Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 9
Radiesse®: a novel rejuvenation treatment for the upper arms
Authors Amselem Belilty M
Received 26 July 2015
Accepted for publication 13 September 2015
Published 29 December 2015 Volume 2016:9 Pages 9—14
DOI https://doi.org/10.2147/CCID.S93137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Moisés Amselem
Clinica Dr. Moisés Amselem, Madrid, Spain
Abstract: Nonsurgical esthetic improvement of the upper arms is a desirable goal for many individuals. Radiesse® (calcium hydroxylapatite) is an effective dermal filler for a number of indications because of its volumizing effect and the ability to stimulate neocollagenesis. No studies have reported on its safety and effectiveness for the treatment of the upper arm. In a prospective, open-label study, 30 subjects seeking improvement in the esthetic appearance of their upper arms received injections with Radiesse® (1.5 mL/arm) at two separate visits, 1 month apart. Subjects returned for a follow-up visit 4 months after the second treatment. The primary endpoint was the degree of overall subject and evaluator (investigators and study nurses) satisfaction assessed using the 5-point Global Satisfaction Scale (ranging from "1" very dissatisfied to "5" very satisfied). Secondary endpoints included assessments of skin quality (flaccidity and volume distribution) using a new Visual Analog Scale for upper arms and overall assessment of treatment using the Global Aesthetic Improvement Scale. All (100%) of both subjects and evaluators were "satisfied" or "very satisfied" with treatment. The mean Global Satisfaction Score for investigators and study nurses was 4.60 and for subjects 4.53 (satisfied to very satisfied). Assessments of flaccidity and volume improved significantly compared with baseline at the post-treatment visit and also between visits. Compared with baseline, 77% of subjects were rated as considerably improved (good or great improvement) by the investigator and study nurse; 73% of subjects rated themselves as considerably improved and 43% of these rated a "great improvement." All stated they would repeat the treatment and recommend it to others. No adverse events were reported. Radiesse® is an effective minimally invasive treatment option for improving upper arm contours and was associated with a 100% satisfaction rate for both subjects and evaluators.
Keywords: esthetic improvement, calcium hydroxylapatite, contouring, Radiesse®, upper arm
Introduction
As part of the natural aging process, elasticity and firmness in the skin of the upper arm begin to decrease, and the underlying layers of fat, muscle, and bone in this area also begin to deteriorate.1,2 These biological changes frequently trigger flaccidity in the upper arm skin, along with visible signs of aging in this area, such as loose-hanging skin and wrinkles. While exercising may help improve muscle tone and strength of the upper arms, it will not improve arm volume changes and skin laxity, which have traditionally been corrected by excision of excess skin and removal of subcutaneous fat. Brachioplasty was first introduced in 19543 and has since undergone a series of modifications to improve the appearance of the scar and the resulting contour of the arm. While it remains an effective procedure for patients with massive weight loss and severe skin laxity, it is an invasive procedure requiring an extensive incision and can be associated with complications such as infection, damage to the subcutaneous tissue, and lymphatic networks.4
With the introduction of dermal fillers, a less invasive option for arm contouring became available. Radiesse® (calcium hydroxylapatite [CaHA]; Merz North America, Inc., Raleigh, NC, USA) is an ideal agent for this indication because of its ability to provide both replacement volume and collagen biostimulation as a primary mechanism of action. When it is injected, the gel that carries the CaHA microspheres fills areas that have lost volume, providing a lifting effect shortly after treatment and giving immediate volume. The benefits develop over time as the CaHA microspheres stimulate the production of the body’s own collagen in the skin.5–7 This is thought to regenerate the skin and improve its elasticity and firmness. The esthetic effects of Radiesse® are well established and have been studied in a wide range of indications,8 but to date there are no published data describing its benefits for the upper arm. Given the lack of nonsurgical esthetic treatments and the increasing number of people seeking improvements in this area, this pilot study sought to develop a new scale for upper arm evaluation and to determine whether the collagen-stimulating properties of Radiesse® would make it an effective treatment for improving upper arm contours and firmness in individuals with age-associated upper arm skin changes.
