Back to Journals » Infection and Drug Resistance » Volume 12
Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: a systematic review and meta-analysis
Authors Jabbari Shiadeh SM, Pormohammad A , Hashemi A , Lak P
Received 20 February 2019
Accepted for publication 8 August 2019
Published 2 September 2019 Volume 2019:12 Pages 2713—2725
DOI https://doi.org/10.2147/IDR.S206084
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Seyedeh Marzieh Jabbari Shiadeh,1 Ali Pormohammad,2 Ali Hashemi,1 Parnian Lak3
1Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Student Research Committee, Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 3Department of Cell and Tissue Biology, University of California San Francisco, San Francisco, CA, USA
Correspondence: Ali Pormohammad
Clinical Microbiology, Shahid Beheshti University of Medical Sciences, Koodakyar Street, Tabnak Blv, Yaman Avenue, Chamran Highway, Tehran, Iran
Tel +98 212 387 2556
Fax +98 212 243 9964
Email [email protected]
Introduction: One of the global concerns is the increasing trend toward antimicrobial resistance and the consequent lack of efficient antimicrobials. Nosocomial infections present a big threat for patients all over the world and treatment with broad-spectrum antibiotics leads to outgrowth of hospital-associated resistant Enterococci clones that are very important in bloodstream infections. We surveyed the frequency and time trend of antibiotic resistance in Enterococci blood isolates from hospitalized patients in different regions of the world.
Methods: Literature from January 1, 2000 to May 20, 2018 was searched systematically using Medline (via PubMed), Embase, and Cochrane Library and all original publications on the antibiotic resistance prevalence in blood-isolated Enterococci strains with standard laboratory tests were included. Quality of the included studies was assessed with the modified Critical Appraisal Checklist recommended by the Joanna Briggs Institute. Depending on the heterogeneity test, we used either random or fixed effect models to assess the appropriateness of the pooled prevalence of drug resistance.
Results: A total of 291 studies were enrolled in the meta-analysis. Between all antibiotics, based on the WHO original offices, American countries showed the lowest prevalence of resistance for linezolid in Enterococcus faecalis. Regarding the prevalence of vancomycin resistance, Western Pacific, European, and American countries had the lowest level of resistance and South-East Asia and Eastern Mediterranean countries showed the highest level of resistance. Moreover, our findings for Enterococcus faecium indicated that America and South-East Asia had the lowest and the highest levels of resistance for linezolid, respectively.
Conclusion: Based on our findings, the prevalence of vancomycin-resistant E. faecium in bloodstream infections is significantly high, especially in Eastern Mediterranean countries, which is a massive warning signal for resistance to this broad-spectrum antibiotic. Therefore, the establishment of appropriate antibiotic usage guidelines should be essential in these countries.
Keywords: drug resistance, Enterococcus faecalis, Enterococcus faecium, prevalence, meta-analysis
Introduction
One of the global concerns is the increasing trend toward antimicrobial resistance and the consequent lack of efficient antimicrobials.1 Enhanced resistance to antimicrobials is a true menace to communal health, and realization of the systematic origin of antimicrobial resistance is important to cope with this public health menace.2 Also, antibiotic-resistant infections cause more morbidity and mortality and are associated with more health-care expenses.3 On the other hand, the availability of second-line antibiotics in developing countries diminishes the usage of next-generation antibiotics and causes more morbidity and mortality after antibiotic-resistant infections.4
Hospital-acquired infections emerging at least 48–72 h after admission of the patient are named nosocomial infections (NIs) and present a big threat for patients all over the world.5,6 Three kinds of infections that cause more than 60% of NIs are urinary tract infections, pneumonia, and primary bloodstream infections (BSIs), that are usually associated with using medical devices such as ventilators and catheters.7 Among the pathophysiological mechanisms that contribute to the emerging NIs, bacterial colonization and immunodeficiency are the main culprits.5
