Back to Journals » Journal of Pain Research » Volume 10
Pain reporting and analgesia management in 270 children with a progressive neurologic, metabolic or chromosomally based condition with impairment of the central nervous system: cross-sectional, baseline results from an observational, longitudinal study
Authors Friedrichsdorf SJ , Postier AC, Andrews GS, Hamre KES, Steele R, Siden H
Received 28 March 2017
Accepted for publication 2 June 2017
Published 31 July 2017 Volume 2017:10 Pages 1841—1852
DOI https://doi.org/10.2147/JPR.S138153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Stefan J Friedrichsdorf,1,2 Andrea C Postier,1 Gail S Andrews,3 Karen ES Hamre,4 Rose Steele,5 Harold Siden6,7
1Department of Pain Medicine, Palliative Care and Integrative Medicine, Children’s Hospitals and Clinics of Minnesota, MN, USA; 2Department of Pediatrics, University of Minnesota Medical School, Minneapolis, MN, USA; 3Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada; 4Department of Research and Sponsored Programs, Children’s Hospitals and Clinics of Minnesota, Minneapolis, MN, USA; 5School of Nursing, Faculty of Health, York University, Toronto, ON, Canada; 6BC Children’s Hospital Research Institute, Vancouver, BC, Canada; 7Canuck Place Children’s Hospice, Vancouver, BC, Canada
Abstract: Little is known about the prevalence, characterization and treatment of pain in children with progressive neurologic, metabolic or chromosomal conditions with impairment of the central nervous system. The primary aims of this study were to explore the differences between parental and clinical pain reporting in children with life-limiting conditions at the time of enrollment into an observational, longitudinal study and to determine if differences in pain experiences were associated with patient- or treatment-related factors. Pain was common, under-recognized and undertreated among the 270 children who enrolled into the “Charting the Territory” study. Children identified by their parents as experiencing pain (n=149, 55%) were older, had more comorbidities such as dyspnea/feeding difficulties, were less mobile with lower functional skills and used analgesic medications more often, compared to pain-free children. Forty-one percent of children with parent-reported pain (21.8% of all patients) experienced pain most of the time. The majority of clinicians (60%) did not document pain assessment or analgesic treatment in the medical records of patients who were experiencing pain. Documentation of pain in the medical record was positively correlated with children receiving palliative care services and being prescribed analgesics, such as acetaminophen, nonsteroidal anti-inflammatory drugs and opioids, as well as the adjuvant analgesics gabapentin and amitriptyline.
Keywords: pediatric palliative care, hospice, neuropathic pain, palliative, life-limiting
Plain language summary
To date, this prospective study is the largest study exploring pain in children with nonmalignant life-limiting diseases. Pain in children with progressive neurologic diseases was common, under-recognized and undertreated. Analgesia management in this vulnerable group currently lacks standard assessment tools, consensus treatment guidelines and prospective randomized controlled trials.
Introduction
Children living with serious illnesses commonly experience pain, which is among the most distressing and prevalent symptoms.1–3 Nearly all studies of pain and other distressing symptoms in pediatric palliative care (PPC) were undertaken in children with malignancies and show a significant symptom burden to this population.4–7 However, the majority of children living with or dying from a serious illness do not have cancer.8 In 2013, a total of 42,328 children aged 0–19 years died in the US, of whom >23,440 (55%) were infants <1 year of age. The most common life-limiting conditions for children living in the US include congenital malformations and chromosomal abnormalities (5,740) followed by malignancies (1,850).9
According to the Declaration of Montreal, access to pain management is a fundamental human right.10 Yet, pain in hospitalized children in general, as well as in pediatric patients with advanced cancer, has been characterized as common, under-recognized and undertreated.4–7,11–14 Existing data suggest that pain processing is altered in most individuals with cognitive impairment, compared with cognitively intact matched controls.15 Nociception may be based on the underlying condition and/or treatment (including procedures/interventions) of that disease. Underlying pain pathologies in this group of nonverbal children with progressive neurologic, metabolic or chromosomal conditions, where the central nervous system (CNS) is impaired, often remain enigmatic. One might speculate that many of these children may have not one, but several underlying conditions simultaneously: acute somatic nociceptive pain (such as otitis media), visceral pain (such as bladder spasms, constipation), chronic postoperative pain,16–19 autonomic disorders,20 chronic pain beyond the expected time of healing or primary pain disorder (such as primary headaches, centrally mediated abdominal pain syndrome, chronic musculoskeletal pain),21–25 medication overuse headaches,26 visceral hyperalgesia,27 psycho-social-spiritual pain and/or neuropathic pain.20,28–30
Little is known about pain prevalence, characterization and treatment in children with a progressive neurologic, metabolic or chromosomally based condition with impairment of the CNS. The primary aims of this study were to explore whether there were differences in pain experiences associated with patient- or treatment-related factors, and there were differences in parental and clinician pain reporting in children with life-limiting conditions at the time of enrollment into a prospective, observational, longitudinal study.
Methods
Data were obtained from a 3-year, multicenter, prospective cohort study of children living with progressive CNS conditions and their families (see Siden et al31 for details regarding study design and methods). Subjects were recruited from clinics that followed patients with CNS conditions in nine child health centers (seven in Canada and two in the US). The conditions of interest were progressive, nontreatable and likely fatal; they affected the nervous system and potentially other organ systems. Children could be at any point in their disease trajectory. Extensive baseline information was obtained from a health records review by a trained research assistant and a baseline interview with a parent. Baseline data included a medication profile and list of interventions. From enrollment onward, monthly data on symptoms were obtained from parents. Follow-up information, including medications and interventions, was obtained either upon notification of the patient’s death or at the time of the study’s conclusion for those who were alive.
