Back to Journals » Infection and Drug Resistance » Volume 11
Noninvasive screening identifies patients at risk for spontaneous bacterial peritonitis caused by multidrug-resistant organisms
Authors Ferstl PG , Müller M, Filmann N, Hogardt M, Kempf VAJ, Wichelhaus TA, Lange CM, Vermehren J, Zeuzem S, Reinheimer C, Waidmann O
Received 28 April 2018
Accepted for publication 29 June 2018
Published 2 November 2018 Volume 2018:11 Pages 2047—2061
DOI https://doi.org/10.2147/IDR.S172587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Philip G Ferstl,1,2 Mona Müller,1 Natalie Filmann,3 Michael Hogardt,2,4,5 Volkhard AJ Kempf,2,4,5 Thomas A Wichelhaus,2,4,5 Christian M Lange,1,2 Johannes Vermehren,1,2 Stefan Zeuzem,1,2 Claudia Reinheimer,2,4,5 Oliver Waidmann1,2
1Department for Internal Medicine I/Gastroenterology and Hepatology, University Hospital Frankfurt, Frankfurt am Main, Germany; 2University Center for Infectious Diseases (UCI), University Hospital Frankfurt, Frankfurt am Main, Germany; 3Institute of Biostatistics and Mathematical Modeling, University Hospital Frankfurt, Frankfurt am Main, Germany; 4Institute of Medical Microbiology and Infection Control, University Hospital Frankfurt, Frankfurt am Main, Germany; 5University Center of Competence for Infection Control at the Universities Frankfurt, Giessen, and Marburg, Frankfurt am Main, State of Hesse, Germany
Background and aims: Spontaneous bacterial peritonitis (SBP) is a severe complication of decompensated cirrhosis. The prevalence of multidrug-resistant organisms (MDROs) in patients with cirrhosis is increasing. Identification of patients at risk for SBP due to MDROs (ie, SBP with the evidence of MDROs or Stenotrophomonas maltophilia in ascitic culture, MDRO-SBP) is crucial to the early adaptation of antibiotic treatment in such patients. We therefore investigated whether MDROs found in ascitic cultures can also be found in specimens determined by noninvasive screening procedures.
Patients and methods: This retrospective study was conducted at the liver center of the University Hospital Frankfurt, Germany. Between 2011 and 2016, patients with cirrhosis were included upon diagnosis of SBP and sample collection of aerobic/anaerobic ascitic cultures. Furthermore, the performance of at least one complete MDRO screening was mandatory for study inclusion.
Results: Of 133 patients diagnosed with SBP, 75 (56.4%) had culture-positive SBP and 22 (16.5%) had MDRO-SBP. Multidrug-resistant Escherichia coli (10/22; 45.5%) and vancomycin-resistant enterococci (7/22; 36.4%) resembled the major causatives of MDRO-SBP. Rectal swabs identified MDROs in 17 of 22 patients (77.3%) who developed MDRO-SBP with a time-dependent sensitivity of 77% and 87% after 30 and 90 days upon testing, while negative predictive value was 83% and 76%, respectively. The majority of patients were included from intensive care unit or intermediate care unit.
Conclusion: MDRO screening may serve as a noninvasive diagnostic tool to identify patients at risk for MDRO-SBP. Patients with decompensated cirrhosis should be screened for MDROs from the first day of inpatient treatment onward.
Keywords: multidrug resistance, liver cirrhosis, ascites, screening routine, antibiotic therapy
Plain language summary
Patients with liver cirrhosis are prone to develop bacterial infections, which mitigate quality of life and survival. The occurrence of multidrug-resistant organisms (MDROs) puts these patients at additional risk and is therefore a severe life event. Since repetitive antibiotic therapies promote the occurrence of MDROs due to further selection pressure, the antibiotic regimen needs to be chosen carefully based on individual patient data. Therefore, the identification of patients at risk for infections caused by MDROs is vital. MDROs are mostly harbored in the large intestine or on the skin and can easily be diagnosed using screening swabs.
