Back to Journals » International Journal of Nanomedicine » Volume 14
Modulated electro-hyperthermia-enhanced liposomal drug uptake by cancer cells
Authors Tsang YW, Chi KH, Huang CC, Chi MS, Chiang HC, Yang KL, Li WT , Wang YS
Received 27 September 2018
Accepted for publication 17 January 2019
Published 18 February 2019 Volume 2019:14 Pages 1269—1279
DOI https://doi.org/10.2147/IJN.S188791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Yuk-Wah Tsang,1,2 Kwan-Hwa Chi,3,4 Cheng-Chung Huang,3,5 Mau-Shin Chi,3,6 Hsin-Chien Chiang,7 Kai-Lin Yang,3,4,8 Wen-Tyng Li,2 Yu-Shan Wang3,6
1Department of Radiation Oncology, Chiayi Christian Hospital, Chiayi, Taiwan; 2Department of Biomedical Engineering, Chung Yuan Christian University, Taoyuan, Taiwan; 3Department of Radiation Therapy and Oncology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan; 4Institute of Radiation Science and School of Medicine, National Yang-Ming University, Taipei, Taiwan; 5Department of Biochemistry and Molecular Biology, College of Medicine, National Taiwan University, Taipei, Taiwan; 6Institute of Molecular Medicine and Bioengineering, National Chiao Tung University, Hsinchu, Taiwan; 7Institute of Veterinary Clinical Science, School of Veterinary Medicine, National Taiwan University, Taipei, Taiwan; 8School of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
Purpose: Modulated electro-hyperthermia (mEHT) stands to be a significant technological advancement in the hyperthermia field, utilizing autofocusing electromagnetic power on the cell membrane to create massive apoptosis. Since mEHT possesses the unique ability to excite cell membranes, we hypothesized that mEHT could enhance the uptake of liposomal drugs by enhancing phagocytic activity.
Materials and methods: Water bath control and mEHT were used to compare the enhancement of liposome-encapsulated doxorubicin (Lipodox®) uptake by cancer cells. Cancer cells were made visible by doxorubicin fluorescence to investigate drug uptake. Viable cell yield was determined via the Trypan Blue exclusion method. Various substrates were used to investigate the mechanism of drug-uptake enhancement. The murine colon carcinoma model, CT26, was used to confirm the tissue infiltration of Lipodox® and its therapeutic effect.
Results: mEHT treatment showed a significant enhancement of Lipodox® uptake of doxorubicin fluorescence compared with 37°C or 42°C water bath treatment. Tumor tissue sections also confirmed that mEHT treatment achieved the highest doxorubicin concentration in vivo (1.44±0.32 µg/g in mEHT group and 0.79±0.32 µg/g in 42°C water bath). Wortmannin was used to inhibit the macropinocytosis effect and 70 kDa dextran-FITC served as uptake substance. The uptake of dextran-FITC by cancer cells significantly increased after mEHT treatment whereas such enhancement was significantly inhibited by wortmannin.
Conclusion: The result showed mEHT-induced particle-uptake through macropinocytosis. mEHT-enhanced uptake of Lipodox® may amplify the therapeutic effect of liposomal drugs. This novel finding warrants further clinical investigation.
Keywords: hyperthermia, cancer treatment, liposome, doxorubicin, micropinocytosis
Corrigendum for this paper has been published
Introduction
Hyperthermia (HT) has a long history of use as a cancer treatment. One specific form of HT is modulated electro-hyperthermia (mEHT),1–4 which utilizes capacitively (impedance) couplled 13.56 MHz amplitude-modulated radiofrequency energy.4 The trade name for mEHT is oncothermia. The electric field energy can concentrate and accumulate in the tumor area due to the higher ionic conductivity around the cancer cell and induce cancer cell apoptosis in relatively low fever-range temperatures (at or below 42°C).3–6 mEHT has been applied as clinical cancer treatment worldwide for more than 20 years.7–9 Numerous clinical trials and retrospective analyses have shown that mEHT can be applied to multiple cancer types, including brain, gastrointestinal, gynecological, liver, lung, and pancreatic cancers.10 mEHT has shown a synergistic effect with some chemotherapy agents.11 In general, mEHT is not recommended as monotherapy, but rather in combination with radiotherapy, chemotherapy, or immunotherapy.