Enterococci are normal flora of the gastrointestinal tract. When a hospitalized patient is treated with broad-spectrum antibiotics, Enterococci are eliminated which leads to a decrease in the thickness of the protective gastrointestinal mucus layer. This enables unusual outgrowth of hospital-associated Enterococcal clones which are resistant to antibiotics.8–12 Enterococcus species have a broad range of resistance genes, and are able to exchange the resistance genes.13 Enterococci are the third common cause of NIs and health-care-associated BSIs14–16 and have been estimated to be responsible for 25–50% of the mortality rate among hospitalized patients.17–22 Also, simultaneous multiple resistance mechanisms lead to the emergence of multiresistant or pan-resistant Enterococci that do not respond to the common first-line antibiotics, leading to amplified morbidity and mortality rates and eventually more financial burdens on hospitals and patients.23
Among Enterococcus species, two major species (Enterococcus faecalisand Enterococcus faecium) are particularly pathogenic for humans: 85–90% of Enterococci infections are caused by E. faecalis, whereas 5–10% are caused by E. faecium;24 however, E. faecium BSI has higher rates of antibiotic resistance and mortality than E. faecalis BSI.25,26 Some of the important hospitalized infections which are life-threatening are BSIs because of vancomycin-resistant Enterococcal (VRE) species.16,27–29
In laboratory animals such as mice, the usage of antibiotics including cefoxitin, ceftriaxone, ampicillin, and vancomycin causes outgrowth of drug-resistant E. faecium isolates.30 Moreover, E. faecium is one of the ESKAPE pathogens (E. faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacterspecies) and along with E. faecalisis responsible for the most Enterococcus infections.13 It is worth mentioning that pathogenic E. faecalis strains can produce cytolysin, which exacerbates the toxicity caused by the infections and leads to a fivefold extreme threat of death in nosocomial bacteremia.31–33
According to our systematic review, there is no comprehensive study regard antibiotic resistance in isolated Enterococci. There are some review articles with limited data on the domain of special countries or exclusive to special antibiotics, such as vancomycin, linezolid, and tigecycline.34–38 The goal of this systematic review is to survey the frequency of antibiotic resistance to some common antibiotics in Enterococci isolated from blood isolates of hospitalized patients worldwide. Consequently, these data could be helpful in tackling drug resistance problems and illuminating the best antibiotic options that are effective for the treatment of the infections caused by Enterococci.
Methods
Sources of information and search strategies
From January 1, 2000 to May 20, 2018, two reviewers (SMJS and AP) searched systematically all publications including antibiotic resistance in Enterococci isolated from NIs via Medline (via PubMed), Embase, and Cochrane Library independently with the MeSH terms “Enterococcus faecalis”, “Enterococcus faecium”, “drug resistance”, “sepsis”, and “antimicrobial resistance”. MeSH terms were combined with text word searches that included “E. faecium”, “E. faecalis”, “septicemia”, “bloodstream”, “antibiotic(s)”, and their synonyms. In addition, we searched related reviews and selected articles, citation lists (backward citation), and references (forward citation) by hand and corresponded with authors (recommended by the Cochrane guideline).39 We did not contact the expert authors regarding previous experiences.40,41 We conducted our study according to the systematic review of prevalence PRISMA guidelines.42
Eligibility
Inclusion criteria
All original cross-sectional articles published in English and other languages, without language restriction, on different antibiotic resistance prevalence in blood-isolated Enterococci strains with standard laboratory tests were included for further analysis. All strains were isolated from hospitalized patients. We found studies in languages other than English that were translated using Google Translate (http://translate.google.com). Standard laboratory tests for determination of E. faecium and E. faecalis drug resistance included phenotypical microbiological methods such as the disk diffusion method, minimum inhibitory concentration, VITEK 2 system (bioMérieux), plate/replicator method, Isosensi test agar (Oxoid), and Trek Diagnostic Systems Inc. (Westlake, OH, USA). Additionally, molecular methods such as PCR, real-time PCR, PCR-restriction fragment length polymorphism, and sequencing assays had been used as the detection method in some studies.