Additional information was acquired on an annual basis regarding the child’s functional status and on a semi-annual basis for the psychosocial and health status of the parents and siblings. These data will contribute to other analyses and publications.
This study was approved by the University of British Columbia Clinical Research Ethics Board, whose approval jointly covered the BC Children’s Hospital in Vancouver, BC. Further ethics approval was obtained at eight other clinical sites (six in Canada and two in the US) (see Supplementary Material section). In addition to obtaining ethical approval from each of the clinical study settings, ethical review and approval was also obtained from five universities at which the researchers were affiliated and/or their clinical hospices in which their primary clinical appointment resided. Confirmation of informed consent was provided by the parents or guardians of the children.
Analysis plan
In order to address the two aims of this paper, four separate analyses were undertaken. To explore whether there were differences in patient- or treatment-related factors associated with pain experiences of children with progressive neurologic, metabolic or chromosomal conditions, we used the following four frames: 1) exploring whether there were differences between children for whom parents reported pain during the baseline study interview versus those for whom pain was not reported; 2) for cases where parents reported their child was in pain, data were analyzed to determine if there were differences between children based on how often they were experiencing pain (most of the time vs. not most of the time); 3) for children whose parents reported pain, data were analyzed to determine if there were differences between children with pain documented in their medical record versus those without chart-documented pain and 4) differences between children by whether their pain was reported in the medical record (yes/no) were explored, regardless of whether the parent reported pain. Figure 1 depicts pain reporting by the source of pain report (parent/medical record).
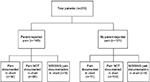  | Figure 1 Study flow diagram organized by source of pain reporting. |
The following child characteristics (independent variables) were assessed: age, sex, ethnicity, PPC team involvement, income, parent education, pain percentage across lifetime, number of medication classes received, number of symptoms, number of disease groups, artificial feeding status (total parenteral nutrition [TPN], gastrostomy [G]-/jejunostomy [J]-tube or tube feeding), mobility/disability (Pediatric Evaluation of Disability Inventory [PEDI]),32 do-not-attempt-to-resuscitate status, suctioning use and analgesia use (including opioids and adjuvant analgesia).
Statistical analysis
Medians and interquartile ranges were calculated for continuous variables because most were not normally distributed in this sample. Frequency distributions were calculated for categorical outcomes. Wilcoxon rank sum tests were used to determine whether statistically significant differences existed between the distribution of the comparison groups for continuous variables, and Pearson’s chi-square tests or Fisher’s exact tests, as appropriate, were used to evaluate the differences for all categorical outcomes.
Data from the multiple sites were entered into Daciforms, a web-based database (Dacima Software, Inc., Montreal, QC, Canada). From Daciforms, data were exported into SPSS v23 (IBM Corporation, Armonk, NY, USA). All analyses were performed using Stata SE version 14.33
Results
Differences between children with “no pain” versus “pain” as reported by parents at the time of study enrollment
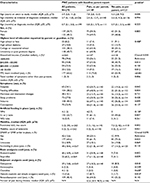
At baseline (time of study enrollment), parents of 149/270 (55%) children reported their child was in pain. Children with parent-reported pain were significantly older (median=7.8 years [2.8, 12.0] vs. 5.3 years [2.8, 9.0], p=0.03), more likely to be from a lower-income household (p=0.04) and more likely to have dyspnea (30.7% vs. 19.5%, p=0.04) and feeding difficulties (48.9% vs. 31.0%, p=0.004) than the children whose parents did not report pain (Table 1).
Children with parent-reported pain were more likely than parents who indicated their child was not in pain to be receiving care from a PPC team (75.8% vs. 52.9%, p<0.001). They were also more likely to be prescribed the following classes of medications: antacids (54.1% vs. 36.4%, p=0.004), laxatives (41.2 vs. 28.1%, p=0.03), antispasticity medications (15.5% vs. 3.3%, p=0.001), acetaminophen/nonsteroidal anti-inflammatory drugs (NSAIDs; 25.0% vs. 10.7%, p=0.003), opioids (12.8% vs. 1.7%, p<0.001) and glucocorticosteroids (39.7% vs. 25.0%, p=0.03). When examined in more detail, children whose parents reported they were experiencing pain were more likely to be prescribed the World Health Organization (WHO)34 step 1 basic analgesics (acetaminophen=20.9% vs. 7.4%, p=0.002; ibuprofen=11.5% vs. 4.1%, p=0.03), gabapentin as adjuvant analgesia (18.2% vs. 8.3%, p<0.02), and they were more likely to be prescribed a combination of opioids and basic analgesics (8.7% vs. 1.7%, p=0.01).
When artificial feeding status and mobility were compared between groups, children with parent-reported pain were more likely to be receiving nutrition through a G- and/or J-tube (61.1% vs. 44.6%, p<0.01). When mobility status as measured by PEDI was examined, children with parent-reported pain were less mobile based upon how many modifications they required to be mobilized or transported (median [interquartile range {IQR}]: 0 [0, 3] vs. 0 [0, 2], p=0.02). Children with parent-reported pain also had lower functional skills compared to those whose parents did not report pain at baseline (PEDI raw functional skill score median [IQR]: 3[1, 16] vs. 7.5 [2, 42], p=0.002).
Finally, children whose parents reported pain were significantly more likely to have a do-not-attempt-to-resuscitate order in place (20.1% vs. 9.9%, p=0.02).
