Back to Journals » Journal of Inflammation Research » Volume 9
Vitamin D and the immunomodulation of rotator cuff injury
Authors Dougherty K, Dilisio M, Agrawal D
Received 11 February 2016
Accepted for publication 31 March 2016
Published 14 June 2016 Volume 2016:9 Pages 123—131
DOI https://doi.org/10.2147/JIR.S106206
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Kaitlin A Dougherty,1 Matthew F Dilisio,2 Devendra K Agrawal1
1Department of Clinical & Translational Science, 2Department of Orthopedic Surgery, Creighton University School of Medicine, Omaha, NE, USA
Abstract: Tendon-to-bone healing after rotator cuff repair surgery has a failure rate of 20%–94%. There has been a recent interest to determine the factors that act as determinants between successful and unsuccessful rotator cuff repair. Vitamin D level in patients is one of the factors that have been linked to bone and muscle proliferation and healing, and it may have an effect on tendon-to-bone healing. The purpose of this article is to critically review relevant published research that relates to the effect of vitamin D on rotator cuff tears and subsequent healing. A review of the literature was conducted to identify all studies that investigate the relationship between vitamin D and tendon healing, in addition to its mechanism of action. The data were then analyzed in order to summarize what is currently known about vitamin D, rotator cuff pathology, and tendon-to-bone healing. The activated metabolite of vitamin D, 1α,25-dihydroxyvitamin D3, affects osteoblast proliferation and differentiation. Likewise, vitamin D plays a significant role in the tendon-to-bone healing process by increasing the bone mineral density and strengthening the skeletal muscles. The 1α,25-dihydroxyvitamin D3 binds to vitamin D receptors on myocytes to stimulate growth and proliferation. The form of vitamin D produced by the liver, calcifediol, is a key initiator of the myocyte healing process by moving phosphate into myocytes, which improves function and metabolism. Investigation into the effect of vitamin D on tendons has been sparse, but limited studies have been promising. Matrix metalloproteinases play an active role in remodeling the extracellular matrix (ECM) of tendons, particularly deleterious remodeling of the collagen fibers. Also, the levels of transforming growth factor-β3 positively influence the success of the surgery for rotator cuff repair. In the tendon-to-bone healing process, vitamin D has been shown to successfully influence bone and muscle healing, but more research is needed to delve into the mechanisms of vitamin D as a factor in skeletal tendon health and healing.
Keywords: bone, calcium, 1,25-dihydroxyvitamin D, matrix metalloproteinases, muscle, rotator cuff tear, tendon
Introduction
Rotator cuff repair surgery is a common procedure to restore function and relieve pain in patients with a symptomatic rotator cuff tear. However, the procedure is accompanied by a high failure rate (20%–94%) of tendon-to-bone healing.3
Vitamin D is an important regulator of matrix metalloproteinase (MMP)-9, varying inversely with the inflammatory factor.6 also found a positive correlation between vitamin D levels and the strength of tendon-to-bone healing. With the advancement in the scientific enquiry and the findings on the effects of vitamin D on the bone component in tendon-to-bone healing, a critical role of vitamin D in tendon healing cannot be ruled out. However, there are currently limited reports investigating the specific role of vitamin D on tendon-to-bone healing.
In this article, we summarize the role of vitamin D in bone, muscle, and tendon physiology, and critically review the published studies that investigated the role of vitamin D in tendon-to-bone healing and discuss outstanding questions and future directions.