In a previous study, we performed a three-armed, direct comparison between water bath, 8 MHz conventional HT (Thermotron RF-8), and mEHT. We observed the respective biological effects on tumor cell lines. In the same treatment conditions (42°C for 30 minutes), mEHT gave rise to a higher apoptosis rate than other HT methods. Moreover, mEHT also induced the release of Heat Shock Protein 70 (Hsp70) from cancer cell cytosol to its extracellular domain.12 These results indicate that mEHT may trigger anti-tumor responses on cell membranes and disturb the biological effects of cell membranes. Liposomal chemotherapy drugs (chemo-drugs) are a relatively new form of chemo-drugs, with many years of clinical application. They have many advantages when compared with conventional chemo-drugs. The use of liposome-encapsulated doxorubicin (Lipodox®) allows the drug to become trapped within the tumor site, enhancing its killing effect on tumor cells. Lipodox® can also reduce side effects induced by conventional doxorubicin, specifically cardiac toxicity. Approved cancer indications for Lipodox® include Kaposi sarcoma, multiple myeloma, and breast and ovarian cancers. Lipodox® has not been approved as a substitute for conventional doxorubicin in adjuvant treatment of breast cancer.13 Furthermore, therapeutic efficacy in application has not matched expectations from development phases.14 Thus, there have been many studies conducted to enhance the therapeutic efficacy of liposomal chemo-drugs. Thermo-sensitive liposome, a new form of doxorubicin, has been proposed as remedy,15 but this new formulation drug has yet to pass clinical trials, and is years away from clinical bedside application. As of now, no proven method is available to enhance the therapeutic efficacy of US Food and Drug Administration-approved Lipodox® or its class of liposomal chemo-drugs.16
mEHT has been mentioned as a nano-heating method on cell membranes without utilizing artificial nanoparticles.17 The radiofrequency energy transmitted from mEHT could stimulate the membrane, specifically the membrane rafts of the tumor cells.18 Thus, in this study, we hypothesized that the ability of mEHT to stimulate cell membranes may enhance the phagocytosis of cancer cells. This may apply to macromolecular drugs such as liposomal chemo-drugs.
Materials and methods
Cell culture
HepG2 (hepatocellular carcinoma) and A549 (lung carcinoma) cells were grown in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% heat-inactivated FBS with 100 units/mL penicillin and 100 μg/mL streptomycin (Thermo Fisher Scientific). U87MG (glioblastoma astrocytoma) cells were maintained in minimum essential medium (Thermo Fisher Scientific) containing 10% heat-inactivated FBS with 1 mM sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin. CT26 (murine colorectal carcinoma) cells were maintained in RPMI 1640 (Thermo Fisher Scientific) containing 10% heat-inactivated FBS with 4.5 g/L D-glucose, 10 mM HEPES, 1 mM sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin. All of the cell lines were purchased from Bioresource Collection and Research Center, Hsinchu, Taiwan (BCRC). The BCRC number of each cell line is listed as follows: BCRC 60025 (HepG2), BCRC 60074 (A549), BCRC 60360 (U87MG), and BCRC 60447 (CT26).
mEHT treatment
Electromagnetic heating was generated by capacitively-coupled, amplitude-modulated, 13.56 MHz radiofrequency (LabEHY, Oncotherm Ltd, Troisdorf, Germany). An in vitro heating model was set up in an electrode chamber (LabEHY in vitro applicator). The chamber contained a cell bag (1×106 cells) heated to 42°C for 30 minutes with average power of 10–12 W. Temperature was maintained at approximately 42°C on the treated side, as measured with optical sensors (Luxtron FOT Lab Kit, LumaSense Technologies, Inc., Santa Clara, CA, USA). The in vitro model setup was shown previously.12 The power patterns were repeated three times. We checked the power pattern each time to verify the accuracy and similarity of the experiments. Our experiment followed the same precision procedure setup for experimentation with mEHT as used by Andocs et al.19 For water bath control, 1×106 cells were placed in a tube with culture medium and incubated at 42°C for 30 minutes.