Exclusion criteria
Articles were excluded from review for any of the following reasons:
- No accessible full text, or insufficient data in the abstract.
- Studies which just focused on treatment, without any clear report of drug resistance prevalence.
- Case report and review studies.
- Isolates were from healthy people as colonization.
- Surviving in special disease groups (eg, HIV-positive or any other immunodeficient patients).
- Isolated from surfaces, food, soil, animal, or sewage origins.
- Studies which had our aimed keywords, but without relevant data.
- Reports from mixed-infection patients without any Enterococci drug resistance pattern.
- Studies that investigated the prevalence of antibiotic resistance in vancomycin linezolid, ampicillin, and gentamycin-resistant Enterococci.
- The total number of Enterococci was not specified.
Data extraction and data collection
Data were extracted by two reviewers (SMJS and AP) independently and results were checked by a third reviewer (AH). Contradictions among the investigators were discussed to obtain agreement. The following data were extracted: name of first author, publication time, sample size, time and location of the study, type of participants, numbers of E. faecium and E. faecalis culture-positive isolates, and number of resistance E. faecium and E. faecalis isolated for each of the antibiotics. Studies identified by the search strategy were reviewed for eligibility based on the title and abstract by two investigators (SMJS and AP). Full manuscripts of the papers kept based on the title and abstract were assessed by the same investigator. Studies identified by the search strategy were reviewed for eligibility based on the title and abstract by one investigator (SMJS). Full manuscripts of the papers were kept based on the title and abstract and were further assessed by the same investigator. For both steps, a 10% random sample was assessed by a second investigator and was compared to the assessment of the first reviewer (AP). Extracted data were further reviewed and inconsistencies in the assessments discussed, and disagreements were resolved by consensus.
Quality assessment
Quality assessment of studies was performed by two reviewers independently according to the modified Critical Appraisal Checklist recommended by the Joanna Briggs Institute,43 and disagreements were resolved by consensus. The checklist is composed of 9 questions that reviewers addressed for each study. The “Yes” answer for each question received a score of 1. Thus, final scores for each study could range from 0 to 9 (Table S1).
Meta-analysis approach
Cleaning data and preparing them for analysis were done in Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) and further analyses were carried out via Comprehensive Meta-Analysis Software Version 2.0 (Biostat, Englewood, NJ, USA). Determination of heterogeneity among the studies was undertaken using the chi-squared test (Cochran’s Q) to assess the appropriateness of pooling data. Depending on the heterogeneity test, we used either random or fixed effect models for pooled prevalence of drug resistance. In the case of high heterogeneities (I2>50%), a random effect model (heterogeneity) was applied, while in low heterogeneities (I2<50%) a random effect model was used.44 The pooled resistance prevalence of each antibiotic was calculated. We also used the Begg’s and Egger’s test based on the symmetry assumption described for publication bias. The point estimates of the effect size and the resistance prevalence of each antibiotic, along with its 95% CI, were estimated for each study. Complementary analyses such as time trend, continent, and subgroup pooled prevalence were generated for countries and WHO regional offices.
Result
Study selection
We retrieved about 24,862 studies in a systematic search and excluded 1016 after the first screening. Finally, 291 studies were included in the meta-analysis (Figure 1). Characteristics of the selected articles are summarized in Table S1. Of the 291 included studies, 103 of them reported antibiotic susceptibility test with the minimum inhibitory concentration method and 100 of them reported by the disc diffusion method. A total of 143 studies interpreted and reported antimicrobial sensitivity tests according to Clinical and Laboratory Standards Institute (CLSI) guidelines, 22 were according to National Committee for Clinical Laboratory Standards (NCCLS), 6 were according to the Committee of the French Society of Microbiology (CASFM), 5 were according to the European Committee on Antimicrobial Susceptibility (EUCAST), 2 were according to the British Standard for Antimicrobial Chemotherapy (BSAC), and 108 were not reported. There were no differences in resistance between studies that did report the use of antimicrobial sensitivity test with different guidelines and those that did not.