Vitamin D
Vitamin D deficiency affects approximately 1 billion people worldwide.3 Many people who suffer from this deficiency lead lifestyles that keep them indoors or they live in a region where sunlight is sparse (such as northwestern Europe). Generally, serum 25-hydroxy vitamin D levels define vitamin D status, and vitamin D deficiency (defined by the Institute of Medicine to be at <12 ng/mL of serum 25-hydroxy vitamin D) correlates with decreased bone density and rigidity (rickets), as well as adverse effects on muscle health and healing.6,7
When sunlight hits the skin, the ultraviolet radiation turns 7-dehydrocholesterol to pre-vitamin D3. The liver then metabolizes pre-vitamin D3 to 25-hydroxyvitamin D3 (calcifediol). Finally, calcifediol is metabolized into 1,25-dihydroxyvitamin D3 (vitamin D) in the kidneys.6–9 The vitamin D precursor, calcifediol, produced by the liver influences the accumulation of phosphate into muscle cells, along with the binding of vitamin D to vitamin D receptors (VDRs) on the myocyte plasma membrane. The phosphate is then metabolized to creatine phosphate, which supports the metabolism and function of myocytes.6–9 Activated VDRs result in the absorption of calcium to regulate the circulating levels of calcium and phosphate for normal mineralization of bone, which is intimately related to parathyroid hormone. The relationship of vitamin D and bones is one of the most critical and well-investigated functions of vitamin D within the body.
The parathyroid gland releases the parathyroid hormone when the levels of blood calcium are low. The released parathyroid hormone pulls calcium from the bone to blood and signals the kidney to convert 25-hydroxyvitamin D to 1,25 dihydroxyvitamin D (the active form), which ultimately results in an increase in the transcription of genes responsible for calcium absorption.12 using 25-hydroxyvitamin D3-1α-hydroxylase–knockout mice. When the parathyroid is removed, vitamin D cannot function properly within the patient, resulting in hypocalcemia.10 In order for a patient to have adequate and efficient blood calcium levels, both vitamin D and parathyroid hormone must be present at sufficient levels and function together; otherwise, the strength of the bones is compromised and could result in rickets.10,11
Rotator cuff pathology and surgical repair
The function of the rotator cuff is to provide a stable fulcrum about which the deltoid can elevate the shoulder and raise the arm in space. A rotator cuff injury can be painful due to direct tendon injury and associated inflammation, and can also lead to significant disability due to the weakness than can result from disruption of the tendon and alteration in shoulder biomechanics.13 Rotator cuff pathology exists in a spectrum, ranging from inflammation of the overlying bursa and tendon to partial-thickness tears to full-thickness tears to massive, chronic, irreparable rotator cuff tears with arthritis. Once a full-thickness tear develops, there is minimal evidence to suggest that it will ever heal spontaneously.14
Shoulder pathology is common, accounting for over 4.5 million patient visits per year in the US.17
The basic principle of rotator cuff repair is to repair the torn tendon back to the humeral tuberosities where it has been avulsed from the bone. However, the incidence of the tendon–bone healing is only ∼20%,1 with other investigators reporting a failure of healing to be as high as 94%.2 While advancements in surgical techniques and implants allow shoulder surgeons to obtain a more biomechanically stable rotator cuff repair,13 failure of tendon healing is still a significant problem. In response to this failure, the forefront of orthopedic research has shifted to biologic augmentation of the tendon–bone interface in order to enhance the ability of the body to heal a rotator cuff tear.6 There is encouraging early evidence that supports the role of vitamin D in tendon–bone healing.3,8
Vitamin D and inflammation
One of the major beneficial functions of vitamin D in the healing process is to decrease inflammation. Vitamin D has been shown to significantly downregulate cellular response to tumor necrosis factor-α (TNF-α), a major inflammatory cytokine.18 It is thought that vitamin D blocks the c-Jun N-terminal kinase (a member of the mitogen-activated protein kinase family) and the nuclear factor-κB (NF-κB) pathway to block the cellular response to TNF-α.18 The exact mechanisms underlying this action are still unknown, but many possibilities exist because vitamin D has the ability to bind to receptors on both the cell membrane and the nuclear membrane. Along with TNF-α, a recent study found that the levels of interleukin (IL)-6 and C-reactive protein were lower in patients who had sufficient levels of serum vitamin D, as compared to a group that was deficient in vitamin D.19 A recent study in BALB/c mice showed that mice with induced vitamin D deficiency had a higher incidence of airway hyperresponsiveness when exposed to antigens than the vitamin D-sufficient mice.20 Agrawal et al20 also found that sufficient levels of vitamin D significantly reduced the levels of IL-5 and IL-13 and increased the levels of the anti-inflammatory cytokine, IL-10, within the airways of sensitized mice.20 Because of its anti-inflammatory nature, vitamin D is sometimes used as an adjunct therapy for patients with arthritis, psoriasis, and some types of cancer.21 More investigation is needed to fully understand the specific mechanisms by which vitamin D exerts the anti-inflammatory effects.
