Back to Journals » Infection and Drug Resistance » Volume 11
Integrated pharmacokinetic–pharmacodynamic modeling to evaluate empiric carbapenem therapy in bloodstream infections
Authors Lim TP , Wang R, Poh GQ, Koh TH, Tan TY, Lee W, Teo JQ , Cai Y, Tan TT, Ee PLR, Kwa AL
Received 16 March 2018
Accepted for publication 30 May 2018
Published 27 September 2018 Volume 2018:11 Pages 1591—1596
DOI https://doi.org/10.2147/IDR.S168191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Joachim Wink
Tze-Peng Lim,1,2 Reyna Wang,1 Gang Quan Poh,3 Tse-Hsien Koh,4 Thean-Yen Tan,5 Winnie Lee,1 Jocelyn Qi-Min Teo,1 Yiying Cai,1 Thuan-Tong Tan,6 Pui Lai Rachel Ee,3 Andrea L Kwa1,3,7
1Department of Pharmacy, Singapore General Hospital, Singapore, Singapore; 2SingHealth Duke-NUS Medicine Academic Clinical Programme, Singapore, Singapore; 3Department of Pharmacy, Faculty of Science, National University of Singapore, Singapore, Singapore; 4Department of Microbiology, Singapore General Hospital, Singapore, Singapore; 5Department of Laboratory Medicine, Changi General Hospital, Singapore, Singapore; 6Department of Infectious Diseases, Singapore General Hospital, Singapore, Singapore; 7Emerging Infectious Diseases, Duke-NUS Medical School, Singapore, Singapore
Objectives: Treatment for nosocomial bloodstream infections (BSI) caused by multidrug-resistant (MDR) Gram-negative bacteria (GNB) is challenging. Rising antimicrobial resistance, especially in extended spectrum beta-lactamase production, inadvertently increases empiric carbapenem consumption. Three antipseudomonal carbapenems (imipenem, meropenem [MER], and doripenem [DOR]) are available commercially against MDR GNB in Singapore. The study aims to determine the most optimal empiric carbapenem dosing regimens (CDR) and evaluate their cost-effectiveness for GNB-BSI in the face of increasing MDR GNB.
Methods: Carbapenem minimum inhibitory concentrations (MICs) were generated for non-repeat GNB-BSI obtained in 2013–2014 from two hospitals. Monte Carlo simulations were used to assess the cumulative fraction of response (CFR) of various CDRs using the percentage of time above MIC for 40% (%T > MIC of 40%) as the pharmacokinetic (PK)–pharmacodynamic (PD) parameter for efficacy. Carbapenem costs were based on patient antibiotic costs. Antibiotic cost-effectiveness was calculated as total daily drug cost/CFR.
Results: A total of 1,140 bloodstream isolates were collected. They comprised 116 Acinetobacter baumannii, 237 Pseudomonas aeruginosa, and 787 Enterobacteriaceae. All CDRs achieved ~40, ~80, and ≥90% CFRs against A. baumannii, P. aeruginosa, and Enterobacteriaceae, respectively. Against P. aeruginosa, MER 2 g every 8 h infused over 3 h and DOR 1 g every 8 h infused over 4 h achieved CFRs 84 and 81%, respectively. Against Enterobacteriaceae, the cost of MER 2 g every 8 h infused over 3 h was the lowest among the three carbapenems at $0.40/percentage of CFR.
Conclusion: This study demonstrates the utility of PK–PD modeling to formulate the optimal selection of a cost-effective empiric CDR in antibiotics guidelines and formulary inclusion. The findings support the selection of high MER doses of prolonged infusions as empiric coverage for GNB-BSI in our institutions.