 |
Figure 1 Flow diagram of the literature search and study selection (PRISMA flow chart). |
The prevalence of antibiotic resistance in blood isolates of E. faecalis
Of the 291 included studies, 125 studies reported the prevalence of E. faecalis antibiotic resistance in blood isolates. The total pooled number of E. faecalis blood isolates was 24,913. Linezolid had the lowest prevalence rate at 0.6% (95% CI: 0.3–15) while quinupristin/dalfopristin had the highest rate at 97% (95% CI: 89–99.2) (Table 1).
 |
Table 1 Prevalence of antibiotic resistance in blood-isolated Enterococcus spp. |
Out of the six WHO original offices, America had the lowest prevalence of antibiotic resistance at 0.3% (95% CI: 0.2–0.6) for linezolid (Table 2). Table 3 shows the pooled prevalence of the most important antibiotic resistance of blood-isolated E. faecalis from 2000–2016. Most of the antibiotics were subject to increased drug resistance of up to more than twofold. Figure 2 demonstrates the time trend of E. faecalis vancomycin resistance prevalence by WHO regional offices. American, European, and Western Pacific countries showed the lowest level of vancomycin resistance with totally decreasing resistance prevalence in this period of time. However, South-East Asia and Eastern Mediterranean countries showed the highest level of vancomycin resistance with an increasing trend in resistance prevalence at the same time. Figure 3 presents the pooled prevalence of E. faecalis vancomycin resistance by country. Sweden had the lowest prevalence at 0.2%, while Japan and Nepal had the highest prevalence at 18% and 11%, respectively.
 |
Table 2 Prevalence of antibiotic resistance in blood-isolated Enterococcus spp. by WHO regional offices |
 |
Table 3 Prevalence of antibiotic resistance in blood-isolated Enterococcusspp. by study time groups |
 |
Figure 2 Time trend meta-analysis: prevalence of vancomycin resistance in blood-isolated Enterococcus faecalis by WHO regional offices. |
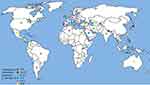 |
Figure 3 Global prevalence of vancomycin resistance in blood-isolated Enterococcus faecalis. |
The prevalence of antibiotic resistance in blood isolates of E. faecium
Of the 291 included studies, 114 studies reported the prevalence of E. faecium antibiotic resistance in blood isolates. The total pooled number of E. faecium blood isolates was 13,238. Tigecycline and linezolid had the lowest prevalence at 1% (95% CI: 0.2–4.5) and 1.7% (95% CI: 1–2.8), respectively; while penicillin had the highest prevalence of 85% (95% CI: 81–89) (Table 1).
Of the WHO original offices, America had the lowest prevalence of antibiotic resistance at 0.8% (95% CI: 0.4–2) for linezolid (Table 2). Table 3 describes the pooled prevalence of the most important antibiotic-resistant blood-isolated E. faecium from 2000–2016. Most of the antibiotics were subject to increased drug resistance of up to more than twofold, in particular vancomycin, whose time trend of resistance in E. faecium by WHO regional offices is described in Figure 4. Figure 5 shows the pooled prevalence of E. faecium vancomycin resistance by country. The Netherlands had the lowest prevalence at 0.1%, while Brazil had the highest prevalence at 67%. Table S2–S43 demonstrate the prevalence of drug resistance in E. faecalis and E. faecium for each antibiotic with more detail.