Methods
This prospective, open-label study enrolled 30 women seeking improvement in the esthetic appearance of their upper arms. Eligible subjects were aged 45−65 years old, expressed a desire and willingness for the correction of their upper arm, could comply with the study requirements, and signed an informed consent form. Individuals who were not compatible with the prescribing criteria for the product, such as those receiving anticoagulant or immunosuppressor treatment, those with autoimmune diseases, and pregnant or breast-feeding women, were excluded. A formal ethics committee approval was not sought; patients requesting rejuvenation of the upper arms at the practice were evaluated as part of a pilot study. The results of which, if positive, would lead to the initiation of a full-scale clinical trial with formal ethic committee approval.
Each subject made three visits to the center. At the first visit and before their first treatment, subjects underwent a pre-treatment evaluation by the investigator. This included an evaluation of skin quality using a Visual Analog Scale (VAS) to determine the flaccidity (firmness of the area to be treated) and the distribution of volume, where “0” was very bad and “10” very good. The subjects received treatment at visit 1 (V1) and visit 2 (V2) with Radiesse® 1.5 mL mixed with 0.5 mL lidocaine solution per arm per session, 1 month apart. The total volume injected was 8 mL (4 mL/arm over two sessions). A follow-up visit (V3) was scheduled 4 months after the second treatment. The quality of skin was assessed at all visits. To the authors knowledge, the only scale for the assessment of the upper arms to date was developed for use with brachioplasty and liposuction procedures and is based on the amount of adipose tissue deposit and degree of ptosis.9 In the absence of a tool for use with nonsurgical esthetic procedures, the image bank from the current study was used to develop a new visual analog scale for aging of the upper arms to enable the investigator to classify the arm based on the degree of flaccidity and loss of volume (Arm VAS).
Before injection, the areas to be treated were marked with a pen with the subject standing upright and arms extended away from the body. Clinical photographs were obtained at each visit using standardized patient positioning, camera angles, and room lighting. All subjects were injected while lying down and received injections of Radiesse® plus lidocaine (2.0 mL/arm/session over an area of ~150 cm2) at V1 and V2, 1 month apart. In general, the first injection was performed with a 27 gauge needle over ~50 injection points with a distance of 1−2 cm between injection points; injections were at the level of the deep dermis. If bruising occurred, the second injection was performed with a 25 gauge cannula over one or two injection points at the level of the subdermis. With both needle and cannula, a retrograde fanning technique was used. The arm was gently massaged after treatment to ensure that the product was evenly dispersed. Visit 3 occurred 4 months after the second treatment; at this last visit only assessments were done. Adverse events were collected at all clinic visits.
The primary objective was to evaluate the degree of overall subject and investigator satisfaction after two sessions of treatment with Radiesse® in the region of the upper arm. This was assessed using the 5-point Global Satisfaction Scale, which ranges from “1” very dissatisfied to “5” very satisfied. The degree of improvement and overall satisfaction with the skin changes were assessed by three different observers – subject, investigator, and study nurse – at each visit. Secondary variables were also determined to assess the degree of improvement in skin quality and included the redistribution of volume, and improvements in elasticity and firmness (flaccidity).
At V3, investigators, study nurses, and subjects provided their assessment of upper arm treatment using the Global Aesthetic Improvement Scale, a 5-point subjective scale for efficacy analysis. This was achieved by comparing photographs of the arm taken before treatment and at visit 3 and asking the question: “How would you describe the pre-treatment photograph with the treated arm?” The available choices were: 1) worse (the appearance is worse than at baseline), 2) no change (the appearance is basically the same as at baseline), 3) improvement (the appearance is better than at baseline, but a follow-up assessment is required), 4) good improvement (the appearance is markedly better than at baseline, but could be better), and 5) great improvement (excellent cosmetic result).
Statistical analyses were primarily descriptive. Quantitative variables were described using the mean, standard deviation, and range. Clinical Grading and Instrumentation Scores at each visit were statistically compared with baseline scores using a paired t-test. Changes from baseline were considered significant at the P<0.05 level.
Results
Demographic data
A total of 30 women took part in the study and completed all three study visits. The mean age of the women was 55.6 years. The images collected for this study were used to create a new scale for aging of the upper arms, the Arm VAS (Figure 1). Using this scale, the majority of subjects had type III or type IV arms at baseline, and only a minority had type I or type II arms (Table 1).