Vitamin D and bone healing
Gorter et al22 found vitamin D to be invaluable to bone density and repair, both postsurgery and during normal metabolic activity. Vitamin D does not directly signal the osteocytes to divide, thereby stimulating the healing process by generic pathways; rather, it increases the bone mineral density (BMD).23 BMD is the amount of mineral matter per square centimeter in bones and, when increased, has been shown to result in better quality of healing.24 Specifically, an increase in BMD correlates to a greater degree of healing in patients who are postprocedural, as confirmed with dual-energy X-ray absorptiometry scans.25 Calcium and parathyroid hormone, along with vitamin D, have been shown to influence BMD, but it is not exactly clear how these chemicals act within the osteocyte to facilitate the healing process.23 Currently, BMD levels can be measured using a dual-energy X-ray absorptiometry scan, which can help the physician determine timelines and best courses of action following a procedure.23 The next step in this investigation of BMD would be to have a larger-scale test to further validate the results obtained by Chung et al,23 since their study only consisted of 272 people. Also, in vitro studies in cells and in vivo testing in experimental animals should be done to determine the relationship of vitamin D with BMD on a microscale and in a situation where the variables can be more tightly controlled. By understanding the cellular processes, improved therapeutic approaches could be developed for physicians and surgeons to better manage and improve the predicted outcome of their postprocedural patients.
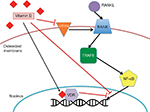
A direct connection between 1,25-dihydroxyvitamin D and the formation and growth of bone is the relationship between vitamin D and receptor activator of NF-κB (RANK) (Figure 1). The RANK receptor, a member of the TNF-α receptor molecular subfamily, functions to signal osteoclastogenesis and bone resorption.26 Recently, research has been done to examine the therapeutic effects of anti-RANK antibodies in treating osteoporosis as well as pediatric osteosarcomas. Interestingly, a huge increase in anti-RANK antibodies decreased the rate of suture mineralization in mice and had adverse effects on long bone formation and tooth eruption.27 Vitamin D also suppresses the expression of RANK, but possesses more potential for therapeutic use than the anti-RANK antibodies in chemotherapy.28 Vitamin D decreases the levels of colony-stimulating factor-1 receptor (c-Fms), a receptor for macrophage colony-stimulating factor, which, in turn, downregulates RANK and osteoclastogenesis.28 Yoskovitz et al29 found that vitamin D acts on the regions marginal to the RANK gene (specifically single nucleotide polymorphism [SNP] rs9594738) by reducing the inhibition on the genomic region, affecting the transcription and translation of RANK protein.
Vitamin D exerts an indirect effect on the genetic expression within the osteoblasts. Serum vitamin D levels affect the expression of both bone morphogenetic proteins (BMPs) and transforming growth factor-β. BMPs are signaling molecules that regulate the stem cell activity and differentiation in various tissues, including bone and connective tissue.30 Both BMP-2 and BMP-4 show decreased expression relative to vitamin D, while BMP-3 mRNA expression is increased in osteoblasts. Vitamin D also affects the levels of transforming growth factor-β, but there exists some dispute as to the exact nature of this relationship.22 In early development and differentiation of osteoblasts, sufficient levels of vitamin D (or 1,25-dihydroxyvitamin D3) have been associated with beneficial osteogenic differentiation and mineralization.9 found in cell culture studies that 1α,25-dihydroxyvitamin D3 directly stimulated matrix mineralization and osteoblast differentiation.9 Also, these investigators found the expression of VDRs on the nuclei of osteoblasts which, potentially, provide a relationship between vitamin D and gene expression.9
An adequate calcium level, as well as vitamin D, is necessary to reduce the risk of bone fracture. In a study by Avenell et al,31 there was a reduced incidence of hip fractures in elderly patients with sufficient calcium and vitamin D levels in comparison to a group with deficient levels of serum vitamin D. Hedström et al32 found similar results in their investigation of 63 randomized elderly women with hip fracture. It was found that the patients who received a mixed dose of vitamin D, calcium, and anabolic steroids had a steadier gait and better functions at both the 6- and 12-month postprocedural checkups. However, in this study, it was unclear whether the vitamin D had any tangible effect on muscle mass and healing or if the steroid treatment was solely responsible for the finding.