 |
Figure 4 Time trend meta-analysis: prevalence of vancomycin resistance in blood-isolated Enterococcus faecium by WHO regional offices. |
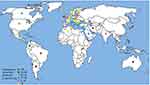 |
Figure 5 Global prevalence of vancomycin resistance in blood-isolated Enterococcus faecium. |
Discussion
This study illuminates the high rate of antibiotic resistance in Enterococcal bloodstream isolates in most regions of the world, especially in Eastern Mediterranean countries. Based on our findings, the prevalence of vancomycin-resistant E. faecium in BSIs is significantly high, especially in Eastern Mediterranean countries, which is an outstanding warning in treatment and control of this bacterium. Our findings, summarized in Table 1, revealed that the resistance level to the most common treatments was significantly higher in E. faecium compared to E. faecalis in Enterococcal infections. Inherently, the antibiotic resistance rate in E. faecium is higher than in E. faecalis; specifically, high levels of resistance in E. faecium pathogenic types to ampicillin, vancomycin, and high-level aminoglycosides are documented. Therefore, treatment of the infections caused by these species is considered a serious problem that requires more precise treatment strategies. Quinupristin/dalfopristin combination is a member of streptogramin antibiotics that is broadly effective against VRE in Europe and the USA.45 Based on this study the pooled prevalence of resistance to quinupristin/dalfopristin was significantly higher in E. faecalis, which is in concord with previous publication results.46
We observed that resistance to linezolid, daptomycin, and tigecycline was nearly at the same level for E. faecalis and E. faecium (pooled prevalence ≤1%), confirming that tigecycline and linezolid are effective against these species in low concentrations.47 Moreover, in our review, the lowest resistance in E. faecalis appeared to be to nitrofurantoin, amoxicillin/clavulanic acid, ampicillin, and imipenem which are critical in the treatment process.
Out of the six WHO original offices, America had the lowest prevalence of antibiotic resistance at 0.3% (95% CI: 0.2–0.6) for linezolid.
Based on our findings in America, in comparison with African countries, the least resistance to linezolid and gentamicin was seen in E. faecium and E. faecalis, indicating good therapeutic methods in America. Therefore, linezolid is highly effective against VRE and recommended for the infections caused by VRE.48,49 Moreover, vancomycin is useful for the treatment of VRE bacteremia in mice.50 However, due to linezolid’s bacteriostatic activity, there are some limitations in its prescription.51
Based on our review, the highest level of vancomycin resistance was observed in the Eastern Mediterranean region. This is in the main due to poor hygiene, defective health control management, insufficient control, and scarcity of antimicrobial stewardship programs in this region.52,53 However, in South-East Asian countries, the highest resistance was found in E. faecium isolates to linezolid. As these countries are the major importer of chickens and livestock,54 the excessive use of antibiotics in these products would result in the spread of multiple-drug-resistant Enterococci, which would get transmitted to humans through consumption of these products in these countries.55
An increasing trend of vancomycin-resistant E. faecium was seen in most regions except in America. However, the trend is different for vancomycin-resistant E. faecalis and it was noticed to be increasing only in the Eastern Mediterranean (Figures 1 and 2). There are many reasons which lead to this trend in developing countries, such as poor antibiotic usage strategies,and inappropriate usage of antibiotics especially vancomycin in the treatment of Enterococcal infections.
Based on our study, resistance to tetracycline in E. faecalis was at the highest level. However, tetracycline is not the first-choice drug for the treatment of Enterococcal infections, it is a broad-spectrum antibiotic that can cause resistance in Enterococci during the treatment of other infections.56 Therefore, broad-spectrum antibiotics such as tetracycline are not recommended for treatment of Enterococcal infections.
On the other side, in African countries the highest resistance was found to ampicillin in E. faecium, whereas the highest resistance was observed to linezolid and gentamicin in E. faecalis isolates. In addition, the lowest resistance rates were detected in E. faecalis to ampicillin, and in E. faecium to both vancomycin and teicoplanin. The scarcity and extra expense of wide-spectrum antibiotics such as vancomycin led to higher prescriptions of narrow-spectrum antibiotics and thus an increased resistance to those antibiotics in this region.57
For lots of antibiotics examined in our review study, the prevalence of resistance increased around the world from 2010 to 2016 in most regions. Therefore, the establishment of appropriate antibiotic usage guidelines should be essential in these countries. In contrast, decreased resistance was seen in E. faecium and E. faecalis to gentamicin and ampicillin, respectively (Table 3). Probably, inappropriate consumption/prescription of antibiotics by patients/physicians and also in industries is the most important reason for increasing antibiotic resistance in Eastern Mediterranean countries. Instead, antibiotic access is restricted in other regions.