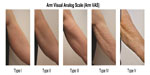  | Figure 1 Arm Visual Analog Scale for aging of the upper arms developed using the image bank from the current study. |
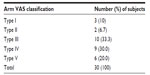  | Table 1 Classification of subjects’ upper arm aging according to the Arm Visual Analog Scale (Arm VAS) |
Satisfaction with treatment
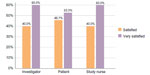
Using the Global Satisfaction Scale to assess overall satisfaction with treatment, investigators, study nurses, and subjects were “satisfied” (4) or “very satisfied” (5) in 100% of cases (Figure 2). The mean Global Satisfaction Score for investigators and study nurses was 4.60 and for subjects 4.53. The mean overall Global Satisfaction Score for investigators, study nurses, and subjects was 4.58. At the end of the study, all subjects stated that they would recommend the treatment to others and all stated that they would repeat the treatment.
Evaluation of skin quality
Assessments of flaccidity and volume were used to evaluate skin quality at each visit. In the overall analysis, both variables improved at the post-treatment visits according to assessments by the investigator, subject, and study nurse. For flaccidity, mean VAS scores for the investigator, subject, and study nurse ranged from 2.8 to 3.5 at V1, and improved to 4.8–5.4 at V2 and 7.5–7.6 at V3 (Table 2). Mean volume scores also improved at each visit from 3.4 to 3.6 at V1, 5.2 to 5.6 at V2, and 7.5 to 7.6 at V3. Improvements from baseline were statistically significant for both flaccidity and volume at V2 and V3 (P<0.05; Table 2). Similar results were observed when the assessments for the two variables were compared between visits. In both cases, significant improvements compared with the previous visit were observed (P<0.05; Figure 3A and B).
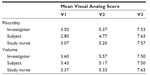  | Table 2 Mean Visual Analog Scores for flaccidity and volume for investigator, subject, and study nurse at each visit |
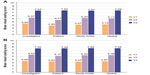  | Figure 3 Comparison of improvement in (A) flaccidity and (B) volume redistribution between visits. |
Overall assessment of treatment
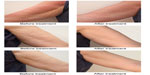
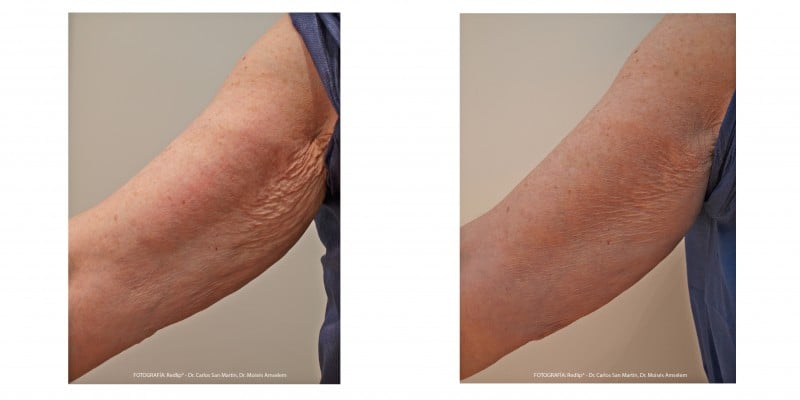
A comparison of subjects’ arms before and after treatment showed a clear benefit of Radiesse® for improving arm appearance (Figure 4). Overall improvement in arm appearance was assessed using the Global Aesthetic Improvement Scale. Whether assessed by investigators, study nurses, or subjects, 100% of treated subjects showed “moderate,” “good,” or “great” improvement. Compared with baseline, 76.6% of subjects showed a considerable (“good to great”) improvement according to the investigator, 73.3% according to the subject, and 76.7% according to the study nurse. Furthermore, 43.3% of subjects assessed the improvement as a “great improvement” (Figure 5).
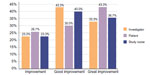  | Figure 5 Overall assessment of treatment by investigator, subject, and study nurse according to the Global Aesthetic Improvement Scale. |
Adverse reactions
None of the study subjects suffered any adverse reactions related to treatment during the study.
Discussion
Radiesse® was very effective at improving upper arm contours in this group of 30 women with visible signs of upper arm aging, including loss of volume and excess flaccidity. All (100%) of both subjects and evaluators were “satisfied” or “very satisfied” with treatment. In addition, all subjects reported that they would repeat the treatment and recommend it to friends.