Keywords: empiric carbapenem regimens, multidrug resistant, Gram-negative bacteria, bloodstream infections
Introduction
Resistance to antimicrobial agents is a serious problem that has increased worldwide over the past decade.1 Gram-negative bacteria (GNB) (eg, Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae) have diverse mechanisms of resistance that result in reduced susceptibilities to almost all classes of available antimicrobial agents. As very few novel antimicrobial agents in the development pipeline have activity against these pathogens, one remaining viable option to combat resistance is to optimize the use of existing antimicrobial agents.2
Carbapenems are broad-spectrum antimicrobial agents that have excellent activity against a wide variety of bacteria. Antipseudomonal carbapenems have increasingly been used as first-line therapy in institutions where there are high levels of resistance to other classes of antimicrobial agents (aminoglycosides, fluoroquinolones, and cephalosporins).3 However, emerging resistance in GNB to carbapenems has been reported.4–7
Sepsis is a leading cause of mortality and morbidity in critically ill patients.8 Infection-related mortality rates for infected patients receiving inadequate antimicrobial treatment can be twice as much when compared with infection-related mortality rates for infected patients receiving adequate antimicrobial treatment.9 In addition, infection and related sepsis costs can account for ~40% of total intensive care unit’s expenditures.8 It is imperative that we advocate the rational use of empiric carbapenem treatment through optimizing their pharmacodynamic (PD) properties to preserve their efficacy, delay emergence of resistance, and provide cost-effective therapy for patients.
Understandably, optimal and cost-effective antimicrobial chemotherapy is dependent on local susceptibility patterns, pharmacokinetic (PK) profiles in the local population, PD of antimicrobials, and pharmacy acquisition costs. PK refers to the achievable antibiotic concentrations at the site of infections in patients while PD describes the relationship between antibiotic exposure and bacteria killing through patient’s response. For example, an antimicrobial standard dosing regimen may not be effective in a hospital with microorganisms having a reduced susceptibility to the antimicrobial agent.
Therefore, the objective of this study is to use Monte Carlo simulation with an integration of prior knowledge of local susceptibility data, PK/PD of carbapenems, and patient antibiotic costs to determine the most appropriate and cost-effective empiric carbapenem dosing regimen (CDR) for GNB bacteremia in the face of increasing MDR GNB.
Methods
Non-repeat isolates from two tertiary hospitals (1,700 beds [A] and 800 beds [B]) in Singapore were collected from January 2013 to December 2014. The first or the only isolate was collected from each patient only. Isolates of A. baumannii, P. aeruginosa, and Enterobacteriaceae were collected from patients with bloodstream infections. The phenotypic profiles of the carbapenems were determined by the microbroth dilution method in accordance with the Clinical Laboratory Standards Institute (CLSI).10
The PD profiles of various CDRs were determined: imipenem (IMI)/cilastatin 0.5 mg every 6 h (q6h) by 0.5 and 3 h infusion and 1 g q6h by 0.5 and 3 h infusion; meropenem (MER) 1 and 2 g every 8 h (q8h) by 0.5 and 3 h infusion; and doripenem (DOR) 0.5, 1, and 2 g q8h by 1 and 4 h infusion. These regimens were selected based on their utilization patterns at both institutions. Continuous infusion regimens were not evaluated as they are often logistically challenging to administer by nurses. A 5000-subject Monte Carlo simulation was performed for each dosing regimen, using the ADAPT II software.11 Steady-state exposures for each carbapenem were determined using PK mean point estimates obtained from previous population PK studies in infected and critically ill patient cohorts.12–14
Carbapenems have been reported to demonstrate concentration-independent bactericidal activity. The percentage of time above minimum inhibitory concentration (MIC) for 40% (%T > MIC of 40%) of the dosing interval of the serum unbound drug concentration has been shown to be the PK/PD parameter delineating drug exposure to antimicrobial effect in carbapenems in vivo.15 Therefore, we evaluated the probability of target attainment (PTA) of %T > MIC of 40% for various CDRs at specific MICs. Subsequently, we used a weighted approach to determine the overall PD target attainment based on our institution-specific MIC distribution, which is defined as cumulative fraction of response (CFR).16 An optimal regimen was defined as achieving a CFR of ≥90%.
In addition to calculating the CFR for each CDR for each bacteria population, patient drug costs of each carbapenem were obtained from the pharmacy department at the time of analysis. The cost-effectiveness was assessed by daily patient drug cost/percentage of CFR.17 A dosing regimen with the lowest cost/ percentage of CFR ratio would be considered as the most cost-effective (due to the highest PD target attainment rate and a lowest patient drug cost).17 We did not account for the cost of nursing disposables (intravenous solution bags and infusion kits) and labor costs associated with drug preparation and administration as these are already included as part of the daily ward charges.
Ethics approval
All experimental protocols were reviewed and approved by Singapore SingHealth Centralized Institutional Review Board (application number: CIRB/2012/423/D). Informed consent was not required from subjects as all experiments involved microorganisms without patient identifiers.