Differences between patients with “pain most of the time” versus “pain NOT most of the time” as reported by parents
Next, we examined data from the parents who reported their child was in pain at baseline (n=149) to determine if there were any differences between children who were in pain most of the time (n=60) versus not most of the time (n=88). One of the 149 parents did not report pain frequency, leaving 148 children included for this analysis. These groups did not differ sociodemographically. Less than half of parents (n=60, 41%) reported their child was in pain most of the time. However, children in pain most of the time were significantly more likely to be experiencing dyspnea (38.3% vs. 22.7%, p=0.04) and were less likely to be using “metabolic” medications such as carnitine, Coenzyme Q10, folic acid, omega-3 fish oil, cholecalciferol, and vitamins A, B6, C, E, K (28.3% vs. 47.1%, p=0.02). There were no other differences found between the groups for frequency of pain.
Children in pain at baseline according to their parents: comparison between those with and without clinician-entered pain documentation in chart
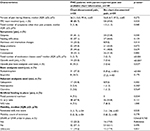
There were no sociodemographic differences between children whose pain was documented in their charts (n=56) compared to children whose pain was not documented in their charts (n=84) whose parents reported pain at baseline. Children with chart-documented pain were more likely to be receiving care from a PPC team than the children whose pain was only reported by their parents (i.e., not in the chart; 85.7% vs. 69.0%, p=0.02). In other words, children whose parents reported that they were experiencing pain were less likely to receive support from a PPC team if their pain was not also documented in their medical record (Table 2).
When symptom experience of children with parent-reported pain was compared between those with chart-documented pain and those without, the total number of symptoms present (other than pain) was significantly higher in the group of children with chart-documented pain (median [IQR]: 2[1, 4] vs. 1.5[1, 3], p=0.04). Children with chart-documented pain were significantly more likely to be experiencing dyspnea (41.1% vs. 23.8%, p=0.03) and changes in alertness/interaction (25.0% vs. 9.5%, p=0.01). Children with chart-documented pain were also more likely to be receiving TPN (7.1% vs. 0%, p=0.02), as shown in Table 2.
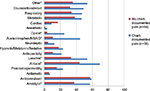
Children whose pain was reported by parents at baseline and whose pain was documented in their medical chart were more likely to be prescribed more classes of medications than those prescribed for children with parent-reported pain only (i.e., their pain was not captured in the medical chart; median [IQR]: 5 [3, 7] vs. 3 [2, 5], p<0.001). The following medication classes were prescribed significantly more often in these children with chart-documented pain: anxiolytics (57.1% vs. 37.3%, p=0.02), antacids (69.6% vs. 44.0%, p=0.003), laxatives (50.7% vs. 33.3%, p=0.02), WHO-step 1:34 acetaminophen/NSAIDs (44.6% vs. 10.7%, p<0.001), WHO-step 2: opioids (25.0% vs. 3.6%, p<0.001) and other medications (53.6% vs. 35.7%, p=0.04), as shown in Figure 2. Finally, benzodiazepine use was higher in the children with chart-documented pain (57.1% vs. 36.9%, p=0.02).
Differences between children with or without pain as documented in their medical record
Regardless of whether parents reported pain for their child, of the 253 children with pain status data available in their medical record at baseline, pain was recorded for only 67 (26.5%) by a clinician (physician or nurse). The groups did not differ sociodemographically. Children with chart-documented pain were significantly more likely to be receiving care from a PPC team (83.6% vs. 59.7%, p<0.001) and to be experiencing more symptoms overall, not including pain (median [IQR]: 2 [1, 4] vs. 1 [0, 3], p=0.003). When we compared specific symptom experiences between groups, we found that children with pain documented in their charts were more likely to have dyspnea (40.3% vs. 20.4%, p<0.01) and feeding difficulties (55.2% vs. 37.2%, p=0.01) documented, as compared to children without chart-documented pain.
Children with chart-documented pain were more likely to be prescribed medications representing a wider range of medication classes (median [IQR]: 5 classes [3, 7] vs. 3 classes [1, 4], p<0.001). Specifically, children assessed by a clinician as experiencing pain were more likely to be prescribed anxiolytics (55.2% vs. 34.2%, p=0.003), antacids (65.7% vs. 38.7%, p<0.001), laxatives (50.7% vs. 29.8%, p=0.002), antispasticity medications (17.9% vs. 6.8%, p=0.008), acetaminophen/NSAIDs (43.3% vs. 9.4%, p<0.001), opioids (22.4% vs. 2.1%, p<0.001), glucocorticosteroids (47.5% vs. 28.4%, p=0.01) and other medication classes (55.2% vs. 33.5%, p=0.002). Basic WHO-step 1 analgesia (ibuprofen and acetaminophen) was significantly more likely to be prescribed for children with chart-documented pain (acetaminophen=35.8% vs. 7.4%, p<0.001; ibuprofen=14.9% vs. 4.7%, p=0.006). Adjuvant analgesia was more highly prescribed for children who had clinician-documented pain, with both gabapentin (28.4% vs. 17.9%, p<0.001) and amitriptyline (6.0% vs. 0.5%, p=0.017) being prescribed significantly more often. A combination of WHO-step 2 opioids and WHO-step 1 basic analgesia (i.e., acetaminophen or ibuprofen) was prescribed more often for children with chart-documented pain, compared to those without chart-documented pain (13.4% vs. 2.1%, p=0.001).