Spontaneous bacterial peritonitis (SBP) is the second most frequent bacterial infection in patients with cirrhosis and ascites and is caused by intestinal bacteria migrating from the gut to the abdomen. Due to the late onset of symptoms, diagnosis may be delayed, and special attention is warranted in the care of these patients. So far, studies determining the outcome of SBP caused by MDROs are scarce. We retrospectively compiled a cohort of patients with SBP who underwent at least one complete MDRO screening (rectal, nasopharyngeal and cutaneous). In this study, we described the clinical impact of ascitic MDRO detection and aimed to assess the utility of such noninvasive screening to identify patients at risk for SBP caused by MDROs.
Introduction
The prevalence of multidrug-resistant organisms (MDROs) in patients with cirrhosis is rapidly increasing.1,2 Bacterial infections have a devastating clinical impact in patients with decompensated cirrhosis, including prolonged and repeated hospitalization as well as reduced survival.3–5 Spontaneous bacterial peritonitis (SBP) is the second most common bacterial infection in patients with cirrhosis and ascites.6 Studies from different continents have determined cirrhotic patients’ outcome as particularly poor in the case of infection by MDROs.2,3,7–13
MDRO-mediated infections in cirrhosis have mostly been attributed to multidrug-resistant gram-negative bacteria (MDRGN).14,15 Secondary SBP prophylaxis with fluoroquinolones has been recommended for more than 2 decades, but multidrug resistance narrows therapeutic options.15–18 In addition, non-fermenting gram-negative bacteria expressing multidrug resistance, such as Stenotrophomonas maltophilia, might bloom under recurrent administration of antimicrobials.4,19,20 In addition, gram-positive pathogens such as enterococci are increasingly contributing to the poor prognosis of SBP, since patients with cirrhosis are at high risk to acquire vancomycin-resistant enterococci (VRE).15,21–23 The burden and spread of VRE are associated with extensive and repetitive antibiotic therapies.24,25 The occurrence of these pathogens is being promoted by intestinal dysbiosis, putting patients with cirrhosis at high risk for acquiring SBP by MDROs (ie, SBP with the evidence of MDROs or S. maltophilia in ascitic culture, MDRO-SBP).19,20
Among the entities of bacterial infection in cirrhosis, SBP is particularly harmful due to the late onset of symptoms, risk of recurrence and the wide spectrum of causative bacteria.4,10,15,21,22,26 To guarantee optimal patient care and, at the same time, minimize selective pressure on further MDRO development, an individualized antibiotic regimen based on concise patient-specific data may be warranted. Therefore, the anticipation of expectable pathogens would be vital. Since SBP is derived from the intestinal microflora, it seems feasible to investigate whether these pathogens may be detected earlier by noninvasive procedures. In this retrospective study, we assessed the diagnostic utility of rectal, nasopharyngeal and cutaneous MDRO screening in a cohort of patients with decompensated cirrhosis, and we described the microbiological characteristics and clinical impact of MDRO-SBP in a tertiary liver transplant center in Germany.
Patients and methods
Study characterization
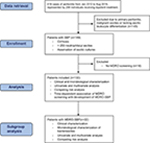
This study was conducted at the liver transplant center at University Hospital Frankfurt (UHF), Germany. From an electronic database, we retrieved all cases coded as “acute peritonitis” (ICD codes such as K65.0, K65.8 and K65.9) from 2012 to 2016. We retrieved 419 cases in total, which are represented by 299 individuals receiving inpatient treatment in the given period. Inclusion criteria were as follows: 1) diagnosis of SBP as defined by polymorphonuclear leukocyte count of >250/µL in ascites in patients with cirrhosis. Patients were included upon the first SBP episode diagnosed at UHF. 2) Sample collection of at least one pair of aerobic/anaerobic ascitic cultures at the bedside at the time of diagnosis of SBP. 3) Complete MDRO screening (rectal, throat, skin, nose swabs). Exclusion criteria were as follows: patients with primary peritonitis or malignant ascites due to any abdominal metastases or peritoneal carcinosis. Biometric, clinical, laboratory and microbiological data as well as empiric antibiotic therapy were collected at the date of study inclusion. If more detailed patient characterization was deemed necessary, the corresponding data were retrieved at defined time points after study inclusion. All the patient data were retrieved from electronic patient charts. Importantly, further microbiological data were collected from study inclusion until death or lost to follow up, resulting in multiple microbiological test results for most patients.