Results
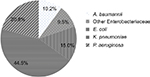
A total of 1,140 clinical bloodstream isolates (including 828 from Hospital A and 312 from Hospital B) were collected. The isolates were collected mainly from the surgical, medical oncology, hematology, and intensive care units. The majority of isolates collected were Enterobacteriaceae (69%), followed by P. aeruginosa (20.8%) and A. baumannii (10.2%) (Figure 1). Enterobacteriaceae isolates consisted of K. pneumoniae (44.5%), E. coli (15.0%) and “other Enterobacteriaceae” (Citrobacter spp., Serratia spp., Enterobacter spp., and Proteus spp.; 9.5%). K. pneumoniae and E. coli were susceptible to the three carbapenems at >95 and 97%, respectively. Both their MIC50 and MIC90 values were similar. The “other Enterobacteriaceae” group had a higher (two-fold) IMI MIC90 than those of MER and DOR (Table 1). The P. aeruginosa susceptibility rates were 48.1, 68.8, and 70.5% for IMI, MER, and DOR, respectively. P. aeruginosa had an IMI MIC90 that was two-fold higher than those of MER and DOR as well. A. baumannii isolates exhibited high resistance rates to all the carbapenems tested with susceptibility rates of only ~35%. Susceptibility results were not significantly different between the two hospitals. Hence, results from the two hospitals were pooled and analyzed together.
The CFRs for the various CDRs are shown in Table 2. Results using normal and log-normal distributions of the parameter estimates in the Monte Carlo simulations were not considerably different (<1% difference, data not shown). The data were thus presented using a log-normal distribution for brevity sake. Regardless of dose or infusion rate, all CDRs obtained optimal exposures as defined by CFR ≥90% against E. coli, K. pneumoniae populations, and “other Enterobacteriaceae” except for IMI 0.5 g q6h by 0.5 h infusion. However, only the DOR regimens of 1 g q8h by 4 h infusion and 2 g q8h by 1 and 4 h infusion and the MER regimen of 2 g q8h by 3 h infusion achieved CFRs ≥80% against P. aeruginosa. None of the CDRs obtained an optimal CFR against A. baumannii. Against the pooled MIC distribution of all isolates, MER 2 g q8h by 3 h infusion and DOR 2 g q8h by 4 h infusion were the only optimal dosing regimens that achieved ≥90% CFRs.
The antibiotic cost-effectiveness of the various regimens is summarized in Table 2. Using the normalized cost/percentage of CFR ratio, MER 2 g q8h by 3 h infusion was the most cost-effective and optimal regimen at $1.20 in the overall pooled isolates’ analysis. MER 1 g q8h by 3 h infusion and IMI 0.5 g q6h by 3 h infusion were the most cost-effective dosing regimen against K. pneumoniae, E. coli, and other Enterobacteriaceae with no significant difference between the two regimens. Against P. aeruginosa, MER 2 g q8h by 3 h infusion and DOR 1 g q8h by 4 h infusion were the most cost-effective regimens with achieving modest CFR. No cost-effective regimens were evaluated against A. baumannii, as no regimens achieved optimal CFRs.
Discussion
Antimicrobial resistance is an emerging public health problem in Singapore, and preserving our existing antibiotic armamentarium is critical. The cost of antimicrobial agents represents a significant portion in institutional pharmacy budgets; therefore, management of drug formularies have profound economic implications. In addition to limited resources and the global trend toward increasing health care costs, it is essential to develop efficacious treatment strategies that are cost-effective as well.
It had been reported that the bacteriostatic and bactericidal activities of carbapenems are dependent on the percentage of time > MIC in animal infection models.15 A prolonged infusion strategy may be beneficial in achieving a more favorable PD exposure when compared with intermittent infusion administration. A retrospective study by Feher et al18 reported that extended infusion of MER was associated with a higher treatment success (68.4 vs 40.9%) compared to short-term infusion in neutropenic patients who presented with fever after receiving hematopoietic stem-cell transplantation or induction chemotherapy for acute myeloid leukemia. Lorente et al19 also reported that continuous infusion of MER achieved a higher clinical cure rate in ventilator-associated pneumonia patients (90.4 vs 59.6%) compared to intermittent infusion.