Viability assay
To determine cell viability, cells were cultured for 24 hours after treatment, and viable cell yield was determined via the Trypan Blue exclusion method.
Lipodox® size determination and doxorubicin concentration
Lipo-Dox® (Lipodox®) was purchased from Taiwan Tung Yang Biopharm (TTY Biopharm Company, Ltd, Taipei, Taiwan). Lipodox® contains 20 mg/10 mL of doxorubicin and 14 mol/mL phospholipid per vial. The lipid compositions included distearoylphosphatidylcholine, cholesterol, and polyethylene glycol-(average molecular weight, 2,000) derived distearoylphosphatidylethanolamine (molar ratio, 3:2:0.3).20,21 The diameter of the Lipodox® was determined by dynamic light scattering (DLS) (HORIBA SZ-100, HORIBA, Kyoto, Japan). Total doxorubicin content in the medium was detected fluorometrically using a 96-well microplate reader (Sunrise, Tecan, Männedorf, Switzerland) (excitation: 470 nm; emission: 582 nm). Fluorescence intensity was translated to doxorubicin concentration, by a standard curve prepared from doxorubicin original solutions. Results are represented by the mean and SD of at least three replicates for each experiment.
Doxorubicin-release detection
In this study, the doxorubicin-release detection was performed by flow cytometry and immunofluorescence. Cells were collected at 10 minutes after treatment. For flow cytometric analysis, the drug-loaded cells were washed with ice cold PBS twice and detected in the FL-2 channel depending on the red fluorescence of doxorubicin. In the inhibition experiments, different inhibitors for each endocytosis pathway were used and cells were pre-treated with them for 30 minutes prior to mEHT treatment, including 200 μg/mL NaN3 (Sigma S2002, ATPase-inhibitor, Sigma-Aldrich Co., St Louis, MO, USA), 4 μg/mL filipin (Sigma F4767, caveolae-mediated pathway), 0.1 μM wortmannin (Sigma W3144, macropinocytosis), and 7 μg/mL chlorpromazine (CPZ) (Sigma C8138, clathrin-mediated endocytosis pathway). For immunofluorescence staining, the drug-loaded cells were washed with ice cold PBS twice and stained with DAPI for nuclear staining after fixing with 3.7% paraformaldehyde for 15 minutes. The fixed cells were washed with ice cold PBS twice, re-suspended in mounting solution, and dropped onto the cover slide for analysis by fluorescence microscopy (iRiS™ digital cell imaging system, Logos Biosystems, Inc., Seoul, South Korea).
Substrate assay
To investigate the increasing uptake ability by mEHT treatment, various endocytosis pathway substrates were used in this study including Alexa-fluor 488-conjugated transferrin (5 μg/mL, Invitrogen T-13342) for clathrin-mediated pathway, Alexa-fluor 488-conjugated cholera toxin subunit-B (2 μg/mL, Invitrogen C-34775) for caveolin-mediated pathway, and FITC-labeled 70 kDa dextran (1 mg/mL, Sigma 46945) for macropinocytosis. All these substrates were used for pre-treating cells for 10 minutes before mEHT treatment and analyzed by Accuri C6 flow cytometer and CFlow Software (Accuri cytometers Inc., Ann Arbor, MI, USA) at 10 minutes after treatment.
Western blot assay
For protein analysis, cells were lysed 10 minutes after treatment with RIPA buffer (Sigma R0278) containing phosphatase inhibitor (Hoffman-La Roche Ltd., Basel, Switzerland) and protease inhibitor (Hoffman-La Roche Ltd.). The total protein concentration was measured using the BCA protein assay kit (Thermo Fisher Scientific). Cell lysates (20 μg) were separated using SDS-PAGE. After transfer, proteins were probed with antibodies against p-EGFR, Tyr1068 (Cell Signaling Technology [#2234], Danvers, MA, USA), and α-tubulin (9F3) (Cell Signaling Technology, #2128). Bound antibodies were visualized using HRP-linked anti-rabbit antibodies.
