measures: their role in measuring and improving patient experience; 2012. Available from: http://patientexperienceportal.org/export/document/1273.
24. Anhang Price R, Elliott MN, Cleary PD, Zaslavsky AM, Hays RD. Should health care providers be accountable for patients’ care experiences? J Gen Intern Med. 2015;30(2):253–256. doi:10.1007/s11606-014-3111-7
25. Clancy C, Brach C, Abrams M. Assessing patient experiences of providers’ cultural competence and health literacy practices: CAHPS item sets. Med Care. 2012;50(9 Suppl 2):S1–S2. doi:10.1097/MLR.0b013e3182641e7f
26. Cleary PD, McNeil BJ. Patient satisfaction as an indicator of quality care. Inquiry. 1988;25(1):25–36.
27. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
28. Jenkinson C, Coulter A, Bruster S. The picker patient experience questionnaire: development and validation using data from in-patient surveys in five countries. J Tradit Complement Med. 2002;14(5):353–358. doi:10.1093/intqhc/14.5.353
29. Riskind P, Fossey L, Brill K. Why measure patient satisfaction? J Med Pract Manage. 2011;26(4):217–220.
30. Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44(3):166–206. doi:10.2307/3348969
31. Pettersen KI, Veenstra M, Guldvog B, Kolstad A. The patient experiences questionnaire: development, validity and reliability. Int J Qual Health Care. 2004;16(6):453–463. doi:10.1093/intqhc/mzh074
32. Sitzia J, Wood N. Patient satisfaction: a review of issues and concepts. Soc Sci Med. 1997;45(12):1829–1843. doi:10.1016/S0277-9536(97)00128-7
33. Tarlov AR, Ware JE
34. Anhang Price R, Elliott MN, Zaslavsky AM, et al. Examining the role of patient experience surveys in measuring health care quality. Med Care Res Rev. 2014;71(5):522–554. doi:10.1177/1077558714541480
35. Lyu H, Wick EC, Housman M, Freischlag JA, Makary MA. Patient satisfaction as a possible indicator of quality surgical care. JAMA Surg. 2013;148(4):362–367. doi:10.1001/2013.jamasurg.270
36. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368(3):201–203. doi:10.1056/NEJMp1211775
37. Trentman TL, Cornidez EG, Wilshusen LL, et al. Patient satisfaction in an academic chronic pain clinic. Pain Pract. 2013;13(5):372–379. doi:10.1111/papr.12004
38. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41–48.
39. Glickman SW, Boulding W, Manary M, et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3(2):188–195. doi:10.1161/CIRCOUTCOMES.109.900597
40. Leininger BD, Evans R, Bronfort G. Exploring patient satisfaction: a secondary analysis of a randomized clinical trial of spinal manipulation, home exercise, and medication for acute and subacute neck pain. J Manipulative Physiol Ther. 2014;37(8):593–601. doi:10.1016/j.jmpt.2014.08.005
41. Chang JT, Hays RD, Shekelle PG, et al. Patients’ global ratings of their health care are not associated with the technical quality of their care. Ann Intern Med. 2006;144(9):665–672. doi:10.7326/0003-4819-144-9-200605020-00010
42. Fenton JJ, Jerant AF, Bertakis KD, Franks P. The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med. 2012;172(5):405–411. doi:10.1001/archinternmed.2011.1662
43. Lee DS, Tu JV, Chong A, Alter DA. Patient satisfaction and its relationship with quality and outcomes of care after acute myocardial infarction. Circulation. 2008;118(19):1938–1945. doi:10.1161/CIRCULATIONAHA.108.792713
44. Rao M, Clarke A, Sanderson C, Hammersley R. Patients’ own assessments of quality of primary care compared with objective records based measures of technical quality of care: cross sectional study. BMJ. 2006;333(7557):19. doi:10.1136/bmj.38874.499167.7C
45. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1):e001570. doi:10.1136/bmjopen-2012-001570
46. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4.