In our study, using a prolonged infusion of the carbapenems (compared to giving high doses infrequently) was a more rational dosing strategy to achieve a carbapenem concentration consistently above the MIC. When used as an empiric treatment strategy against the pooled isolates, our findings showed that MER 2 g q8h by 3 h infusion is the most optimal and cost-effective carbapenem regimen in our local setting. Clinical settings where patients are at higher risk for P. aeruginosa and/or multidrug-resistant (MDR) GNB bacteremia suggested the need for aggressive dosing for empirical therapy until the pathogen identification and susceptibilities are available as the standard CDRs were unable to achieve optimal CFRs against all the isolates.
Most pharmacoeconomic analyses usually compare two different antimicrobial agents in a single patient population of a certain infection (eg, community-acquired pneumonia) in a clinical trial setting. As broad-spectrum antibiotics may be used in a variety of infections and each microorganism can cause several types of infections, there are numerous possible microorganism–antibiotic combinations. The possibility of many such scenarios makes it a challenge to extrapolate results from such pharmacoeconomic evaluations from clinical trial to clinical practice. In the choice of empiric antibiotic therapy, we have used PK/PD (%T>MIC of 40% of the dosing interval of the serum unbound drug concentration) as a surrogate for the efficacy of antimicrobial treatment to perform the cost-effective analyses. Unlike most cost-effectiveness analyses that mainly examine the costs and efficacy of a treatment, our analysis also measured whether the marginal CFR (PD exposure of a drug) gained was worth the cost of treatment. Our method is a general approach to an institution-specific analysis, incorporating local susceptibility patterns (PD), different patient cohorts (PK), and varying patient antibiotic costs (economics) to evaluate an antibiotic regimen’s cost-effectiveness. Additional comparisons can also be made between a single antibiotic against different bacteria to enhance existing institution antibiograms. Evaluations of various antimicrobial agents against a single organism are also useful in developing institution treatment guidelines and provide objective cost-effective information on the addition of antimicrobial agents into the drug formulary. All these form part of an ongoing quality improvement process where we can regularly update the latest susceptibility data to carbapenems and antibiotic costs to reflect the most optimal and cost-effective CDRs in each institution in an evidence-based manner.
Despite its potential utility, our approach has a few limitations. Our analysis did not evaluate various PK/PD thresholds of the proportion of the dosing interval during which the concentration of unbound (free) drug in plasma remains above the MIC of a specific pathogen (the percentage of time > MIC) as the target for optimal therapy. For carbapenems, the PK/PD threshold is widely considered to be 40%T > MIC.15,20 Institution-specific cost-effectiveness studies should also be considered to validate our modeling approach. The design of such studies should take into account the PK–PD of the antimicrobial agent as well as the patients’ co-morbidities and the severity of illness. As the PK data used in the analysis were obtained from a Caucasian population, the extrapolation of the results may not be fully applicable to local patient populations. Pooling of data from different organisms from only two local hospitals has yielded a recommendation to use aggressive dosing regimens empirically and did not account for sub-strata patient populations with lower risks for MDR GNB. Generalizability may be a concern, given these susceptibility patterns may not reflect many other practice settings locally and globally. While our results had suggested the use of extended infusions in our local setting, carbapenem stability after reconstitution in room temperature may be a limiting factor for administration. Therefore, critical consideration has to be taken at pharmacy & therapeutics (P&T) or procurement committees to include carbapenem formulations that have good stability data in the clinical ward setting where extended infusion of carbapenems will be administered.
Future research should include local PK studies in the target patient population to verify whether published PK profiles are relevant. Pathogen susceptibility, clonality patterns, and patient antibiotic costs can be updated on a periodic basis based on individual institution-specific data. This will improve the applicability of the data over time in various settings. A multicenter study examining the PK of MER in the critically ill patients is currently ongoing in Singapore.21
Conclusion
A comprehensive pharmacoeconomic analysis to evaluate the cost-effectiveness of antimicrobial agents should encompass local susceptibility patterns, population PK, PD, and antibiotic acquisition costs. This approach can guide clinicians in selecting the most appropriate antibiotic regimens and guide P&T committees beyond mere drug acquisition costs in formulary inclusion, especially for agents in a functional class.