Colonization, infection and case definition
If MDROs were detected only in rectal, throat or cutaneous swabs, patients were defined as “colonized”. If the MDRO strain was detected only or additionally in ascites, blood, urine, bronchial or pleural secretion, bile, pus, wounds or surgical sites, on medical devices or catheters, the patient was classified as “infected” by the strain. “Nosocomial SBP” was defined as SBP diagnosed at least 3 days after hospitalization according to the current definition27,28 or as SBP acquired up to 7 days after the last hospital discharge. Sepsis was defined as suspected or evident bacterial infection combined with two of the following clinical parameters according to the quick sequential organ failure assessment (qSOFA) score29: hemodynamic instability with systolic blood pressure below 100 mmHg (including patients receiving noradrenaline with systolic blood pressure >100 mmHg), respiratory rate >22/min or PaCO2 <32 mmHg and/or altered mental status.
Screening for MDROs at UHF
Screening routine was performed for MDRGN, VRE and methicillin-resistant Streptococcus aureus (MRSA). No regular screening was scheduled for S. maltophilia. MDRO screening was performed according to the infection control protocol at UHF which again is according to the German infection protection law (§23, Infektionsschutzgesetz30). Screening measures apply to, eg, patients arriving from hospitals in high prevalence countries/high prevalence local hospitals for MDRGN and/or MRSA as well as patients admitted to intensive care unit/intermediate care unit (ICU/IMC) on the day of admission. In addition, MDRO screening is scheduled weekly on ICU and IMC at UHF, and patients included may therefore have undergone repetitive MDRO screening. As of 2011–2016, no regular MDRO screening was scheduled on normal hepatologic wards.
Definition of MDRO-SBP
MDRO-SBP was defined as SBP due to MDRGN, VRE and MRSA as well as due to S. maltophilia. Although S. maltophilia intrinsically exhibits a broad spectrum of antibiotic resistance, eg, against antibiotic resistance, eg against carbapenems, it does not genuinely qualify to be included in the collective term “MDRO”. However, S. maltophilia, MDRGN, VRE and MRSA all require treatment with broad-spectrum antibiotics. We therefore decided to include S. maltophilia in the collective term “MDRO-SBP”.
Definition of MDRGN
MDRGN were defined as Enterobacteriaceae with extended-spectrum ß-lactamase (ESBL) phenotype as well as Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter baumannii resistant against piperacillin, any third- or fourth-generation cephalosporin and fluoroquinolone resistance (QR) ± CR.31,32
Detection of MDROs and molecular resistance analysis
All laboratory procedures were performed under quality-controlled criteria (laboratory accreditation according to ISO 15189:2007 standards; certificate number D-ML-13102-01-00, valid through January 25, 2021). To identify MDRGN and VRE, rectal swabs were collected using culture swabs with Amies collection and transport medium (Hain Lifescience, Nehren, Germany) and streaked onto selective CHROMagar ESBL plates (Mast Diagnostica, Paris, France) and ChromID VRE agar (bioMérieux, Nürtingen, Germany), respectively. Species identification was performed by matrix-assisted laser desorption ionization–time-of-flight analysis (VITEK MS; bioMérieux). Antibiotic susceptibility testing was performed according to Clinical and Laboratory Standards Institute guidelines using VITEK 2 and/or antibiotic gradient tests (bioMérieux). In the case of gram-negative CR isolates, the detection of genes encoding carbapenemases was routinely performed via PCR analysis and subsequent sequencing including the bla genes for carbapenemases NDM, VIM, IMP, OXA-48 like, and KPC as well as OXA-23, OXA-24 and OXA-58 for Acinetobacter baumannii.33,34 For the detection of MRSA, moistened nasal swabs were inoculated on Brilliance MRSA Agar (Oxoid, Wesel, Germany), and identification and antimicrobial susceptibility testing were performed as described earlier. The detection of MDROs from screening and microbiological culture of clinical specimens (eg, ascites) were performed according to German microbiological procedure quality standards (MiQ), for example, current versions such as MiQ 2, 3a/b (2007), MiQ 25 (2006), MiQ 32 (2012).