Wrinkles and tissue laxity in the upper arm area are often tell-tale signs of aging and difficult to camouflage, other than with clothing. Esthetic improvement of the upper arms has therefore become a desirable goal, with more and more people seeking treatment to achieve firm, toned, and youthful appearing arms. Indeed, the American Society for Aesthetic Plastic Surgery reports that brachioplasty is an increasingly popular esthetic surgery procedure in the US, having increased by more than 800% from 1997 to 2014.10
While brachioplasty procedures are effective at improving arm contours, many individuals whose arms have become wrinkled and a little saggy with age are reluctant to accept a visible scar that may affect the activity of the upper arm or clothing choices.11 Yet few nonsurgical treatments for aging upper arms exist, even though they are a visible and important area of cosmetic concern.
This pilot study demonstrates that Radiesse® fills a clinical niche for subjects who have mild to moderate upper arm laxity, but are not accepting a brachioplasty scar. Its dual mechanism of action provides both immediate (replacement volume) and long lasting (collagen biostimulation) volume enhancement. In the current study, improvements were seen at each treatment visit compared with baseline. Only one other study has reported on the use of Radiesse® in the upper arms, which evaluated a body-vectoring technique to correct skin flaccidity in the thighs and abdomen as well as the upper arms. Improvements in skin density and thickness were observed in this short-term study only 5 weeks after treatment.12
Although Radiesse® is completely biodegradable, its clinical esthetic effects are long lasting. At visit 3, 5 months after the first injection, statistically significant improvements in skin flaccidity were observed compared with both baseline and V2. The benefits of treatment therefore continue to increase with time after injection. A recent study, which performed punch biopsies 4 and 9 months after supraperiosteal injection of Radiesse® into the postauricular area, has shown that Radiesse® stimulates the production of collagen type III and type I in a two-step process, thereby collagen type I gradually replaces collagen type III.7 This is consistent with the process of remodeling and collagen production that occurs under physiologic conditions and which provides natural-looking volume replacement and hence younger-looking arms. In this manner, Radiesse® may offer advantages for the upper arm area, over hyaluronic-acid treatments,13 which while effective at providing initial improvement in arm contours, do not have the collagen-stimulating effect of Radiesse® for long-term results. To the author’s knowledge, no studies have evaluated the rejuvenating effects of a hyaluronic acid compared with Radiesse® on the upper arms, and this is an area that warrants further research.
Other nonsurgical treatment procedures that induce neocollagenesis and which may be suitable for individuals with mild skin laxity in the upper arm include energy-based body-contouring devices. These rely on the laser or radiofrequency energy to increase connective tissue temperature and thereby cause collagen formation and tissue tightening.14,15 Although beneficial effects have been shown with these devices, clinical outcomes can be inconsistent, and the treatments require multiple sessions and can be painful. Intense, focused ultrasound is the most recent energy-based therapy to become available. It is able to target much deeper dermal elements than the other devices, which can lead to skin lifting as well as tightening, and it has been used successfully to improve the clinical appearance (texture and contour) of the upper arms for at least 6 months.16
In addition to immediately restoring volume loss, studies of Radiesse® in other treatment areas indicate that it has an average duration of effect of 12–18 months.17 Human histological studies, which examined the distribution of CaHA microspheres after intradermal injection into the forearm, have shown that it is absorbed by the skin at 12 months.18 As other studies have shown that Radiesse® stimulates neocollagenesis,7 it is hypothesized that the duration of effect of Radiesse® is a result of long-term deposition of new collagen and not due to the continued presence of the microspheres. The treatment also has a very well-established safety profile19 and is associated with no down time.
This study has a number of limitations. It was designed as a preliminary study to determine whether Radiesse® would make an effective treatment for improving upper arm contours and firmness. The long-term maintenance of any esthetic effects beyond 4 months was not evaluated. The evaluation of treatment effects was subjective and was not performed by independent raters, which may have introduced a potential source of bias. However, the study has also identified a number of future lines of research. The beneficial effects were seen after only two treatment sessions, 1 month apart. Additional studies are now required to determine whether the beneficial effects of Radiesse® on fibroblasts7 can maintain the observed improvements in upper arm contours and firmness in the long term. Further studies are also warranted to determine the types of subjects best suited to arm contouring with Radiesse®. Even individuals who exercise regularly and have respectable muscle tone can have excess skin that has lost elasticity along with weakened tissue and localized fat deposits. Brachioplasty is likely to continue to be recommended for individuals with severe skin laxity, for example, after massive weight loss, but scarring is an issue, particularly in individuals with higher Fitzpatrick skin types. Liposuction may be effective when minimal skin laxity is present, but can be challenging due to the difficulties of achieving symmetry and the high risk of contour irregularities due to fat structures in the region.20 Long-term studies will also be useful to determine the repeat treatment interval for optimal effect and optimal dose. Finally, the pre- and post-treatment images in this paper illustrate the efficacy of Radiesse® for improving upper arm contours and the value of the new Arm VAS for assessing clinical outcomes. Further work is now required to produce a validated scale for objectively quantifying the severity of aging in the upper arm area. This would be used in future studies and in everyday clinical practice for the evaluation and follow-up of subjects receiving esthetic treatment for the upper arm.