Acknowledgments
This study was supported in part by SingHealth Foundation SHF/FG473P/2011, Singapore General Hospital Research Grant SRG#16/2012, SingHealth Allied Health Publication Fund, National Medical Research Council Centre Grant NMRC/CG/M011/2017 and NMRC/CG/C005/2017. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. RW is no longer at the institution where the work was performed. G-QP is no longer at the institution where the work was performed and is currently at National University Health System, 1E Kent Ridge Road, Singapore 119228, Singapore. This study was presented in part at the 24th European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain, May 10–13, 2014.
Data availability
All data generated or analysed during this study are included in this published article.
Author contributions
T-PL and ALK conceived and designed the experiments. T-PL, RW, G-QP, WL, JQ-MT, and YC performed the experiments. T-PL, G-QP, T-HK, T-TT, T-YT, and ALK analyzed the data. T-PL, G-QP, ALK, and PLRE contributed reagents/materials/analysis tools. T-PL, G-QP, and ALK wrote the article. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Livermore DM. Introduction: the challenge of multiresistance. Int J Antimicrob Agents. 2007;29(suppl 3):S1–S7. | ||
Talbot GH, Bradley J, Edwards JE Jr, Antimicrobial Availability Task Force of the Infectious Diseases Society of America, et al. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42(5):657–668. | ||
Rahal JJ. The role of carbapenems in initial therapy for serious Gram-negative infections. Crit Care. 2008;12(suppl 4):S5. | ||
Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165(12):1430–1435. | ||
Bratu S, Brooks S, Burney S, et al. Detection and spread of Escherichia coli possessing the plasmid-borne carbapenemase KPC-2 in Brooklyn, New York. Clin Infect Dis. 2007;44(7):972–975. | ||
Lolans K, Rice TW, Munoz-Price LS, Quinn JP. Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40. Antimicrob Agents Chemother. 2006;50(9):2941–2945. | ||
Villegas MV, Kattan JN, Correa A, et al. Dissemination of Acinetobacter baumannii clones with OXA-23 carbapenemase in Colombian hospitals. Antimicrob Agents Chemother. 2007;51(6):2001–2004. | ||
Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. | ||
Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(2):462–474. | ||
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Testing: Seventeenth Informational Supplement M100-S17. Wayne, PA, USA: CLSI; 2007. | ||
D’Argenio DZ, Schumitzky A, Wang XA. ADAPT II user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource. 1997:45–52. | ||
Ikawa K, Morikawa N, Uehara S, et al. Pharmacokinetic-pharmacodynamic target attainment analysis of doripenem in infected patients. Int J Antimicrob Agents. 2009;33(3):276–279. | ||
Lomaestro BM, Drusano GL. Pharmacodynamic evaluation of extending the administration time of meropenem using a Monte Carlo simulation. Antimicrob Agents Chemother. 2005;49(1):461–463. | ||
Sakka SG, Glauner AK, Bulitta JB, et al. Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem–cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob Agents Chemother. 2007;51(9):3304–3310. | ||
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–10;quiz11–12. | ||
Tam VH, Louie A, Lomaestro BM, Drusano GL. Integration of population pharmacokinetics, a pharmacodynamic target, and microbiologic surveillance data to generate a rational empiric dosing strategy for cefepime against Pseudomonas aeruginosa. Pharmacotherapy. 2003;23(3):291–295. | ||
Tam VH, Adams S, LaRocco MT, Gerard LN, Gentry LO, Garey KW. An integrated pharmacoeconomic approach to antimicrobial formulary decision-making. Am J Health Syst Pharm. 2006;63(8):735–739. | ||
Feher C, Rovira M, Soriano A, et al. Effect of meropenem administration in extended infusion on the clinical outcome of febrile neutropenia: a retrospective observational study. J Antimicrob Chemother. 2014;69(9):2556–2562. | ||
Lorente L, Lorenzo L, Martin MM, Jimenez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to Gram-negative bacilli. Ann Pharmacother. 2006;40(2):219–223. | ||
Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis. 2008;47(suppl 1):S32–S40. | ||
Kwa AL, Chua NG, Chua S, et al. Therapeutic Drug Monitoring of Meropenem and Piperacillin-Tazobactam Among Critically Ill Patients in Singapore. European Conference on Clinical Microbiology and Infectious Diseases; 2017; Vienna, Austria. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.