Ethics statement
For this retrospective study, approval from the clinical ethics committee of the Goethe University Frankfurt was obtained prior to research (file number: 391/15). The database was anonymized, and informed consent was waived due to the retrospective nature of the study.
Statistical analysis
BiAS software (v. 11.06; Epsilon-Verlag, Darmstadt, Germany) and R (v. 3.2.0, R Core Team [2015], R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria) were used for statistical calculations. Fatal outcome due to sepsis, fatal outcome due to other causes and the appearance of MDRO-SBP were defined as end points, and patients who did not reach an end point were censored from the last day of inpatient treatment. Model for End-stage Liver Disease (MELD), Albumin–Bilirubin (ALBI), Child–Pugh–Turcotte and Chronic Liver Failure–Acute Decompensation (CLIF-AD) scores were calculated with the laboratory parameters taken at the day of SBP detection. Standard deviation (SD) was calculated for age, MELD and CLIF-AD scores and laboratory parameters. SE of the mean was calculated for all observational intervals given in days. Clinical scores were compared using the Wilcoxon–Mann–Whitney U-test. Kaplan–Maier curve was employed for the display of comparative survival analysis, and Cox–Mantel chi-squared test was used for the calculation of HR and P-values. Considering the appearance of MDRO-SBP as the event of interest, fatal outcome due to sepsis and fatal outcome due to other causes than sepsis were defined as competing risks, using the Aalen–Johansen estimator. In case that death fatal due to sepsis was considered as the event of interest, fatal outcome due to other causes than sepsis was defined as competing risk. Independent-risk factors for death were calculated using the multivariate Cox regression model. For calculating unbiased sensitivity and specificity for censored data with competing risks, the method proposed by Blanche et al35 was utilized.
Results
Clinical characterization of the cohort
One hundred and thirty-three patients diagnosed with SBP between December 2011 and August 2016 were included in the analysis (Figure S1). Of these, 72 (54.1%) were positive for MDRO colonization or infection in any location, while MDRO-SBP was diagnosed in 22 patients (16.5%). Biometric and laboratory parameters as well as liver scores (Child–Pugh–Turcotte, MELD, CLIF-AD, ALBI) did not differ between patients with or without the detection of MDROs or MDRO-SBP, respectively. Clinical features of hepatic decompensation were equally distributed (Table 1). MELD score and ICU admission at the time of SBP diagnosis were independent predictors of survival, and MELD, CLIF-AD and ALBI scores were higher in patients who died than those in patients who were lost to follow up (Table S1). Patients with MDRO-SBP were at increased risk of death (HR =2.17, P=0.007) and were more likely to develop lethal sepsis (HR =5.67, P<0.001). While MELD and CLIF-AD scores were marginally better in patients with culture-negative SBP, severe clinical courses due to bacterial infections were more frequent in patients with MDRO-SBP (Figure 1). As calculated by multivariate regression, liver scores or clinical features were not associated with the occurrence of MDRO-SBP.
  | Figure 1 Competing risk analysis of 22 patients with MDRO-SBP compared to 111 patients without MDRO-SBP. Notes: Patients were included from the day of first SBP detection, and death due to sepsis and death due to other reasons were defined as competing risks. Lethal sepsis was significantly increased in patients with MDRO-SBP (HR =5.67, P<0.001). Of note, MDRO-SBP was included as a time-dependent variable in this analysis. Therefore, the number at risk cannot be displayed in this plot, since the time-dependent calculation has been performed with censored data.35 MDRO-SBP, SBP with the evidence of MDROs or S. maltophilia in ascitic culture. Abbreviations: MDRO, multidrug-resistant organism; SBP, spontaneous bacterial peritonitis; S. maltophilia, Stenotrophomonas maltophilia. |
Microbiological characterization of the cohort
The first MDRO screening was performed before or upon study inclusion in 105/133 patients (78.9%) and in timely fashion in the rest of the patients, resulting in MDRO detection in the majority of patients (Figure 2A and B). Among 72/133 patients with MDROs, MDRGN were detected in 42/133 cases (31.6%), VRE in 50/133 cases (37.6%) and MRSA in 5/133 cases (3.8%). Colonization – defined as positivity for MDRO screening without detection in invasive materials – was detected in 16/42 patients (38.1%) with MDRGN, 39/50 patients (78%) with VRE and 2/5 patients (40%) with MRSA. MDRO-associated ascitic or extra-ascitic infection was found in the remaining patients within the body compartments as given in Table 2. Multiple MDRO strains were detected within 24 individuals, resulting in 22 patients tested positive for MDRGN and VRE, one patient for MRSA and VRE and one patient with MDRGN, MRSA and VRE.