Animal study
BALB/c mice were purchased from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan) and housed at 20°C–22°C, with 50%–60% humidity and 12-hour light/dark cycles. Sterile rodent food and water were given ad libitum. Five-week old mice at weights of 20–25 g were used. CT26 murine colorectal carcinoma cells were cultured as described previously. CT26 murine colorectal carcinoma cells (3×105) were injected subcutaneously in the right femoral areas of mice. The mice were used for experiments when their tumor volume grew to approximately 150–200 mm3. The mice received local water bath or mEHT treatment (as described previously) immediately after 10 mg/kg Lipodox® intravenous injection through the tail vein as a one-time treatment. For water bath control, tumor-bearing legs were fixed on a rack to maintain a stable position in a water bath during the HT treatment. The size of tumors was measured on the day of experiment, and once every 2 days. Length (L) and width (W) were recorded and the tumor volumes were calculated as (L×W2/2). Mice were sacrificed when their tumors reached the maximum allowed volume of 3,000 mm3.
Statistical analysis
All statistical analyses were performed using the statistical software program Prism 4 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons among groups were made with one-way ANOVA or unpaired Student’s t-test (two-tailed). Differences were considered statistically significant at P<0.05. Data are given as mean and SD of experiments, independently repeated at least three times.
Ethics statement
The studies were approved by the Institutional Animal Care and Use Committee of the National Yang-Ming University prior to initiation (approval no. 1060602). The welfare of the animals was based on the Guide for the Care and Use of Laboratory Animals, 8th edition.
Results
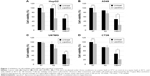
mEHT enhanced cytotoxicity of Lipodox® in various cancer cell lines
In order to investigate the cytotoxicity of mEHT combined with Lipodox® in cancer cells, HepG2, A549, U87MG, and CT26 cells were treated for 10 minutes with or without 50 μM of Lipodox®, and then separated into groups to be either incubated in a water bath at 37°C (control), in a water bath at 42°C, and mEHT at 42°C for 30 minutes. After 24 hours incubation without Lipodox®, cell number was counted and the number relative to the 37°C control was indicated as cell viability. Cell viability of the mEHT group decreased in comparison with the 37°C control, whereas mEHT combination with Lipodox® treatment further decreased the cell viability to 10.7%±2.7% from 52.3%±6.2% with mEHT treatment alone in HepG2 cells (Figure 1A, P<0.001). This result was consistent among other cell lines. For A549 cells, cell viability under mEHT treatment with Lipodox® was 23.4%±4.1%, while without Lipodox® was 49.5%±13.2%, (P<0.05) (Figure 1B). In U87MG cells, cell viability was 45.0%±1.7% with mEHT treatment alone and 25.6%±4.9% with mEHT plus Lipodox®, (P<0.01) (Figure 1C). In CT26 cells, cell viability was 47.3%±8.7% with mEHT treatment alone and 22.3%±8.9% with mEHT plus Lipodox®, (P<0.05) (Figure 1D).
Size distribution and doxorubicin-release rate of Lipodox® unaffected by mEHT treatment
To investigate if mEHT treatment affects the liposomal size and structure of Lipodox®, Lipodox® was separated into groups and treated under three conditions: in a water bath at 37°C (control), in a water bath at 42°C, and with mEHT at 42°C for 30 minutes. Liposomal size was then measured by DLS. Size distributions showed no significant difference among the three groups, with 84.5±18.6 nm at 37°C, 67.2±15 nm in 42°C water bath, and 90.3±25.5 nm with mEHT incubation (Figure 2A). The release rate of doxorubicin concentration in medium also showed no significant difference among the three groups, with 4.24% at the 37°C water bath, 4.38% at the 42°C water bath, and 5.87% with mEHT treatment (Figure 2B). This result indicated that both HT treatment by water bath and mEHT did not affect the liposomal size and structure of Lipodox®. Therefore, we can confirm that the cytotoxic enhancement of mEHT plus Lipodox® was due to increased release of doxorubicin in medium.