Conclusion
Esthetic improvement of the upper arms is a desirable goal for many individuals. After only two treatments, Radiesse® provided very effective nonsurgical arm contouring and improvements in the quality of skin with a 100% satisfaction rate for both subjects and evaluators.
Acknowledgments
The sponsor of this study, Redlip Madrid SL, was involved in the design and conduct of the study; the collection, analysis, and interpretation of data; and in the preparation, review, and approval of this manuscript. Photography was provided by Jose Salto SL. The author would like to thank Jenny Grice for drafting the manuscript.
Disclosure
The author reports no conflicts of interest in this work.
Glanz S, Gonzalez-Ulloa M. Aesthetic surgery of the arm: Part I. Aesthet Plast Surg. 1981;5:1–17. | |
Teimourian B, Malekzadeh S. Rejuvenation of the upper arm. Plast Reconstr Surg. 1998;102:545–551. | |
Correa-Iturraspe M, Fernandez JC. Dermolipectomia braquial [Brachial dermolipectomy]. Prensa Med Argent. 1954;41:2432–2436. Spanish. | |
Wolf AM, Kuhlmann HW. Reconstructive procedures after massive weight loss. Obes Surg. 2007;17:355–360. | |
Marmur ES, Phelps R, Goldberg DJ. Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler. J Cosmet Laser Ther. 2004;694:223–226. | |
Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34(Suppl 1):S64–S67. | |
Yutskovskaya Y, Kogan E, Leshunov E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13:1047–1052. | |
Van Loghem J, Yutskovskaya YA, Philip Werschler W. Calcium hydroxylapatite: over a decade of clinical experience. J Clin Aesthet Dermatol. 2015;8:38–49. | |
El Khatib HA. Classification of brachial ptosis: strategy for treatment. Plast Reconstr Surg. 2007;119:1337–1342. | |
American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank Statistics 2014. Available from: http://www.surgery.org/sites/default/files/2014-Stats.pdf. Accessed August 23, 2015. | |
Knoetgen J, Moran SL. Long-term outcomes and complications associated with brachioplasty: a retrospective review and cadaveric study. Plast Reconstr Surg. 2006;117:2219–2223. | |
Cogorno Wasylkowski V. Body vectoring technique with Radiesse(®) for tightening of the abdomen, thighs, and brachial zone. Clin Cosmet Investig Dermatol. 2015;8:267–273. | |
Distante F, Pagani V, Bonfigli A. Stabilized hyaluronic acid of non-animal origin for rejuvenating the skin of the upper arm. Dermatol Surg. 2009;35(Suppl 1):389–393. | |
Brightman L, Weiss E, Chapas AM, Karen J, et al. Improvement in arm and post-partum abdominal and flank subcutaneous fat deposits and skin laxity using a bipolar radiofrequency, infrared, vacuum and mechanical massage device. Lasers Surg Med. 2009;41:791–798. | |
Blyumin-Karasik M, Rouhani P, Avashia N, Miteva M, et al. Skin tightening of aging upper arms using an infrared light device. Dermatol Surg. 2011;37:441–449. | |
Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754–759. | |
Bass LS, Smith S, Busso M, McClaren M. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and efficacy results. Aesthet Surg J. 2010;30:235–238. | |
Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthet Plast Surg. 2003;27:354–366. | |
Pavicic T. Calcium hydroxylapatite filler: an overview of safety and tolerability. J Drugs Dermatol. 2013;12:996–1002. | |
Hoyos A, Perez M. Arm dynamic definition by liposculpture and fat grafting. Aesthet Surg J. 2012;32:974–987. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.