Microbiological characterization of SBP and MDRO-SBP
Ascitic culture was positive in 60/133 patients (45.1%) at first paracentesis and in 75/133 patients (56.4%) altogether. Gram-positive bacteria were detected in 29/75 patients (38.7%), followed by gram-negative bacteria in 25/75 patients (33.3%). Both gram-positive and gram-negative bacteria were found in 21/75 patients (28%), which were due to sequential detection in 10/21 patients (47.6%) and simultaneous, ie, polymicrobial SBP, in 11/21 patients (52.4%). In these patients, abdominal perforation as a cause for peritonitis had been ruled out by computed tomography. Appropriate initial antibiotic therapy according to ascitic resistance profile was administered in 41/60 patients (68.3%) with positive first ascitic culture. Patients carrying MDROs yielded positive ascitic cultures more frequently (52/72 cases, 72.2%; HR =14.6, P<0.001), demonstrating that in these patients ascitic MDRO presence was also more likely to be detected.
In 22/133 patients, MDROs or S. maltophilia were detected in ascitic cultures during at least one episode. This was the case upon the first diagnosis of SBP in 15/22 patients (68.2%), while MDRO-SBP as a recurring episode of SBP occurred in 7/22 patients (31.8%) with an estimated time-dependent probability of 14% (standard error [SE] =1.4%) after 60 days and 28% (SE =2%) after 90 days, respectively. In the cases of MDRO-SBP after study inclusion, mean MELD score did not differ from baseline MELD. MDRO-SBP was nosocomial in 15/22 cases (68.2%). In 13/22 patients with MDRO-SBP (59.1%), further MDROs were detected in other compartments than ascites (Table S2).
Assessing the occurrence of MDRO-SBP upon rectal screening
Since SBP originates from the intestinal microflora, we reasoned that rectal MDRO screening might reveal causative to pathogens in patients with MDRO-SBP. Among all patients with MDRO-positive ascitic culture, the identical strain could be detected in other body compartments; rectal swabs being the most frequent localization with 17/22 cases (77.3%; Table 3). Rectal, cutaneous or nasopharyngeal screening identified the corresponding pathogens in 20/22 cases (90.9%). Of note, one of these did not undergo rectal MDRO screening. The estimated time-dependent probability for the occurrence of MDRO-SBP upon the first positive rectal MDRO screening was 11.5% (SE =2.8%) after 30 days and 12.5% (SE =3%) after 60 days, respectively. Time-dependent sensitivity of MDRO screening before the occurrence of MDRO-SBP was 77% after 30 days and 87% after 90 days, while negative predictive value (NPV) was 83% and 76%, respectively (Table S3; Figure S2).
Antibiotic treatment and secondary prophylaxis
Standard empirical therapy for community-acquired SBP at UHF is intravenous ceftriaxone, while the standard of care for nosocomial SBP is a carbapenem such as meropenem or imipenem. Based on the individual microbial data, diverging antibiotic regimens may have been chosen, and inappropriate initial therapy resulted in decreased survival in 19/60 (31.7%) patients with culture-positive SBP (HR =1.92, P=0.014). Initial treatment of MDRO-SBP comprised of carbapenems in 16/22 patients (72.3%), cephalosporins and piperacillin/tazobactam in 2/22 patients (9.1% each), and a fluoroquinolone in one patient (4.5%). Initial treatment furthermore composed of glycopeptides, oxazolidinones or tigecycline in four of eight patients with the diagnosis of VRE-associated MDRO-SBP. Among these cases, resistance against one or more of these agents was detected in four of eight patients (50%; Table 4), while all cases of MDRO-SBP associated with VRE displayed QR expression. If necessary, antibiotic regimens were adapted immediately according to microbiological findings. Nevertheless, inadequate initial antibiotic therapy was associated with highly increased risk of death in MDRO-SBP patients (14/22 patients, 63.6%, HR =11.4, P=0.002). Clinical courses did not differ notably between different causatives of MDRO-SBP (Table S4).