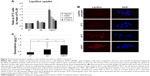
mEHT enhanced Lipodox® uptake by cancer cells in vitro and in vivo
Lipodox® uptake by cells (HepG2, A549, U87MG, and CT26) was detected by the intracellular fluorescence intensity of doxorubicin by using flow cytometry and fluorescence microscopy. As shown in Figure 3A, mEHT significantly increased the uptake of doxorubicin in HepG2 (3.4±0.7 fold to 37°C), A549 (2.2±0.8 fold to 37°C), U87MG (1.8±0.3 fold to 37°C), and CT26 (1.5±0.2 fold to 37°C) in vitro. Flow cytometric assay for detecting fluorescence intensity showed that after mEHT treatment, Lipodox® uptake was significantly increased in all cell lines compared with the 42°C water bath and 37°C control (Figure 3A). Immunofluorescence further showed the uptake of Lipodox® in HepG2 culture water bath at 37°C, in 42°C water bath, and with mEHT treated with 50 μM of Lipodox® for 30 minutes. As shown in Figure 3B, cells cultured with mEHT showed the highest fluorescence intensity compared with the 37°C and 42°C water bath groups, indicating elevated uptake under mEHT treatment.
To investigate the mEHT-increased Lipodox® uptake in vivo, CT26-bearing mice were separated into three groups to be treated with a water bath at 37°C (control), water bath at 42°C, and with mEHT at 42°C for 30 minutes after Lipodox® intravenous treatment (10 mg/kg). The tumors were dissected and the concentrations of doxorubicin were detected. Figure 3C shows the doxorubicin concentrations water bath at 37°C, in 42°C water bath, and with mEHT were 0.35±0.1, 0.79±0.32, and 1.44±0.32 (μg/g), respectively, indicating that mEHT significantly increased Lipodox® retention in tumors.
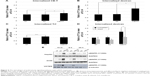
mEHT enhanced Lipodox® uptake by activation of macropinocytosis
Endocytosis pathways are subdivided into four categories: receptor-mediated endocytosis (clathrin-mediated endocytosis), caveolae-dependent endocytosis, macropinocytosis, and phagocytosis.22 To explore which pathway was utilized in mEHT-enhanced Lipodox® uptake, various inhibitors were utilized to block pathways. By blocking energy by supplementation with NaN3 (ATPase-inhibitor), both water bath and mEHT treatment showed a significant decrease of Lipodox® uptake (Figure 4A, P<0.01). Both CPZ (clathrin-mediated endocytosis-inhibitor) and wortmannin (macropinocytosis-inhibitor) significantly reduced the Lipodox® uptake in water bath and mEHT treatment (Figure 4B and C), whereas wortmannin induced a more inhibitory effect with mEHT treatment (P<0.01). Filipin (caveolae-mediated endocytosis-inhibitor) had no effect on both water bath and mEHT treatment groups (Figure 4D). These results indicated that the processes involved in translocation of Lipodox® across cancer cell membranes are energy-dependent and may include macropinocytosis.
The cytotoxic effect after Lipodox® plus HT treatment was further evaluated by wortmannin. Cytotoxicity of Lipodox® under water bath and mEHT treatment could be reversed by wortmannin (Figure 4E). Consistent with the observation of uptake inhibition, mEHT responded to wortmannin more significantly than water bath treatment (P<0.01 vs P<0.05, Figure 4E).
Various substrates for the investigation of endocytosis pathways
Multiple substrates were used for different pathways: cholera toxin subunit for caveolae-dependent endocytosis and transferrin for clathrin-dependent endocytosis were both utilized. Cholera toxin subunit was found to increase levels of substrate internalized significantly after mEHT treatment (Figure 5A, 1.21±0.08 fold compared to 37°C treatment, P<0.05). Transferrin uptake was significantly enhanced with 42°C water bath (Figure 5C, 1.18±0.05 fold compared to 37°C treatment, P<0.01). The substrate of macropinocytosis, dextran, was significantly increased (2±0.5 fold compared to 37°C treatment) in cancer cells after mEHT treatment (Figure 5B). Since the increased uptake was higher in dextran, we further investigated the effect with the macropinocytosis inhibitor, wortmannin. Wortmannin also significantly reduced the internalized dextran under mEHT treatment (Figure 5D). It has been reported that macropinocytosis can be stimulated by triggering tyrosine kinase receptor.23 The activation of EGFR after mEHT treatment was evaluated by Western blot. As shown in Figure 5E, EGFR was activated immediately from 10 minutes up to 6 hours and decreased in 24 hours after mEHT treatment. This result further confirmed that mEHT-induced endocytosis was macropinocytosis.