47. Tan G, Jensen MP, Thornby JI, Anderson KO. Are patient ratings of chronic pain services related to treatment outcome? J Rehabil Res Dev. 2006;43(4):451–460. doi:10.1682/JRRD.2004.10.0128
48. Ahmed S, Eilyyan O, Ware P, Gogovor A, Visca R. Evaluation of an integrated interdisciplinary program for managing sub-acute low back pain. Physiotherapy. 2015;101(Supplement 1):e40. doi:10.1016/j.physio.2015.03.153
49. Glasgow RE, Whitesides H, Nelson CC, King DK. Use of the Patient Assessment of Chronic Illness Care (PACIC) with diabetic patients: relationship to patient characteristics, receipt of care, and self-management. Diabetes Care. 2005;28(11):2655–2661. doi:10.2337/diacare.28.11.2655
50. Iglesias K, Burnand B, Peytremann-Bridevaux I. PACIC instrument: disentangling dimensions using published validation models. J Tradit Complement Med. 2014;26(3):250–260. doi:10.1093/intqhc/mzu042
51. Vrijhoef HJ, Berbee R, Wagner EH, Steuten LM. Quality of integrated chronic care measured by patient survey: identification, selection and application of most appropriate instruments. Health Expect. 2009;12(4):417–429. doi:10.1111/j.1369-7625.2009.00557.x
52. Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care. 2005;43(5):436–444. doi:10.1097/01.mlr.0000160375.47920.8c
53. Koley M, Saha S, Ghosh S, et al. Patient-Assessed Chronic Illness Care (PACIC) scenario in an Indian homeopathic hospital. J Tradit Complement Med. 2016;6(1):72–77. doi:10.1016/j.jtcme.2014.11.020
54. Aung E, Donald M, Coll JR, Williams GM, Doi SA. Association between patient activation and patient-assessed quality of care in type 2 diabetes: results of a longitudinal study. Health Expect. 2015.
55. Noel PH, Jones S, Parchman ML. Patient experience in an era of primary care transformation: revisiting the PACIC. Eur J Pers Cent Healthc. 2016;4(3):528–540. doi:10.5750/ejpch.v4i3
56. Rick J, Rowe K, Hann M, et al. Psychometric properties of the patient assessment of chronic illness care measure: acceptability, reliability and validity in United Kingdom patients with long-term conditions. BMC Health Serv Res. 2012;12:293. doi:10.1186/1472-6963-12-293
57. Gogovor A, Hunt M, Hovey R, Ahmed A. Patients’ experiences in an interdisciplinary primary care program for low back pain. Disabil Rehabil. 2018. Submitted.
58. Fairbank JC, Pynsent PB. The oswestry disability index. Spine (Phila Pa 1976). 2000;25(22):2940–2952. doi:10.1097/00007632-200011150-00017
59. Roland M, Fairbank J. The roland-morris disability questionnaire and the oswestry disability questionnaire. Spine. 2000;25(24):3115–3124. doi:10.1097/00007632-200012150-00006
60. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
61. Hill JC, Whitehurst DGT, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. The Lancet. 2011;378(9802):1560–1571. doi:10.1016/S0140-6736(11)60937-9
62. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/acp.1983.67.issue-6
63. Kroenke K, Spitzer RL, Williams JB. The Phq-9. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
64. Ware JE
65. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138.
66. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. doi:10.1097/00002508-200409000-00005
67. Beaulieu M-D, Dragieva N, Del Grande C, et al. The team climate inventory as a measure of primary care teams’ processes: validation of the French version. Healthcare Policy. 2014;9(3):40–54.
68. Anderson NR, West MA. Measuring climate for work group innovation: development and validation of the team climate inventory. J Organ Behav. 1998;19:235–258. doi:10.1002/(ISSN)1099-1379
69. Kivimaki M, Elovainio M. A short version of the team climate inventory: development and psychometric properties. J Occup Organ Psychol. 1999;72(2):241–246. doi:10.1348/096317999166644
70. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010
71. Sloan JA. Assessing the minimally clinically significant difference: scientific considerations, challenges and solutions. Copd. 2005;2(1):57–62. doi:10.1081/COPD-200053374
72. Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther. 2012;20(3):160–166. doi:10.1179/2042618612Y.0000000001
73. Schmittdiel J, Mosen DM, Glasgow RE, Hibbard J, Remmers C, Bellows J. Patient Assessment of Chronic Illness Care (PACIC) and improved patient-centered outcomes for chronic conditions. J Gen Intern Med. 2008;23(1):77–80. doi:10.1007/s11606-007-0452-5
74. Berkman ND, Sheridan SL, Donahue KE, et al. Health literacy interventions and outcomes: an updated systematic review. Evid Rep Technol Assess (Full Rep). 2011;199:1–941.