One hundred and five patients (78.9%) could be discharged after first inpatient treatment. Secondary antibiotic prophylaxis as recommended by the current hepatologic guidelines34 was prescribed in 86/105 patients (81.9%; Table S5). Recurrent SBP occurred in 35/105 patients (33.3%), and ascitic evidence of bacteria expressing QR was found in 13 of these patients (37.1%) and in 36/133 patients in total (27.1%). This was due to Enterococcus spp. or VRE in 16/36 cases (44.4%), MDRGN in 8/36 cases (22.2%), MRSA in one case (2.8%) and due to others (other pathogens expressing QR composed of coagulase-negative Staphylococcus spp. [four patients], K. pneumoniae [two patients] and Clostridium tertium, E. coli, S. aureus, Streptococcus mitis and S. maltophilia [one patient each]) in 11/36 cases (30.6%). The estimated time-dependent probabilities for recurring SBP were 9.6% (SE =2.8%) after 30 days, 15.7% (SE =3.5%) after 60 days and 17.9% (SE =3.8%) after 90 days, and MDRO-SBP was more likely to occur in patients with recurrent SBP (HR =2.82, P=0.001).
Discussion
The global incidence of MDRO-SBP, especially caused by pathogens expressing resistance to cephalosporins and fluoroquinolones, is a growing health threat.36 We showed that accurate microbiological screening measures helped identify patients who developed MDRO-SBP. These patients are at high risk of death due to sepsis. Although the study was designed in a retrospective fashion, our findings are consistent and clinically substantial due to the following arguments.
First, patients with and without MDRO detection had similar baseline parameters, thus resembling a homogenous population in terms of the severity of disease. Second, survival was impaired if the initial empiric antibiotic therapy did not cover the indicated pathogens, which is in line with previous findings and shows that lethality was mainly associated with bacterial infections.37,38 Third, while liver scores and survival in patients with culture-negative SBP were comparable to culture-positive patients, lethal sepsis was less frequent (Table 1).
Regarding patients with culture-negative SBP, one might argue that cases of ascitic MDRO presence might have been missed in some of these patients. However, It is well known that the sensitivity of ascitic cultures is only around 50%,18 a fact that is reflected by our findings and has to be dealt with throughout the management of SBP. Nevertheless, MDRO-SBP was exclusively detected in patients with carriage of MDROs in other body compartments as well, while patients negative for MDROs also had significantly less positive ascitic cultures (HR =0.07, P<0.001). Considering the significantly better outcome in regard of septic complications in these patients, who are regularly treated with ceftriaxone, these SBP cases have to be considered as nonresistant rather than resistant.
Time-dependent sensitivity of MDRO screening for developing MDRO-SBP was between 77% and 95% including a high NPV, while specificity of the test was only around 50% (Table S3). Thus, negative MDRO screening might rule out MDRO-SBP with a high likelihood. Due to its low specificity and positive predictive value (PPV), though, repetitive diagnostic paracentesis including microbiological sample collection remains mandatory. Patients with nosocomial SBP should receive empiric antibiotic therapy according to local resistance patterns.28 A more “narrow” antibiotic therapy might be justified if MDRO screening was negative, but prospective data are warranted. Notably, the first MDRO evidence was made by the time of diagnosis of MDRO-SBP in almost half of the patients, since no screening results had been available beforehand.
Based on these observations, the ascitic detection of MDROs resembles a major prognostic factor in patients with decompensated cirrhosis.11,36 Because these patients are afflicted by rapid deterioration and death, early MDRO detection is vital.37,38 Since directional spread of MDROs goes from the intestine or skin into the abdomen (and not vice versa), it would be appropriate to screen for MDRO colonization in patients with risk or even history of SBP. Our data give the first evidence that this approach might be valid in the clinical setting and suggest that MDRO screening should be conducted on the day of patients’ admission. However, a study including a larger sample size of patients with MDRO-SBP is warranted.