Artificial feeding devices were significantly more likely to be in place for children with chart-documented pain. Specifically, children with clinician-documented pain were more likely to be receiving TPN (6.0% vs. 0%, p=0.004) and were more likely to be dependent upon a G- and/or J-tube for nutrition (64.2% vs. 49.7%, p=0.04) than those without chart-documented pain. In terms of advanced planning, do-not-resuscitate orders were significantly more likely to be in place for children whose pain was documented in the chart (23.9% vs. 13.6%, p<0.05).
Discussion
To date, this prospective cohort study is the largest to explore prevalence, assessment and treatment of pain in children with life-limiting nonmalignant diseases. Pain was common, under-recognized and undertreated among the 270 children from Canada and the USA who were suffering from neurologic, genetic or metabolic conditions with impairment of the CNS.
Overwhelmingly, published evidence of pain prevalence, assessment and treatment in PPC focuses on children with a malignancy and there is a dearth of research on pain in children with life-limiting, nonmalignant diseases. A prospective study describing patient-reported outcomes in pediatric patients with advanced cancer showed that 39% of all children were self-reporting high distress from pain, which increased to 58% at the end of life.7 However, the majority of children living with, and dying of, life-limiting diseases do not have cancer.8 Higher pain prevalence rates than in the normal pediatric population have been reported in children with developmental disabilities,35,36 cerebral palsy,37,38 Noonan syndrome,39,40 progressive neurodegenerative and metabolic conditions,41–43 as well as in children dying of nonmalignant diseases.44,45 In 2011, data from an observational cohort study of 515 patients served by six PPC teams in North America showed that 31% of the children experienced pain at the time of consultation.3 The predominant primary clinical conditions of these children were genetic/congenital (41%), neuromuscular (39%), followed by cancer (20%).
The majority of the 270 children with progressive neurologic, metabolic or chromosomal conditions in our study experienced pain (n=149, 55%) at the time of study enrollment according to their parents. The analysis of all 149 children experiencing parent-reported pain revealed that they were significantly more likely to have received PPC services, to have been more symptomatic (increased dyspnea and changes in alertness/interaction), to have received TPN and to have received basic analgesics, opioids and benzodiazepines.
In our study, children experiencing parent-reported pain were more likely than those without pain to receive artificial nutrition and hydration through a G- or J-tube. Children receiving enteral tube feeding usually have a higher morbidity and appear more prone to developing feeding intolerance and/or visceral hyperalgesia.46,47 Pain caused by artificial feeding, unresponsive to conservative intervention, appears to be a leading symptom, suggesting that a child with advanced serious illness may have entered the end-of-life period.48 Compared to children without pain, children with parent-reported pain were more likely to use the WHO pain ladder34 of basic (acetaminophen, NSAIDs) and opioid analgesics in our study. Also, adjuvant analgesia, especially gabapentin,49 was used significantly more often. However, other adjuvants, which might play a role especially in the pharmacologic treatment of neuropathic and/or visceral pain50–53 (such as the alpha-agonists54–56 clonidine or dexmedetomidine, tricyclic antidepressants57,58 such as amitriptyline or nortriptyline, N-methyl-d-aspartic acid-channel blockers59,60 such as ketamine or methadone and sodium channel blockers such as lidocaine60–63) were rarely administered to the children in pain in this study. This finding points to a paucity of evidence and established treatment guidelines in the management of these challenging patients.
Our study also showed that children with parent-reported pain were sicker and of lower socioeconomic status than the children who were pain free. They were more likely to be from low-income households and to have associated comorbidities such as dyspnea, impaired mobility, lower functional skills and feeding difficulties. Health disparities are well described in the literature, and income differences correlate strongly with health outcomes among children. For instance, families with low incomes have a higher prevalence of abdominal pain among their children with increased pain intensity.64,65 Our results support existing literature on differences in health outcomes due to socioeconomic status.48,53,66–70
Due to their CNS impairment, self-report was not feasible, but more than one out of five parents reported pain as occurring most of the time. Stallard et al reported a similar number in a small study, with 8 (23.5%) out of 34 cognitively impaired, noncommunicating children experiencing daily pain according to their parents.36 The children in our study who experienced pain most of the time were more likely to experience dyspnea, suggesting a higher morbidity.
The 67 children with pain documentation in their charts compared to patients who did not have any pain documented by clinicians were more likely to experience more distressing nonpain symptoms, including dyspnea and feeding difficulties, in our study, and they were more likely to have a G- or J-tube. They were more likely to receive basic analgesics (acetaminophen, NSAIDs), opioids and adjuvant analgesia (gabapentin and amitriptyline), as well as benzodiazepines, glucocorticosteroids and muscle relaxants. In addition, among the study patients, documentation of pain was correlated with a higher likelihood of children receiving care from a PPC team and to have a do-not-resuscitate order. It would be difficult to extrapolate causality: one might speculate that the inclusion of a PPC team might result in increased pain assessment and analgesic prescription, and/or that the realization of increased pain and symptom burden result in children with serious illness increases the chance of referral to a PPC team.