Some limitations can be introduced by the literature search procedure and we might have missed some relevant studies. Furthermore, we have excluded articles in which the total number of Enterococci was not specified. But the main strength of our study that distinguishes it from previous studies is investigating the prevalence of antibiotic resistance in two common strains of Enterococci that were isolated from blood samples in different parts of the world during 18 years.
Spit meta-regression, subgroup, meta-regression, and sensitivity analyses to detect the sources of heterogeneity, and publication bias and heterogeneity should be considered when interpreting the outcomes of the reports.
Conclusion
Rates of resistance in South-East Asia and Eastern Mediterranean countries were at the highest level, especially for vancomycin and quinupristin/dalfopristin. There were increasing trends of antibiotic resistance in blood isolates of Enterococcus up to more than twice from 2000 to 2018. Therefore, the establishment of appropriate antibiotic usage guidelines should be essential in these countries.
Acknowledgments
This study is related to project no. 1396/3618 from the Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The authors also appreciate the Student Research Committee and Research & Technology Chancellor at Shahid Beheshti University of Medical Sciences for their financial support of this study.
The Student Research Committee and “Research & Technology Chancellor” at Shahid Beheshti University of Medical Sciences financially supported this study (Grant/Award Number: 1396/3618).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi:10.1016/S0140-6736(15)00474-2
2. Holmes AH, Moore LS, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–187. doi:10.1016/S0140-6736(15)00473-0
3. Holmberg SD, Solomon SL, Blake PA. Health and economic impacts of antimicrobial resistance. Rev Infect Dis. 1987;9(6):1065–1078.
4. Planta MB. The role of poverty in antimicrobial resistance. J Am Board Fam Med. 2007;20(6):533–539. doi:10.3122/jabfm.2007.06.070019
5. Vincent J-L. Nosocomial infections in adult intensive-care units. lancet. 2003;361(9374):2068–2077. doi:10.1016/S0140-6736(03)13644-6
6. Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. Jama. 2003;290(14):1868–1874. doi:10.1001/jama.290.14.1868
7. Agaba P, Tumukunde J, Tindimwebwa J, Kwizera A. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: a cross sectional study. BMC Res Notes. 2017;10(1):349. doi:10.1186/s13104-017-2695-5
8. Brandl K, Plitas G, Mihu CN, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455(7214):804. doi:10.1038/nature07270
9. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci. 2008;105(39):15064–15069. doi:10.1073/pnas.0803124105
10. Ruiz-Garbajosa P, de Regt M, Bonten M, et al. High-density fecal Enterococcus faecium colonization in hospitalized patients is associated with the presence of the polyclonal subcluster CC17. Eur J Clin Microbiol Infect Dis. 2012;31(4):519–522. doi:10.1007/s10096-011-1342-7
11. Tedim AP, Ruiz-Garbajosa P, Corander J, et al. Population biology of intestinal Enterococcus isolates from hospitalized and nonhospitalized individuals in different age groups. Appl Environ Microbiol. 2015;81(5):1820–1831. doi:10.1128/AEM.03661-14
12. Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120(12):4332–4341. doi:10.1172/JCI43918
13. MacCallum WG, Hastings TW. A case of acute endocarditis caused by Micrococcus zymogenes (nov. spec.), with a description of the microorganism. J Exp Med. 1899;4(5–6):521–534. doi:10.1084/jem.4.5-6.521
14. Olawale KO, Fadiora SO, Taiwo SS. Prevalence of hospital acquired enterococci infections in two primary-care hospitals in Osogbo, Southwestern Nigeria. Afr J Infect Dis. 2011;5:2. doi:10.4314/ajid.v5i2.66513
15. McBride S, Upton A, Roberts S. Clinical characteristics and outcomes of patients with vancomycin-susceptible Enterococcus faecalis and Enterococcus faecium bacteraemia—a five-year retrospective review. Eur J Clin Microbiol Infect Dis. 2010;29(1):107–114. doi:10.1007/s10096-009-0830-5