Conclusion
Due to the retrospective design of the study, it must be stressed that prospective data are warranted to validate the accuracy of MDRO screening in association with MDRO-SBP. Therefore, general treatment recommendations cannot be made based on our findings. Furthermore, patients were mainly recruited from ICU/IMC. This translates into comparably high liver scores upon inclusion and an overall reduced survival during inpatient treatment as well as under secondary prophylaxis.39 Yet, differences between patients with and without MDRO-SBP were significant and comprehensible. Importantly, microbiological resistance data might depend on local resistance patterns, which is reflected by a high proportion of VRE, and may therefore not be transferable unrestrictedly. However, our microbiological data are comparable to reports from other mono-center studies.1,2,11–14,21,22,40 On a more general basis, we suggest a prospective multicenter setting to determine the long-term clinical impact of MDRO-SBP and the benefit of MDRO screening in patients with decompensated cirrhosis.
Author contributions
Compiling data, data interpretation and drafting and writing the manuscript: PGF, MM and OW: Biostatistical analysis and interpretation: PGF and NF. Data interpretation and critical review of the manuscript: SZ, VAJK, MH, TAW, CML, JV, CR and OW. All the authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
SZ has provided consultancy for Abbvie, BMS, Gilead, Intercept, Janssen and Merck (all outside the scope of this study). OW has provided consultancy for Amgen, Bayer, BMS, Celgene, Eisai, Merck, Novartis, Roche, Servier and Shire (all outside the scope of this study). VAJK was partially supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, research unit 2251). The authors report no other conflicts of interest in this work.
References
Tandon P, Delisle A, Topal JE, Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10(11):1291–1298. | ||
Fernández J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55(5):1551–1561. | ||
Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56(6):2328–2335. | ||
Garcia-Tsao G. Current Management of the Complications of Cirrhosis and Portal Hypertension: Variceal Hemorrhage, Ascites, and Spontaneous Bacterial Peritonitis. Dig Dis. 2016;34(4):382–386. | ||
Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4):1246–1256. | ||
Singal AK, Salameh H, Kamath PS. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalised patients in the United States. Aliment Pharmacol Ther. 2014;40(1):105–112. | ||
Ferstl PG, Filmann N, Brandt C, et al. The impact of carbapenem resistance on clinical deterioration and mortality in patients with liver disease. Liver Int. 2017;37(10):1488–1496. | ||
Waidmann O, Kempf VA, Brandt C, Zeuzem S, Piiper A, Kronenberger B. Colonisation with multidrug-resistant bacteria is associated with increased mortality in patients with cirrhosis. Gut. 2015;64(7):1183–1184. | ||
Salerno F, Borzio M, Pedicino C, et al. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017;37(1):71-79. | ||
Lutz P, Nischalke HD, Strassburg CP, Spengler U. Spontaneous bacterial peritonitis: The clinical challenge of a leaky gut and a cirrhotic liver. World J Hepatol. 2015;7(3):304–314. | ||
Lutz P, Nischalke HD, Krämer B, et al. Antibiotic resistance in healthcare-related and nosocomial spontaneous bacterial peritonitis. Eur J Clin Invest. 2017;47(1):44–52. | ||
Merli M, Lucidi C, di Gregorio V, et al. The spread of multi drug resistant infections is leading to an increase in the empirical antibiotic treatment failure in cirrhosis: a prospective survey. PLoS One. 2015;10(5):e0127448. | ||
Alexopoulou A, Vasilieva L, Agiasotelli D, et al. Extensively drug-resistant bacteria are an independent predictive factor of mortality in 130 patients with spontaneous bacterial peritonitis or spontaneous bacteremia. World J Gastroenterol. 2016;22(15):4049–4056. | ||
Ariza X, Castellote J, Lora-Tamayo J, et al. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56(4):825–832. | ||
Fernández J, Navasa M, Gómez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35(1):140–148. | ||
Ginés P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12(4 Pt 1):716–724. | ||