Indeed, more well-designed studies are warranted to further examine the actual healing of bones in relation to vitamin D. Avenell et al31 and Hedström et al32 made significant progress investigating how vitamin D strengthens bones, but not much work has been done to investigate the specific effect of vitamin D on the quality and speed of bone healing.22 Studies in relevant animal models would allow for greater control of vitamin D levels and type of bone break in order to have a less confounding study, which could then be adapted to human testing at minimal risk (since vitamin D supplements already exist with negligible adverse effects). By establishing the role of vitamin D in fracture healing, physicians would be in a better position to prescribe patients with a more nuanced treatment and healing plan.
Vitamin D and muscle healing
Along with bone repair and healing, vitamin D levels also positively correlate with muscle growth and metabolism.6 Although underlying mechanisms are not currently well understood, vitamin D acts on specific VDRs on myocytes. Activation of these receptors triggers a signaling pathway cascade that ultimately activates specific genes in the nucleus of the myocyte.33 While current evidence has shown that myocytes are a direct target for vitamin D,34,35 it remains unclear whether vitamin D acts by a direct or indirect mechanism on the genetic transcription of myocytes. This question could be answered by focused in vitro studies on myocytes with fluorescent or radioactive labeled vitamin D.
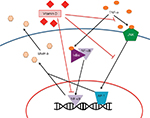
Sufficient or increased levels of vitamin D in patients correlate with an increase in the size, number, and strength of type II muscle fibers.33 In fall-prone poststroke patients, a 59% reduction in falls was reported in patients who received a vitamin D supplement (ergocalciferol), when compared to the placebo group.36 In a well-designed, placebo-controlled study, Sato et al36 positively linked serum vitamin D levels (achieved through supplementation) and the diameter of type II muscle fibers (P<0.0001). This increased muscle proliferation can potentially be linked to the Notch/BMP-4 signaling pathway on myocytes (Figure 2). While the exact mechanism is not yet known, decreased vitamin D levels correlated with a decrease in BMP-4 expression on myocyte surface, thereby decreasing myocyte growth and proliferation.37
  | Figure 2 Potential effects of vitamin D on muscle cell differentiation and proliferation. |
Vitamin D can be more effective in these types of patients if they also receive a calcium supplement. Taken together, the vitamin D and calcium have been shown to create higher muscle volume and more stable and solid bones.32
In rotator cuff muscles, vitamin D is a critical factor in muscle performance. Oh et al8 found that increased vitamin D levels in patients decreased the fatty degeneration of the supraspinatus and infraspinatus while increasing isokinetic muscular performance. When the data were analyzed by the muscle tear grade, a negative correlation was found between tear grade number and serum vitamin D levels.8 Although this correlation was not statistically significant (P≥0.05), this is most likely due to the small sample size used in this investigation (N=228).