16. Vergis EN, Hayden MK, Chow JW, et al. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia: a prospective multicenter study. Ann Intern Med. 2001;135(7):484–492. doi:10.7326/0003-4819-135-7-200110020-00007
17. Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis. 2007;58(2):163–170. doi:10.1016/j.diagmicrobio.2006.12.022
18. Hidron AI, Edwards JR, Patel J, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi:10.1086/591861
19. Reik R, Tenover FC, Klein E, McDonald LC. The burden of vancomycin-resistant enterococcal infections in US hospitals, 2003 to 2004. Diagn Microbiol Infect Dis. 2008;62(1):81–85. doi:10.1016/j.diagmicrobio.2008.04.013
20. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi:10.1086/421946
21. van Harten RM, Willems RJ, Martin NI, Hendrickx AP. Multidrug-resistant enterococcal infections: new compounds, novel antimicrobial therapies? Trends Microbiol. 2017;25(6):467–479. doi:10.1016/j.tim.2017.01.004
22. Chlebicki MP, Kurup A. Vancomycin-resistant Enterococcus: a review from a Singapore perspective. Ann Acad Med Singapore. 2008;37(10):861–869.
23. de Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis. 2007;7(1):24. doi:10.1186/1471-2334-7-89
24. Ho C, Lau A, Cimon K, Farrah K, Gardam M. Screening, isolation, and decolonization strategies for vancomycin-resistant enterococci or extended spectrum beta-lactamase producing organisms: a systematic review of the clinical evidence and health services impact. europepmc. 2012.
25. Shenoy ES, Paras ML, Noubary F, Walensky RP, Hooper DC. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): a systematic review. BMC Infect Dis. 2014;14(1):177. doi:10.1186/1471-2334-14-177
26. Ulrich N, Vonberg R-P, Gastmeier P. Outbreaks caused by vancomycin-resistant Enterococcus faecium in hematology and oncology departments: a systematic review. Heliyon. 2017;3(12):e00473. doi:10.1016/j.heliyon.2017.e00473
27. Van Tulder M, Furlan A, Bombardier C, Bouter L; Group EBotCCBR. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976). 2003;28(12):1290–1299. doi:10.1097/01.BRS.0000065484.95996.AF
28. Houri H, Pormohammad A, Riahi SM, et al. Acute bacterial meningitis in Iran: systematic review and meta-analysis. PLoS One. 2017;12(2):e0169617. doi:10.1371/journal.pone.0169617
29. Pormohammad A, Seyed-Mohammad R, Nasiri MJ, et al. Diagnostic test accuracy of adenosine deaminase for tuberculous meningitis: a systematic review and meta-analysis. J Infect. 2017. doi:10.1016/j.jinf.2017.02.012
30. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):
31. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews: addressing questions of prevalence. Int J Health Policy Manag. 2014;3:123. doi:10.15171/ijhpm.2014.71
32. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies. J Natl Cancer Inst. 1959;22(4):719–748.
33. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10(4):266. doi:10.1038/nrmicro2761
34. Vankerckhoven V, Van Autgaerden T, Vael C, et al. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J Clin Microbiol. 2004;42(10):4473–4479. doi:10.1128/JCM.42.10.4473-4479.2004
35. Billington E, Phang S, Gregson D, et al. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis. 2014;26:76–82. doi:10.1016/j.ijid.2014.02.012
36. Caballero-Granado F, Becerril B, Cuberos L, Bernabeu M, Cisneros J, Pachon J. Attributable mortality rate and duration of hospital stay associated with enterococcal bacteremia. Clin Infect Dis. 2001;32(4):587–594. doi:10.1086/318717
37. DiazGranados CA, Zimmer SM, Mitchel K, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41(3):327–333. doi:10.1086/430909
38. Edmond MB, Ober JF, Weinbaum DL, et al. Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis. 1995;20(5):1126–1133. doi:10.1093/clinids/20.5.1126
39. Noskin GA, Peterson LR, Warren JR. Enterococcus faecium and Enterococcus faecalis bacteremia: acquisition and outcome. Clin Infect Dis. 1995;20(2):296–301. doi:10.1093/clinids/20.2.296
40. Patterson JE, Sweeney AH, Simms M, et al. An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine 1995;74(4):191–200.