One surprising finding was that for over half (56%) of cases in which parents reported their child was experiencing pain at baseline, no associated pain documentation was found in the child’s medical chart. Data suggest that pain assessment is critical to optimal pain treatment interventions and that the assessment and documentation of pain results in increased prescription of analgesics.34,71–74
Unlike in adult patients with neuropathic, visceral and/or chronic pain, there are no guidelines, randomized controlled trials or systematic reviews guiding the assessment and treatment of this challenging pediatric population.50,75,76 One may speculate whether lack of pain documentation might be due to missing pain assessment standards, guidelines and/or lack of knowledge about how to differentiate and treat acute nociceptive, visceral, neuropathic, psycho-social-spiritual or chronic pain in seriously ill children suffering from pain. Abdominal discomfort appears to be a common complaint in these children as reported by parents, and feeding intolerance and visceral hyperalgesia27 may not be uncommon. In nonverbal, cognitively impaired children, pain assessment and the ability to differentiate between “episodes of inconsolability”, “neuroirritability” and “pain” remain difficult. Autonomic stress response (i.e., change of heart rate, heart rate variability, mean arterial blood pressure, respiratory rate, plasma levels of norepinephrine and epinephrine) is not significantly correlated with pain severity.77 The absence of signs of sympathetic stimulation cannot be accepted as a guarantee for the absence of significant pain, and traditional pain assessment cannot be replaced with more objective measures of physiologic changes of autonomic and respiratory parameters.78 Validated pain assessment tools for children with impaired communication are available, but are not commonly used in daily practice.79–84 The findings of this study suggest that the lack of appropriate measurement tools is not a barrier to pain assessment by clinicians. Further studies need to determine what system issues limit their use.
Supported by adult and pediatric evidence,85,86 advanced pain treatment postoperatively and also for complex children with advanced serious illnesses is increasingly based on the opioid-sparing concept of “multimodal analgesia”:53 Multiple pharmacologic agents (such as basic analgesics, opioids and adjuvant analgesia), regional anesthesia (such as central neuraxial infusions, peripheral nerve and plexus blocks or infusions, neurolytic blocks and implanted intrathecal ports and pumps for baclofen, opioids, local anesthetics and other adjuvants),87 rehabilitation (such as physical, occupational, speech and music therapy),88,89 psychologic family therapy90–92 and integrative therapies93–95 (such as massage, deep breathing, aromatherapy, yoga), act synergistically for more effective pediatric pain control with fewer side effects than a single analgesic or modality. Although we could describe a higher use of analgesia and adjuvant pain medication in children referred to PPC in our study population, future research is required to evaluate the efficacy and safety of multimodal analgesia in children with progressive, neurodegenerative and chromosomal conditions with impairment of the CNS.
Study limitations
Our study population is heterogeneous in terms of age, underlying condition and disease progression. This heterogeneity complicates intergroup comparisons and interpretation of results. However, many of the conditions in our study population are so rare that it would not be feasible to enroll a sufficient number of children with the same condition and similar disease progression. Also, the timing of a child’s medical visit with respect to the baseline study interview with the child’s parent varied, although the timing of the clinical assessment was as close as possible to the parent pain assessment. Finally, parent proxy ratings of pain were used instead of child self-report, as the cognitive impairment of the mostly nonverbal children excluded self-reporting.
Conclusion and implications
Pain in children with progressive neurodegenerative/chromosomal conditions with CNS impairment is common, under-recognized and undertreated. In our cohort, children who experienced parent-reported pain were prescribed more comprehensive health services, including analgesia and PPC services, when their pain was documented in their medical record by a clinician. Further research should address the gap in pain recognition and reporting in this population of children, in order to ensure optimal pain management throughout their disease trajectory. Clinicians treating this challenging population lack standard assessment tools for pain, consensus treatment guidelines and evidence from prospective randomized controlled trials. Future research should evaluate whether effective prevention and treatment of pain in this large group of children might be more effective if it employs multimodal analgesia strategies.
Acknowledgments
We thank Laurie Foster, Ashley Young, Michele Gilbert and Dr Elizabeth Gilles for their efforts in recruiting families and collecting data. Leanne Feichtinger and Amy Tan graciously undertook data entry and produced initial statistical reports. We also thank Dr Dave Watson and Amanda Nickel for their additional statistical guidance, and the families who generously shared their time and insights into their personal journeys with us. This study was funded by a grant from the Canadian Institutes of Health Research (CIHR MOP-89984).
Disclosure
The authors report no conflicts of interest in this work.