75. US Department of Health and Human Services Food and Drug Administration. Patient-reported outcomes measures: use in medical product development to support labelling claims; 2009. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf.
76. Fixsen DL, Naoom SF, Blase KA, Friedman RM, Wallace F. Implementation Research: A Synthesis of the Literature. Tampa, FL: University of South Florida, Louis de la Parte Florida Mental Health Institute, The National Implementation Research Network (FMHI Publication #231); 2005.
77. Gensichen J, Serras A, Paulitsch MA, et al. The patient assessment of chronic illness care questionnaire: evaluation in patients with mental disorders in primary care. Community Ment Health J. 2011;47(4):447–453. doi:10.1007/s10597-010-9340-2
78. Houle J, Beaulieu MD, Lussier MT, et al. Patients’ experience of chronic illness care in a network of teaching settings. Can Fam Physician. 2012;58(12):1366–1373.
79. Jackson GL, Weinberger M, Hamilton NS, Edelman D. Racial/ethnic and educational-level differences in diabetes care experiences in primary care. Prim Care Diabetes. 2008;2(1):39–44. doi:10.1016/j.pcd.2007.11.002
80. Kuznetsov L, Simmons RK, Sandbaek A, Maindal HT. The impact of intensive multifactorial treatment on perceptions of chronic care among individuals with screen-detected diabetes: results from the ADDITION-Denmark trial. Int J Clin Pract. 2015;69(4):466–473. doi:10.1111/ijcp.2015.69.issue-4
81. Levesque JF, Feldman DE, Lemieux V, Tourigny A, Lavoie JP, Tousignant P. Variations in patients’ assessment of chronic illness care across organizational models of primary health care: a multilevel cohort analysis. Healthc Policy. 2012;8(2):e108–e123. doi:10.12927/hcpol.2012.23105
82. Rosemann T, Laux G, Szecsenyi J, Grol R. The chronic care model: congruency and predictors among primary care patients with osteoarthritis. Qual Saf Health Care. 2008;17(6):442–446. doi:10.1136/qshc.2007.022822
83. Taggart J, Chan B, Jayasinghe UW, et al. Patients Assessment of Chronic Illness Care (PACIC) in two Australian studies: structure and utility. J Eval Clin Pract. 2011;17(2):215–221. doi:10.1111/j.1365-2753.2010.01423.x
84. Markun S, Brandle E, Dishy A, Rosemann T, Frei A. The concordance of care for age related macular degeneration with the chronic care model: a multi-centered cross-sectional study. PLoS One. 2014;9(10):e108536. doi:10.1371/journal.pone.0108536
85. Petersen JJ, Paulitsch MA, Mergenthal K, et al. Implementation of chronic illness care in German primary care practices–how do multimorbid older patients view routine care? A cross-sectional study using multilevel hierarchical modeling. BMC Health Serv Res. 2014;14:336. doi:10.1186/1472-6963-14-336
86. Gibbons CJ, Small N, Rick J, Burt J, Hann M, Bower P. The patient assessment of chronic illness care produces measurements along a single dimension: results from a Mokken analysis. Health Qual Life Outcomes. 2017;15(1):61. doi:10.1186/s12955-017-0638-4
87. Spicer J, Budge C, Carryer J. Taking the PACIC back to basics: the structure of the patient assessment of chronic illness care. J Eval Clin Pract. 2012;18(2):307–312. doi:10.1111/j.1365-2753.2010.01568.x
88. Fok CC, Henry D. Increasing the sensitivity of measures to change. Prev Sci. 2015;16(7):978–986. doi:10.1007/s11121-015-0545-z
89. Gogovor A. Improving Chronic Illness Care: Implementation and Evaluation of Interdisciplinary and Patient-Centred Care [Doctoral dissertation]. Canada: McGill University; 2019.
90. Benoist M, Lenoir T. Natural evolution of nonspecific low-back pain. In: Szpalski M, Gunzburg R, Rydevik B, Le Huec J, Mayer H, editors. Surgery for Low Back Pain. Berlin: Springer; 2010:65–71.
91. Dunn KM, Croft PR. Epidemiology and natural history of low back pain. Eura Medicophys. 2004;40(1):9–13.
92. Lemeunier N, Leboeuf-Yde C, Gagey O. The natural course of low back pain: a systematic critical literature review. Chiropr Man Therap. 2012;20(1):33. doi:10.1186/2045-709X-20-33
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