In vivo evaluation of therapeutic efficacy of the combination of Lipodox® and mEHT
The therapeutic effect of Lipodox® plus mEHT was further confirmed by a mouse cancer model. The effect of tumor growth inhibition was shown in Figure 6. There was no therapeutic effect in groups treated with water bath or with mEHT alone. Lipodox® alone inhibited tumor growth slightly. Lipodox® with water bath was found to increase tumor growth inhibition (P<0.05). Tumors treated with Lipodox® plus mEHT were significantly smaller than those of the control groups (untreated, with water bath alone, mEHT alone, Lipodox® alone, or Lipodox® plus water bath) (P<0.05).
Discussion
In this study, we found that mEHT could enhance the uptake of Lipodox® by disturbing physiologic activity within the cell membrane. A possible pathway to stimulate this endocytosis ability was also investigated. Uptake of particles is limited to a specific size during mEHT treatment. Uptake enhancement is positively related to the killing effect of cancer cells. Our in vivo study also showed a similar enhancement and therapeutic effect.
To the best of our knowledge, this is the first report that directly shows the enhancement of cellular uptake ability of clinical grade Lipodox®. Lipodox® is widely used for the treatment of gynecologic cancer including cervical and ovarian cancers. However, the efficacy of Lipodox® in clinical bedside settings is not as high as expected based on clinical development findings. Although several studies reported that HT could enhance the therapeutic effect of Lipodox®, these studies focused on the tumor liposome extravasation induced by HT.24,25 In this report, we utilized mEHT as a physical method to enhance the cellular uptake of Lipodox® in cancer cells, significantly increasing the killing effect on cancer cells. These findings show promise for future clinical applications.
The cell membrane is one important target for mEHT.17 Due to the specific design of mEHT, the structure of lipid bilayer membranes serves as a good insulator for the cell from the electric field generated by mEHT. The heating of mEHT applies forward energy selectively to the most ionized areas in the tumor microenvironment. The RF current, which flows through the cancerous lesion of ionized areas, was electrically concentrated by its lower impedance. The mEHT generated energy is captured by the extracellular part of the cell membrane.17 The membrane rafts, which are a cluster of functional proteins, have different electromagnetic properties when compared with other parts of the cell membrane. This property allows membrane rafts to absorb more mEHT energy than other lipid bilayer parts of the cell membrane. The higher energy absorption induces elevation of temperature on rafts during mEHT treatment. Therefore, the energy of mEHT could disrupt the membrane arrangement and enhance particles of specific size to penetrate cancer cells.
Conventional HT has been reported to induce hyperpermeable tumor vasculature environment that enhances the therapeutic efficacy of both Doxil and Lipodox®.26 Conventional HT can induce a selective intratumoral accumulation of liposomal drugs, while HT increases vascular permeability in the tumor vasculature.27 Conventional HT may also trigger the release of extravascular liposomal drugs from the liposomal structure, but the release of Doxil may increase the risk of toxicity in healthy tissues. For conventional HT as a treatment consideration for regional tumors, the temperature may need to rise to a higher level (above 41°C) to achieve intravascular drug release. This treatment lacks the selectivity for tumor tissues, thus increasing the possibility of side effects and toxicity to surrounding healthy tissues. Moreover, when compared to mEHT, conventional HT cannot enhance the uptake of Lipodox® by tumor cells directly. mEHT may indicate a particular therapeutic benefit for tumor cells that have more resistance to chemotherapy by inhibiting transportation across the membrane.
Macropinocytosis has been considered as growth factor-induced and actin-driven endocytosis that transfers particles or fluid from outside the cell into cytosol.22 When compared with other cellular endocytosis pathways, macropinocytosis takes up particles in a nonspecific manner and could occur in most cells. This kind of endocytosis is usually triggered by outside stimulations that induce the activation of receptor tyrosine kinase. In this report, we showed that mEHT could be a stimulator to excite EGFR and may induce the formation of membrane ruffles. Dextran testing also confirmed that this mEHT-induced macropinocytosis is nonspecific endocytosis.