References
Goldman A. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342(26):1998. | ||
Hongo T, Watanabe C, Okada S, et al. Analysis of the circumstances at the end of life in children with cancer: symptoms, suffering and acceptance. Pediatr Int. 2003;45(1):60–64. | ||
Feudtner C, Kang TI, Hexem KR, et al. Pediatric palliative care patients: a prospective multicenter cohort study. Pediatrics 2011;127(6):1094–1101. | ||
Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342(5):326–333. | ||
Wolfe J, Hammel JF, Edwards KE, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol. 2008;26(10):1717–1723. | ||
Friedrichsdorf SJ, Postier A, Dreyfus J, Osenga K, Sencer S, Wolfe J. Improved quality of life at end of life related to home-based palliative care in children with cancer. J Palliat Med. 2015;18(2):143–150. | ||
Wolfe J, Orellana L, Ullrich C, et al. Symptoms and distress in children with advanced cancer: prospective patient-reported outcomes from the pediQUEST study. J Clin Oncol. 2015;33(17):1928–1935. | ||
Feudtner C, Hays RM, Hayness G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. | ||
Osterman MJ, Kochanek KD, MacDorman MF, Strobino DM, Guyer B. Annual summary of vital statistics: 2012–2013. Pediatrics. 2015;135(6):1115–1125. | ||
International Association for the Study of Pain (IASP). Declaration of Montréal. 2010; Available from: http://www.iasp-pain.org/DeclarationofMontreal?navItemNumber=582. Accessed May 1, 2017. | ||
Friedrichsdorf SJ, Postier A, Eull D, et al. Pain outcomes in a US children’s hospital: a prospective cross-sectional survey. Hosp pediatr. 2015;5(1):18–26. | ||
Taylor EM, Boyer K, Campbell FA. Pain in hospitalized children: a prospective cross-sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Res Manag. 2008;13(1):25–32. | ||
Zhu LM, Stinson J, Palozzi L, et al. Improvements in pain outcomes in a Canadian pediatric teaching hospital following implementation of a multifaceted knowledge translation initiative. Pain Res Manag. 2012;17(3):173–179. | ||
Stevens BJ, Harrison D, Rashotte J, et al. Pain assessment and intensity in hospitalized children in Canada. J Pain. 2012;13(9):857–865. | ||
Defrin R, Amanzio M, de Tommaso M, et al. Experimental pain processing in individuals with cognitive impairment: current state of the science. Pain. 2015;156(8):1396–1408. | ||
Fortier MA, Chou J, Maurer EL, Kain ZN. Acute to chronic postoperative pain in children: preliminary findings. J Pediatr Surg. 2011;46(9):1700–1705. | ||
Sieberg CB, Simons LE, Edelstein MR, et al. Pain prevalence and trajectories following pediatric spinal fusion surgery. J Pain. 2013;14(12):1694–1702. | ||
Page MG, Stinson J, Campbell F, Isaac L, Katz J. Identification of pain-related psychological risk factors for the development and maintenance of pediatric chronic postsurgical pain. J Pain Res. 2013;6:167–180. | ||
Noel M, Rabbitts JA, Tai GG, Palermo TM. Remembering pain after surgery: a longitudinal examination of the role of pain catastrophizing in children’s and parents’ recall. Pain. 2015;156(5):800–808. | ||
Nickel FT, Seifert F, Lanz S, Maihofner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;22(2):81–91. | ||
Goodman JE, McGrath PJ. The epidemiology of pain in children and adolescents: a review. Pain. 1991;46(3):247–264. | ||
Hechler T, Dobe M, Zernikow B. Commentary: a worldwide call for multimodal inpatient treatment for children and adolescents suffering from chronic pain and pain-related disability. J Pediatr Psychol. 2010;35(2):138–140. | ||
Schechter NL. Functional pain: time for a new name. JAMA Pediatr. 2014;168(8):693–694. | ||
King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738. | ||
Friedrichsdorf SJ, Giordano J, Desai-Dakoji K, Warmuth A, Daughtry C, Schulz CA. Chronic pain in children and adolescents: diagnosis and treatment of primary pain disorders in head, abdomen, muscles and joints. Children (Basel). 2016;3(4): pii:E42. | ||
Lauwerier E, Paemeleire K, Van Damme S, Goubert L, Crombez G. Medication use in patients with migraine and medication-overuse headache: the role of problem-solving and attitudes about pain medication. Pain. 2011;152(6):1334–1339. | ||
Di Lorenzo C, Youssef NN, Sigurdsson L, Scharff L, Griffiths J, Wald A. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr. 2001;139(6):838–843. | ||
Walco GA, Dworkin RH, Krane EJ, LeBel AA, Treede RD. Neuropathic pain in children: special considerations. Mayo Clin Proc. 2010;85(3 Suppl):S33–S41. | ||
Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. | ||
Chu C, Levine E, Gear RW, Bogen O, Levine JD. Mitochondrial dependence of nerve growth factor-induced mechanical hyperalgesia. Pain. 2011;152(8):1832–1837. | ||
Siden H, Steele R, Brant R, et al. Designing and implementing a longitudinal study of children with neurological, genetic or metabolic conditions: charting the territory. BMC pediatr. 2010;10:67. | ||
Feldman AB, Haley SM, Coryell J. Concurrent and construct validity of the pediatric evaluation of disability inventory. Phys Ther. 1990;70(10):602–610. | ||
StataCorp. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP; 2015. | ||
World Health Organization. WHO-Principles of Acute Pain Management for Children 2012; Available from: http://whqlibdoc.who.int/publications/2012/9789241548120_Guidelines.pdf. Accessed May 1, 2017. | ||
Breau LM, Camfield CS. The relation between children’s pain behaviour and developmental characteristics: a cross-sectional study. Dev Med Child Neurol. 2011;53(2):e1–e7. | ||
Stallard P, Williams L, Lenton S, Velleman R. Pain in cognitively impaired, non-communicating children. Arch Dis Child. 2001;85(6):460–462. | ||