Fernández J, Tandon P, Mensa J, Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: Good and bad. Hepatology. 2016;63(6):2019–2031. | ||
Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60(6):1310–1324. | ||
Cobos-Trigueros N, Solé M, Castro P, et al. Acquisition of Pseudomonas aeruginosa and its resistance phenotypes in critically ill medical patients: role of colonization pressure and antibiotic exposure. Crit Care. 2015;19:218. | ||
Winter SE, Bäumler AJ. Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes. 2014;5(1):71–73. | ||
Reuken PA, Pletz MW, Baier M, Pfister W, Stallmach A, Bruns T. Emergence of spontaneous bacterial peritonitis due to enterococci - risk factors and outcome in a 12-year retrospective study. Aliment Pharmacol Ther. 2012;35(10):1199–1208. | ||
Fernández J, Bert F, Nicolas-Chanoine MH. The challenges of multi-drug-resistance in hepatology. J Hepatol. 2016;65(5):1043–1054. | ||
Pan SC, Wang JT, Chen YC, Chang YY, Chen ML, Chang SC. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS One. 2012;7(10):e47297. | ||
Fernández J, Acevedo J. New antibiotic strategies in patients with cirrhosis and bacterial infection. Expert Rev Gastroenterol Hepatol. 2015;9(12):1495–1500. | ||
Acevedo J, Silva A, Prado V, Fernández J. The new epidemiology of nosocomial bacterial infections in cirrhosis: therapeutical implications. Hepatol Int. 2013;7(1):72–79. | ||
European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397–417. | ||
Cheong HS, Kang CI, Lee JA, et al. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48(9):1230–1236. | ||
Piano S, Fasolato S, Salinas F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: Results of a randomized, controlled clinical trial. Hepatology. 2016;63(4):1299–1309. | ||
Bundesministerium der Justiz und für Verbraucherschutz Bundesministerium der Justiz und für Verbraucherschutz, 2000 [homepage on the Internet].Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten beim Menschen (Infektionsschutzgesetz – IfSG). Federal Ministry Justice and Consumer Protection, 2000. Law on Prevention and Control of Infectious Diseases in Humans (Infection Protection Act - IfSG). Available from: http://www.gesetze-im-internet.de/ifsg/BJNR104510000.html. Accessed September 5, 2018. German. | ||
Bundesministerium der Justiz und für Verbraucherschutz Bundesministerium der Justiz und für Verbraucherschutz [homepage on the Internet]. Gesetz zur Verhütung und Bekämpfung von Infektionskrankheiten beim Menschen (Infektionsschutzgesetz – IfSG). Law on Prevention and Control of Infectious Diseases in Humans (Infection Protection Act - IfSG). Federal Ministry Justice and Consumer Protection; 2000]. Available from: http://www.gesetze-im-internet.de/ifsg/BJNR104510000.html. Accessed September 5, 2018. German. | ||
Reinheimer C, Kempf VA, Göttig S, et al. Multidrug-resistant organisms detected in refugee patients admitted to a University Hospital, Germany June-December 2015. Euro Surveill. 2016;21(2):30110. | ||
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. | ||
Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol. 2012;50(12):3877–3880. | ||
Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65(2):233–238. | ||
Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32(30):5381–5397. | ||
Fiore M, Maraolo AE, Gentile I, et al. Nosocomial spontaneous bacterial peritonitis antibiotic treatment in the era of multi-drug resistance pathogens: A systematic review. World J Gastroenterol. 2017;23(25):4654–4660. | ||
Umgelter A, Reindl W, Miedaner M, Schmid RM, Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009;37(1):2–8. | ||
Friedrich-Rust M, Wanger B, Heupel F, et al. Influence of antibiotic-regimens on intensive-care unit-mortality and liver-cirrhosis as risk factor. World J Gastroenterol. 2016;22(16):4201–4210. | ||
Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2016;28(12):1450–1454. | ||
Reuken PA, Pletz MW, Baier M, Pfister W, Stallmach A, Bruns T. Emergence of spontaneous bacterial peritonitis due to enterococci - risk factors and outcome in a 12-year retrospective study. Aliment Pharmacol Ther. 2012;35(10):1199–1208. |
Supplementary materials
Impact of multidrug-resistant organisms in spontaneous bacterial peritonitis
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.