An increase in steatosis of rotator cuff muscles inversely correlates with the amount of healing in muscle tears in the rotator cuff and positively correlates with the tear grade.8 Steatosis of rotator cuff muscles has been found as a result of diabetes, polymyositis, muscular dystrophy, and low levels of serum calcifediol. Specifically, low level of serum calcifediol has been linked to higher level of steatosis in all four of the rotator cuff muscles.8
The study by Oh et al8 demonstrated a promise in linking vitamin D and muscle healing and health. In this study, there were too many confounders to equivocally establish that vitamin D was the major factor in fatty degeneration in muscle tear repair.8 The groups were divided based on the nature of the rotator cuff injury and the vitamin D levels were not treated as a dependent variable. However, more critical and well-designed studies are needed in which the healing of the muscles is the dependent variable and the vitamin D levels are independent variables.
Vitamin D and tendon repair
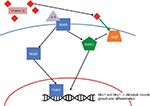
The success of rotator cuff surgery depends on the ability of the torn tendon to heal to the tuberosity of the humerus. This fibrovascular tendon-to-bone scar is weaker than the original attachment before injury and is affected by the age of the patient and the size of the avulsion.3 Specifically in the supraspinatous muscle, there exists four distinct zones on the junction of tendon-to-bone insertion: tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone. Each of these areas has its own unique makeup of cartilages, with the fibrocartilages being made up of primarily fibrillar collagens types I, II, and III in various concentrations. However, following surgery for rotator cuff repair, the four distinct zones disappear and the collagen fibers and other extracellular matrix proteins of the natural insertion form bridges.38 The tendon–bone healing of the rotator cuff is a complex process and requires many factors, including intrinsic and extrinsic cells, cytokines and growth factors, and collagens. The fibrillar type collagens, collagen I and collagen III, are critical in tendon–bone healing; collagen I is present during the remodeling, whereas collagen III is present in the early stage of the healing process and is associated with the degenerative and scar tissue.38 Healing failure is due, in part, to changes in the cartilage concentrations within the fibrovascular scar (composed of mostly type III collagen, which is replaced by type I collagen to achieve a specific ratio).3 Investigators have found that vitamin D positively influences the rotator cuff tendons through MMPs (Figure 3). MMPs are enzymes that contain zinc ion in their active site and are essential in extracellular matrix breakdown and repair, specifically collagen degradation.18 Reider40 reported a correlation between increase in MMPs and poor healing in rotator cuff muscles.40 Likewise, blockade of MMPs in tendon-to-bone healing was shown to improve healing by increasing fibrocartilage and collagen organization as well as improving the scar strength in rats.4 TNF-α stimulates the secretion of MMP-9 (P<0.0005), an inflammatory component that is prevalent in wounds and aids in cutaneous healing (Figure 3). In an in vitro study, Bahar-Shany et al18 found that calcitriol inhibited the activation of MMP-9 by TNF-α (P<0.01) and exerted its effect by inhibiting the extracellular signal-regulated kinase, c-Jun N-terminal kinase, and (potentially) the NF-κB pathways to varying degrees. Angeline et al3 also found an inverse relationship between the levels of MMP-1 and MMP-9 and rotator cuff healing.
Tissue inhibitors of MMPs (TIMPs) inhibit the connective tissue reconstructive properties of MMPs, aiding in rotator cuff healing. Specifically, Timms et al4 found that TIMP-1 levels fluctuated with MMP-9. Vitamin D was shown to be another determinant of the amount of MMP-9 circulating in the body. Angeline et al3 An increase in TIMPs improved collagen organization and decreased collagen degradation, allowing for an increased amount and quality of rotator cuff healing.3
The most recent study by Angeline et al3 in 62 rats demonstrates the potential role of vitamin D in rotator cuff healing in humans. There have been many in vitro studies looking at the cellular mechanisms relating vitamin D and tendons. However, the in vivo studies are very limited. Therefore, in vivo studies with larger sample size are warranted to establish the relationship between vitamin D status and rotator cuff repair, followed by clinical studies to potentially develop a postsurgical therapy for humans.3 Vitamin D has also been associated with strengthening of surgical scars by indirectly affecting the remodeling of fibrocartilage; however, a definitive evidence to support the role of vitamin D in this regard has yet to be established. There have been little to no definitive published articles or research relating vitamin D to either mineralized or unmineralized tendon. Thus, well-designed investigations are needed with a focus on the role of vitamin D in the healing of tendon rather than its role as a strengthening or protective factor on the tendon.