Parkinson KN, Gibson L, Dickinson HO, Colver AF. Pain in children with cerebral palsy: a cross-sectional multicentre European study. Acta Paediatr. 2010;99(3):446–451. | ||
Houlihan CM, O’Donnell M, Conaway M, Stevenson RD. Bodily pain and health-related quality of life in children with cerebral palsy. Dev Med Child Neurol. 2004;46(5):305–310. | ||
Vegunta S, Cotugno R, Williamson A, Grebe TA. Chronic pain in noonan syndrome: a previously unreported but common symptom. Am J Med Genet A. 2015;167A(12):2998–3005. | ||
Croonen EA, Harmsen M, Van der Burgt I, et al. Perceived motor problems in daily life: focus group interviews with people with noonan syndrome and their relatives. Am J Med Genet A. 2016;170(9):2349–2356. | ||
Hunt A, Burne R. Medical and nursing problems of children with neurodegenerative disease. Palliat Med. 1995;9(1):19–26. | ||
Malcolm C, Forbat L, Anderson G, Gibson F, Hain R. Challenging symptom profiles of life-limiting conditions in children: a survey of care professionals and families. Palliat Med. 2011;25(4):357–364. | ||
Malcolm C, Hain R, Gibson F, Adams S, Anderson G, Forbat L. Challenging symptoms in children with rare life-limiting conditions: findings from a prospective diary and interview study with families. Acta Paediatr. 2012;101(9):985–992. | ||
Hunt AM. A survey of signs, symptoms and symptom control in 30 terminally ill children. Dev Med Child Neurol. 1990;32(4):341–346. | ||
Drake R, Frost J, Collins JJ. The symptoms of dying children. J Pain Symptom Manage. 2003;26(1):594–603. | ||
Edwards L, DeMeo S, Hornik CD, et al. Gabapentin use in the neonatal intensive care unit. J Pediatr. 2016;169:310–312. | ||
Miranda A. Early life events and the development of visceral hyperalgesia. J Pediatr Gastroenterol Nutr. 2008;47(5):682–684. | ||
van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Socl Sci Med. 2000;50(6):813–828. | ||
Hauer JM, Solodiuk JC. Gabapentin for management of recurrent pain in 22 nonverbal children with severe neurological impairment: a retrospective analysis. J Palliat Med. 2015;18(5):453–456. | ||
Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. | ||
Anghelescu DL, Faughnan LG, Jeha S, et al. Neuropathic pain during treatment for childhood acute lymphoblastic leukemia. Pediatric Blood Cancer. 2011;57(7):1147–1153. | ||
Friedrichsdorf SJ, Nugent AP. Management of neuropathic pain in children with cancer. Curr Opin Support Palliat Care. 2013;7(2):131–138. | ||
Friedrichsdorf SJ. Prevention and Treatment of Pain in Hospitalized Infants, Children, and Teenagers: From Myths and Morphine to Multimodal Analgesia. Pain 2016: Refresher Courses. 16th World Congress on Pain. Washington, D.C: International Association for the Study of Pain, IASP Press; 2016:309–319. | ||
Horvath R, Halbrooks EF, Overman DM, Friedrichsdorf SJ. Efficacy and safety of postoperative dexmedetomidine administration in infants and children undergoing cardiac surgery: a retrospective cohort study. J Pediatr Inten Care. 2015(3):138–145. | ||
Chrysostomou C, Schulman SR, Herrera Castellanos M, et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164(2):276–282. e271–e273. | ||
O’Mara K, Gal P, Wimmer J, et al. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates. J Pediatr Pharmacol Ther. 2012;17(3):252–262. | ||
Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr. 2008;152(5):685–689. | ||
Saps M, Youssef N, Miranda A, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology. 2009;137(4):1261–1269. | ||
Finkel JC, Pestieau SR, Quezado ZM. Ketamine as an adjuvant for treatment of cancer pain in children and adolescents. J Pain. 2007;8(6):515–521. | ||
Kajiume T, Sera Y, Nakanuno R, et al. Continuous intravenous infusion of ketamine and lidocaine as adjuvant analgesics in a 5-year-old patient with neuropathic cancer pain. J Palliat Med. 2012;15(6):719–722. | ||
Krane E, Leong M, Golianu B, Leong Y. Treatment of pediatric pain with nonconventional analgesics. In: Schechter N, Berde C, Yaster M, eds. Pain in Infants, Children, and Adolescents. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:225–240. | ||
Wallace MS, Lee J, Sorkin L, Dunn JS, Yaksh T, Yu A. Intravenous lidocaine: effects on controlling pain after anti-GD2 antibody therapy in children with neuroblastoma--a report of a series. Anesth Analg. 1997;85(4):794–796. | ||
Massey GV, Pedigo S, Dunn NL, Grossman NJ, Russell EC. Continuous lidocaine infusion for the relief of refractory malignant pain in a terminally ill pediatric cancer patient. J Pediatr Hematol Oncol. 2002;24(7):566–568. | ||
Groholt EK, Stigum H, Nordhagen R, Kohler L. Recurrent pain in children, socio-economic factors and accumulation in families. Eur J Epidemiol. 2003;18(10):965–975. | ||
Schwille IJ, Giel KE, Ellert U, Zipfel S, Enck P. A community-based survey of abdominal pain prevalence, characteristics, and health care use among children. Clin Gastroenterol Hepatol. 2009;7(10):1062–1068. | ||
American_Society_of_Anesthesiologists_(ASA). Barriers lead to poor pain control in Latino children after surgery. 2014; Avilable from: http://www.sciencedaily.com/releases/2014/10/141013112316.htm. Accessed May 1, 2017. | ||
Wang L, Haberland C, Thurm C, Bhattacharya J, Park KT. Health outcomes in US children with abdominal pain at major emergency departments associated with race and socioeconomic status. PLoS One. 2015;10(8):e0132758. | ||
Hennes H, Kim MK, Pirrallo RG. Prehospital pain management: a comparison of providers’ perceptions and practices. Prehosp Emerg Care. 2005;9(1):32–39. | ||
Probst BD, Lyons E, Leonard D, Esposito TJ. Factors affecting emergency department assessment and management of pain in children. Pediatr Emerg Care. 2005;21(5):298–305. | ||