It has been reported that apoptosis can be induced in cancer cells by mEHT treatment alone.12,28,29 The death receptors and caspase signaling pathway are activated in cancer cells after mEHT treatment. Although monotherapy with mEHT is not very promising in clinical applications, the ability of apoptosis induction by mEHT may contribute to the enhancement of cancer cell killing effect when doxorubicin is released from the liposome within cancer cells. Previous studies have shown that thermosensitive liposome-encapsulated doxorubicin (TLED) could achieve a similar therapeutic effect in doxorubicin-resistant cancer cells.30 However, TLED still focuses on a local release of doxorubicin in the tumor site, which differs from our findings of enhanced cellular uptake of doxorubicin. Vujaskovic et al have reported neoadjuvant therapy with Lipodox® and HT as a feasible and well-tolerated treatment strategy in locally advanced breast cancer.31 Their results are consistent with our animal study which showed that conventional HT (water bath) could elevate the therapeutic effect of Lipodox®. We found that mEHT enhanced the effect further.
There are several approaches to enhance the cellular uptake of doxorubicin through various methods of drug-loaded nanoparticles. Two recent studies have shown that treatment with MoS2 nanoparticles followed by photothermal therapy could accelerate drug release and increase efficacy of cancer treatment with doxorubicin.32,33 The application of thermosensitive liposomal doxorubicin and mild HT produced by a clinical high intensity focused ultrasound system has undergone a clinical trial that showed improved anti-tumor efficacy.34,35 These studies have shown the promise of utilizing physics-based therapies in combination with nanoparticle drugs for the treatment of cancer. Our studies also confirmed the value of this treatment approach. Furthermore, the use of clinically-approved Lipodox® and mEHT can have immediate clinical bedside applications without further need for trials.
Conclusion
mEHT treatment was designed to focus on low pH areas in tissues, but mEHT did not induce doxorubicin release from liposomes in the extracellular matrix. mEHT stimulated the activity of receptors and enzymes of cancer cells. Consequently, the uptake of Lipodox by cancer cells significantly increased after mEHT treatment and effectively enhanced the therapeutic effect of the drug. This novel finding warrants further study for application to clinical cancer therapy.
Acknowledgments
The authors would like to thank Mr Winston Han and Dr Tony Tsoi for their editing and proofing of the manuscript. This study was supported by the research fund from the Department of Radiation Oncology, Ditmanson Medical Foundation Chiayi Christian Hospital, Chiayi, Taiwan.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Andocs G, Renner H, Balogh L, Fonyad L, Jakab C, Szasz A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther Onkol. 2009;185(2):120–126. | ||
Szasz A, Vincze G, Szasz O, Szasz N. An energy analysis of extracellular hyperthermia. Electromagn Biol Med. 2003;22(2–3):103–115. | ||
Andocs G, Szasz O, Szasz A. Oncothermia treatment of cancer: from the laboratory to clinic. Electromagn Biol Med. 2009;28(2):148–165. | ||
Szasz A, Szasz N, Szasz O. Oncothermia: a new kind of oncologic hyperthermia. Oncothermia: Principles and Practices. Netherlands: Springer; 2010:173–392. | ||
Szasz A, Vincze G. Dose concept of oncological hyperthermia: heat-equation considering the cell destruction. J Can Res Ther. 2006;2(4):171–181. | ||
Hegyi G, Szigeti GP, Szász A. Hyperthermia versus Oncothermia: cellular effects in complementary cancer therapy. Evid Based Complement Alternat Med. 2013;2013(4):1–12. | ||
Gadaleta-Caldarola G, Infusino S, Galise I, et al. Sorafenib and locoregional deep electro-hyperthermia in advanced hepatocellular carcinoma: a phase II study. Oncol Lett. 2014;8(4):1783–1787. | ||
Fiorentini G, Giovanis P, Rossi S, et al. A phase II clinical study on relapsed malignant gliomas treated with electro-hyperthermia. In Vivo. 2006;20(6A):721–724. | ||