41. Linden P. Can enterococcal infections initiate sepsis syndrome? Curr Infect Dis Rep. 2003;5(5):372–378.
42. Jia W, Li G, Wang W. Prevalence and antimicrobial resistance of Enterococcus species: a hospital-based study in China. Int J Environ Res Public Health. 2014;11(3):3424–3442. doi:10.3390/ijerph110303424
43. Hershberger E, Donabedian S, Konstantinou K, Zervos MJ, Eliopoulos GM. Quinupristin-dalfopristin resistance in gram-positive bacteria: mechanism of resistance and epidemiology. Clin Infect Dis. 2004;38(1):92–98. doi:10.1086/380125
44. Dobbs TE, Patel M, Waites KB, Moser SA, Stamm AM, Hoesley CJ. Nosocomial spread of Enterococcus faecium resistant to vancomycin and linezolid in a tertiary care medical center. J Clin Microbiol. 2006;44(9):3368–3370. doi:10.1128/JCM.00850-06
45. Kristich CJ, Rice LB, Arias CA. Enterococcal infection—treatment and antibiotic resistance. Boston: Massachusetts Eye and Ear Infirmary; 2014.
46. Huycke MM, Sahm DF, Gilmore MS. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4(2):239. doi:10.3201/eid0402.980211
47. Ike Y, Hashimoto H, Clewell D. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987;25(8):1524–1528.
48. Jett B, Jensen H, Nordquist R, Gilmore M. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60(6):2445–2452.
49. Arce JS, Gainza EC, Brusola AG, Estévez RO, Canton E, Gobernado M. In vitro activity of linezolid in combination with doxycycline, fosfomycin, levofloxacin, rifampicin and vancomycin against methicillin-susceptible Staphylococcus aureus. Rev Esp Quimioterap. 2006;19(3):252–47.
50. Beitdaghar M, Ahmadrajabi R, Karmostaji A, Saffari F. In vitro activity of linezolid alone and combined with other antibiotics against clinical enterococcal isolates. Wien Med Wochenschr. 2019;169:215.
51. Gales AC, Sader HS, Ribeiro J, Zoccoli C, Barth A, Pignatari AC. Antimicrobial susceptibility of gram-positive bacteria isolated in Brazilian hospitals participating in the SENTRY program (2005-2008). Braz J Infect Dis. 2009;13(2):90–98.
52. Moellering RC. Linezolid: the first oxazolidinone antimicrobial. Ann Intern Med. 2003;138(2):135–142. doi:10.7326/0003-4819-138-2-200301210-00015
53. Maina D, Omuse G, Revathi G, Adam RD. Spectrum of microbial diseases and resistance patterns at a private teaching hospital in Kenya: implications for clinical practice. PLoS One. 2016;11(1):e0147659. doi:10.1371/journal.pone.0147659
54. Årdal C, Outterson K, Hoffman SJ, et al. International cooperation to improve access to and sustain effectiveness of antimicrobials. Lancet. 2016;387(10015):296–307. doi:10.1016/S0140-6736(15)00470-5
55. Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: a developing country-perspective. Front Microbiol. 2016;7:1881. doi:10.3389/fmicb.2016.01881
56. Del Rosario BP, Aquino AP, Tidon AG, Gerpacio RV. Livestock Sector Training Needs Assessment Report for Southeast Asia, China and Papua New Guinea. 2008.
57. Hammerum A. Enterococci of animal origin and their significance for public health. Clin Microbiol Infect. 2012;18(7):619–625. doi:10.1111/j.1469-0691.2012.03829.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