Conclusion
Much research has been conducted concerning the specific effects of vitamin D on bone and muscle, but some studies have been conducted to study the effects of vitamin D on tendon and tendon healing. Given the nature and prevalence of rotator cuff injury requiring surgery, the potential for better management postoperatively with vitamin D supplementation is encouraging. Studies have investigated the effect of vitamin D on tendon-to-bone healing following rotator cuff repair. Thus far, much of the understanding on the role of vitamin D in the healing process comes from the effects of vitamin D on bone healing and repair with little attention into the effects on the tendon in tendon-to-bone healing. Nonetheless, limited studies, as discussed above, show promise for further research of vitamin D on rotator cuff tendon healing. Vitamin D supplements are a very simple therapy for the patients to take and the physicians to prescribe; therefore, if vitamin D is found to be beneficial in tendon repair, it would greatly benefit rotator cuff surgery patients and, indeed, many other orthopedic surgery patients with least cost.
Acknowledgments
This work was supported by research grants R01 HL116042, R01 HL112597, and R01 HL120659 from the Office of Director, National Institutes of Health, and the National Heart Lung and Blood Institute, NIH, USA to DK Agrawal, and Haddix Grant to MF Dilisio. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96:265–271. | ||
Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004; | ||
Angeline ME, Ma R, Pascual-Garrido C, et al. Effect of diet-induced vitamin D deficiency on rotator cuff healing in a rat model. Am J Sports Med. 2013;42(1):27–34. | ||
Timms PM, Mannan N, Hittman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95(12):787–796. | ||
Robertson CM, Chen CT, Shindle MK, Cordasco FA, Rodeo SA, Warren RF. Failed healing of rotator cuff repair correlates with altered collagenase and gelatinase in supraspinatus and subscapularis tendons. Am J Sports Med. 2012;40(9):1993–2001. | ||
Nossov S, Dines JS, Murrell GA, Rodeo SA, Bedi A. Biologic augmentation of tendon-to-bone healing: scaffolds, mechanical load, vitamin D, and diabetes. Instr Course Lectr. 2014;63:451–462. | ||
Pramyothin P, Holick MF. Vitamin D supplementation. Curr Opin Gastroenterol. 2012;28(2):139–150. | ||
Oh JH, Kim SH, Kim JH, Shin YH, Yoon JP, Oh CH. The level of vitamin D in the serum correlates with fatty degeneration of the muscles of the rotator cuff. J Bone Joint Surg Br. 2009;91(12):1587–1593. | ||
Van Driel M, Koedam M, Buurman CJ, et al. Evidence that both 1α,25-dihydroxyvitamin D3 and 24-dihydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem. 2006;99(3):922–935. | ||
Salinger E, Moore J. Perioperative indicators of hypocalcemia in total thyroidectomy: the role of vitamin D and parathyroid hormone. Am J Surg. 2013;206(6):876–881. | ||
Lioben L, Carmeliet G. The delicate balance between vitamin D, calcium and bone homeostasis: lessons learned from intestinal- and osteocyte-specific VDR null mice. J Steroid Biochem Mol Biol. 2013;136:102–106. | ||
Hoenderop J, Dardenne O, van Abel M, et al. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-dihydroxyvitamin D3-1α-hydroxylase knockout mice. FASEB J. 2002;16(11):1398–1406. | ||
Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333–346. | ||
Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199–203. | ||
Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;455:52–63. | ||
National Ambulatory Medical Care Survey (NAMCS); 2010. Available from: http://www.cdc.gov/nchs/ahcd/ahcd_questionnaires.htm. Accessed March 16, 2015. | ||
Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;2095(22):1993–2000. | ||
Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFα in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2009;222(3):729–737. | ||
Laird E, McNulty H, Ward M, et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J Clin Endocrinol Metab. 2014;99(5):1807–1815. | ||
Agrawal T, Gupta GK, Agrawal DK. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin Exp Allergy. 2013;43(6):672–683. | ||
Nagpal S, Lu J, Boehm MF. Vitamin D analogs: mechanism of action and therapeutic applications. 2001. Curr Med Chem. 2001;8(13): | ||
Gorter EA, Hamdy NAT, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone. 2014;64:288–297. | ||
Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39(10):2099–2107. | ||
NIH Osteoporosis and Related Bone Diseases National Resource Center. “Bone mass measurement: What the numbers mean.” Available from: http://www.niams.nih.gov/health_info/bone/bone_health/bone_mass_measure.asp. Accessed November 28, 2014. | ||
Oh JH, Song BW, Kim SH, et al. Measurement of bone mineral density of bilateral proximal humeri using DXA in patients with unilateral rotator cuff tear. Osteoporos Int. 2014;25(11):2639–2648. | ||
Aoyama E, Kubota S, Khattab HM, Nishida T, Takigawa M. CCH2 enhances RANKL-inducing osteoclast differentiation via direct binding to RANK and OPG. Bone. 2014;73:242–248. | ||
Lézot F, Chesneau J, Navet B, et al. Skeletal consequences of RANKL-blocking antibody (IK22-5) injections during growth: mouse strain disparities and synergic effect with zoledronic acid. Bone. 2014;73: | ||
Kim T, Lee B, Kwon E, et al. 1,25-dihydroxyvitamin D3 inhibits directly human osteoblastogenesis by down-regulation of the c-Fms and RANK expression. Joint Bone Spine. 2013;80(3):307–314. | ||
Yoskovitz G, Garcia-Giralt N, Rodriguez-Sanz M, et al. Analysis of RANK and RANKL in the post-GWAS context: functional evidence of vitamin D stimulation through a RANKL distal region. J Bone Miner Res. 2013;28(12):2550–2560. | ||
Yousef H, Morgenthaler A, Schlesinger C, Bugaj L, Conboy IM, Schaffer DV. Age-associated increase in BMP signaling inhibits hippocampal neurogenesis. Stem Cells. 2014;33(5):1577–1588. | ||
Avenell A, Gillespie WJ, Gillespie LD, O-Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;15;(2):CD000227. | ||
Hedström M, Sjöberg K, Brosjö E, Åström K, Sjöberg H, Dalén N. Positive effects of anabolic steroids, vitamin D and calcium on muscle mass, bone mineral density and clinical function after a hip fracture: a randomized study of 63 women. J Bone Joint Surg. 2002;84(4): | ||
Cipriani C, Pepe J, Piemonte S, Colangelo L, Cilli M, Minisola S. Vitamin D and its relationship with obesity and muscle. Int J Endocrinol. 2014;2014:841248. | ||
Bartoszewska M, Kamboj M, Patel DP. Vitamin D, muscle function, and exercise performance. Pediatr Clin North Am. 2010;57(3):849–861. | ||
Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010;20(2):182–190. | ||
Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20(3): | ||
Domingues-Faria C, Chanet A, Salles J, et al. Vitamin D deficiency down-regulates Notch pathway contributing to skeletal muscle atrophy in old wistar rats. Nutr Metab (Lond). 2014;10:11(1):47. | ||
Schaer M, Schober M, Berger S, Boileau P, Zumstein MA. Biologically based strategies to augment rotator cuff tears. Int J Shoulder Surg. 2012;6(2):51–60. | ||
Bedi A, Kovacevic D, Hettrich C, et al. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010;19:384–391. | ||
Reider B. Big D. Am J Sports Med. 2014;42(1):25–26. | ||
Bedi A, Fox AJS, Kovacevic D, Deng X, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38:308. | ||
Mall NA, Tanaka MJ, Choi LS, Paletta GA. Factors affecting rotator cuff healing. J Bone Joint Surg. 2014;96(9):778–788. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.