Szasz A. Current status of oncothermia therapy for lung cancer. Korean J Thorac Cardiovasc Surg. 2014;47(2):77–93. | ||
Andocs G, Szasz O, Szasz A. Oncothermia treatment of cancer: from the laboratory to clinic. Electromagn Biol Med. 2009;28(2):148–165. | ||
Hegyi G, Szigeti GP, Szász A. Hyperthermia versus Oncothermia: cellular effects in complementary cancer therapy. Evid Based Complement Alternat Med. 2013;2013(4):1–12. | ||
Yang KL, Huang CC, Chi MS, et al. In vitro comparison of conventional hyperthermia and modulated electro-hyperthermia. Oncotarget. 2016;7(51):84082–84092. | ||
Andocs G, Rehman MU, Zhao Q-L, Papp E, Kondo T, Szasz A. Nanoheating without artificial nanoparticles Part II. Experimental support of the Nanoheating concept of the modulated Electro-Hyperthermia method, using U937 cell suspension model. Biology and Medicine. 2015;07(04):247. | ||
Szasz A. Challenges and solutions in oncological hyperthermia. Thermal Medicine. 2013;29(1):1–23. | ||
Zhao M, Ding XF, Shen JY, Zhang XP, Ding XW, Xu B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. J Zhejiang Univ Sci B. 2017;18(1):15–26. | ||
Gabizon AA, Patil Y, La-Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Updat. 2016;29:90–106. | ||
Dicheva BM, Koning GA. Targeted thermosensitive liposomes: an attractive novel approach for increased drug delivery to solid tumors. Expert Opin Drug Deliv. 2014;11(1):83–100. | ||
Shafei A, El-Bakly W, Sobhy A, et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed Pharmacother. 2017;95:1209–1218. | ||
Andocs G, Rehman MU, Zhao QL, Tabuchi Y, Kanamori M, Kondo T. Comparison of biological effects of modulated electro-hyperthermia and conventional heat treatment in human lymphoma U937 cells. Cell Death Discov. 2016;2(1):16039. | ||
Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. | ||
Chang CH, Stabin MG, Chang YJ, et al. Comparative dosimetric evaluation of nanotargeted (188)Re-(DXR)-liposome for internal radiotherapy. Cancer Biother Radiopharm. 2008;23(6):749–758. | ||
Kou L, Sun J, Zhai Y, He Z. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J Pharmaceut Health Sci. 2013;8(1):1–10. | ||
Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. | ||
Kong G, Anyarambhatla G, Petros WP, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60(24):6950–6957. | ||
Heidarli E, Dadashzadeh S, Haeri A. State of the art of Stimuli-Responsive liposomes for cancer therapy. Iran J Pharm Res. 2017;16(4):1273–1304. | ||
Li L, Ten Hagen TL, Haeri A, et al. A novel two-step mild hyperthermia for advanced liposomal chemotherapy. J Control Release. 2014;174:202–208. | ||
Li L, Ten Hagen TL, Bolkestein M, et al. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J Control Release. 2013;167(2):130–137. | ||
Tsang YW, Huang CC, Yang KL, et al. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer. 2015;15(1):708. | ||
Meggyeshazi N, Andocs G, Balogh L, et al. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther Onkol. 2014;190(9):815–822. | ||
Merlin JL, Marchal S, Ramacci C, Notter D, Vigneron C. Antiproliferative activity of thermosensitive liposome-encapsulated doxorubicin combined with 43 degrees C hyperthermia in sensitive and multidrug-resistant MCF-7 cells. Eur J Cancer. 1993;29A(16):2264–2268. | ||
Vujaskovic Z, Kim DW, Jones E, et al. A phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperthermia. 2010;26(5):514–521. | ||
Yang H, Zhao J, Wu C, Ye C, Zou D, Wang S. Facile synthesis of colloidal stable MoS2 nanoparticles for combined tumor therapy. Chem Eng J. 2018;351:548–558. | ||
Zhao J, Xie P, Ye C, et al. Outside-in synthesis of mesoporous silica/molybdenum disulfide nanoparticles for antitumor application. Chem Eng J. 2018;351:157–168. | ||
Staruch RM, Hynynen K, Chopra R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: therapeutic effect in rabbit VX2 tumours. Int J Hyperthermia. 2015;31(2):118–133. | ||
Lyon PC, Griffiths LF, Lee J, et al. Clinical trial protocol for TARDOX: a phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. J Ther Ultrasound. 2017;5(1):28. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.