Cone DC, Richardson LD, Todd KH, Betancourt JR, Lowe RA. Health care disparities in emergency medicine. Acad Emerg Med. 2003;10(11):1176–1183. | ||
Fink R. Pain assessment: the cornerstone to optimal pain management. Proc (Bayl Univ Med Cent). 2000;13(3):236–239. | ||
Wong C, Lau E, Palozzi L, Campbell F. Pain management in children: part 1 – pain assessment tools and a brief review of nonpharmacological and pharmacological treatment options. Can Pharm J (Ott). 2012;145(5):222–225. | ||
Wells N PC, McCaffery M. Improving the Quality of Care Through Pain Assessment and Management. Rockville, MD: Agency for Healthcare Research and Quality (US); 2008. | ||
Hatherley C, Jennings N, Cross R. Time to analgesia and pain score documentation best practice standards for the emergency department – a literature review. Australas Emerg Nurs J. 2016;19(1):26–36. | ||
Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. | ||
Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007. | ||
Ledowski T, Reimer M, Chavez V, Kapoor V, Wenk M. Effects of acute postoperative pain on catecholamine plasma levels, hemodynamic parameters, and cardiac autonomic control. Pain. 2012;153(4):759–764. | ||
Janig W. Autonomic reactions in pain. Pain. 2012;153(4):733–735. | ||
Breau LM, Finley GA, McGrath PJ, Camfield CS. Validation of the non-communicating children’s pain checklist-postoperative version. Anesthesiology. 2002;96(3):528–535. | ||
Breau LM, McGrath PJ, Camfield CS, Finley GA. Psychometric properties of the non-communicating children’s pain checklist-revised. Pain. 2002;99(1–2):349–357. | ||
Breau LM. Non-communicating children’s pain checklist: better pain assessment for severely disabled children. Expert Rev Pharmacoecon Outcomes Res. 2003;3(3):327–339. | ||
Hunt A, Goldman A, Seers K, et al. Clinical validation of the paediatric pain profile. Dev Med Child Neurol. 2004;46(1):9–18. | ||
Hunt A, Wisbeach A, Seers K, et al. Development of the paediatric pain profile: role of video analysis and saliva cortisol in validating a tool to assess pain in children with severe neurological disability. J Pain Symptom Manage. 2007;33(3):276–289. | ||
Voepel-Lewis T, Malviya S, Tait AR, et al. A comparison of the clinical utility of pain assessment tools for children with cognitive impairment. Anesth Analg. 2008;106(1):72–78. | ||
Michelson JD, Addante RA, Charlson MD. Multimodal analgesia therapy reduces length of hospitalization in patients undergoing fusions of the ankle and hindfoot. Foot Ankle Int. 2013;34(11):1526–1534. | ||
Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. | ||
Rork JF, Berde CB, Goldstein RD. Regional anesthesia approaches to pain management in pediatric palliative care: a review of current knowledge. J Pain Symptom Manage. 2013;46(6):859–873. | ||
Jerstad SJ, Boutelle KN, Ness KK, Stice E. Prospective reciprocal relations between physical activity and depression in female adolescents. J Consult Clin Psychol. 2010;78(2):268–272. | ||
Wilson AC, Palermo TM. Physical activity and function in adolescents with chronic pain: a controlled study using actigraphy. J Pain. 2012;13(2):121–130. | ||
Lewandowski AS, Palermo TM, Stinson J, Handley S, Chambers CT. Systematic review of family functioning in families of children and adolescents with chronic pain. J Pain. 2010;11(11):1027–1038. | ||
Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain. 2010;148(3):387–397. | ||
Palermo TM, Holley AL. The importance of the family environment in pediatric chronic pain. JAMA pediatr. 2013;167(1):93–94. | ||
Bussing A, Ostermann T, Ludtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13(1):1–9. | ||
Evans S, Moieni M, Taub R, et al. Iyengar yoga for young adults with rheumatoid arthritis: results from a mixed-methods pilot study. J Pain Symptom Manage. 2010;39(5):904–913. | ||
Evans S, Moieni M, Sternlieb B, Tsao JC, Zeltzer LK. Yoga for youth in pain: the UCLA pediatric pain program model. Holist Nurs Pract. 2012;26(5):262–271. |
Supplementary material
List of IRBs and Ethics Committees
Study sponsor: Canadian Institutes of Health Research (CIHR MOP-89984)
Participating sites
- Calgary (Alberta Children’s Hospital – Dr Sharron Spicer) – The Conjoint Health Research Ethics Board (CHREB) (University of Calgary)
- Edmonton (Stollery Children’s Hospital – Dr Dawn Davies) – Health Research Ethics Board (University of Alberta)
- Toronto (The Hospital for Sick Children – Dr Adam Rapoport) – Research Ethics Board (SickKids)
- Ottawa (Children’s Hospital of Eastern Ontario – Dr Christina Vadeboncoeur) – Research Ethics Board (CHEO Research Institute)
- Montreal (Montreal Children’s Hospital – Dr Stephen Liben – Research Ethics Board (McGill University Health Center)
- Vancouver – The University of British Columbia/Children’s and Women’s Health Centre of British Columbia Research Ethics Board (UBC C&W REB)
- Halifax – Research Ethics Office (IWK Health Centre Research)
- Gillette Children’s in St Paul – Institutional Review Board (IRB) (University of Minnesota)
- Children’s Hospitals and Clinics of Minnesota – Institutional Review Board (IRB) (Children’s Hospitals and Clinics of Minnesota)
Institutions
- York University ON – Office of Research Ethics (ORE), Human Participants Review Sub-Committee
- UCSF – Committee on Human Research (CHR)
- UVIC – Human Research Ethics Board (HREB)
- U Waterloo – Office of Research Ethics
- Laurier – Research Ethics Board (Wilfrid Laurier University)
Hospices
Canuck Place Children’s Hospice Ethics Committee
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.