Back to Journals » OncoTargets and Therapy » Volume 9
Arsenic trioxide inhibits viability and induces apoptosis through reactivating the Wnt inhibitor secreted frizzled related protein-1 in prostate cancer cells
Authors Zheng L, Jiang H, Zhang Z, Wang K, Wang Q, Li Q, Jiang T
Received 10 July 2015
Accepted for publication 24 November 2015
Published 23 February 2016 Volume 2016:9 Pages 885—894
DOI https://doi.org/10.2147/OTT.S92129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Faris Farassati
Lei Zheng,1,2 Hui Jiang,3 Zhi-Wei Zhang,1 Ke-Nan Wang,1 Qi-Fei Wang,1 Quan-Lin Li,1 Tao Jiang1
1Department of Urology, First Affiliated Hospital of Dalian Medical University, 2Department of Urology, The Fifth People’s Hospital of Dalian, Dalian, 3Department of Urology, Third Affiliated Hospital of Beijing University, Beijing, People’s Republic of China
Background: Growing evidence suggests that arsenic trioxide (As2O3) induces apoptosis and inhibits tumor cell growth in prostate cancer (PCa), although details of the mechanism are still inconclusive. We investigated the antitumor effect of As2O3 in human PCa cell lines LNCaP and PC3 and the underlying mechanisms by focusing on the Wnt signaling pathway.
Methods: The effect of As2O3 on the viability and apoptosis of PCa cells was investigated by cholecystokinin-8 and flow cytometry. The expression of the related proteins in the Wnt signaling pathway and the downstream target genes of the Wnt signaling pathway was examined by Western blot and quantitative real-time PCR assay. The methylation status of the SFRP1 gene promoter was assessed by bisulfite sequencing.
Results: As2O3 inhibited the viability of PCa cells and induced apoptosis of PCa cells in a dose-dependent manner. The protein level of phospho-glycogen synthase kinase-3β was upregulated, whereas the protein level of β-catenin and the mRNA levels of c-MYC, MMP-7, and COX-2 were downregulated in a dose-dependent manner in PCa cells treated with As2O3. In addition, As2O3 pregulated the protein and mRNA levels of secreted frizzled related protein-1, and increased the demethylation of the SFRP1 gene promoter.
Conclusion: Our results suggest that As2O3 may inhibit cell viability and induce apoptosis through reactivating the Wnt inhibitor secreted frizzled related protein-1 in both androgen-dependent and -independent human PCa.
Keywords: arsenic trioxide, CpG island methylation, demethylation, prostate cancer, Wnt signaling pathway, SFRP1
Introduction
Prostate cancer (PCa), the most common malignant tumor of the urinary system, has become the second leading cause of cancer-related death in men in Western developed countries.1 The incidence of PCa is gradually increasing due to increased awareness and screening. Clinically, one in six men are diagnosed with PCa over the age of 60.2 Multiple risk factors may induce the occurrence of PCa, including androgens, dietary factors, ethnicity, family history, and old age.3 Although details of the pathogenesis of PCa are still inconclusive, aberrant activation of the Wnt signaling pathway may be involved. Currently, endocrine therapy, radical surgical resection, radiotherapy, and chemotherapy are used as treatments for PCa.4 However, after treatment with endocrine therapy for an average of 18 months, the disease is likely to be castration-resistant PCa with a poor prognosis.5 Radical surgical resection causes great surgical trauma and affects the sexual function of patients.6 Radiotherapy is accompanied by serious complications, such as acute or chronic gastrointestinal reaction, urinary incontinence, and erectile dysfunction.7 Therefore, it is important to identify new chemotherapeutic agents with lower toxicity and stronger antitumor effects and investigate their mechanisms of antitumor activity.
The Wnt signaling pathway is an evolutionarily conserved signaling cascade and plays important roles in multiple biological processes, including cell proliferation, differentiation, migration, apoptosis, and tumor development.8–10 Wnt proteins are secreted cysteine-rich proteins that serve as ligands for the Wnt signaling pathway.11 β-Catenin is the most important component of the Wnt signaling pathway, and its altered localization is a marker of pathway activation. Once the Wnt ligand binds to a specific member of the frizzled (FZD) family of receptors, a stable receptor complex consisting of Wnt protein, coreceptor lipoprotein receptor-related protein (LRP5/6), and FZD is formed. The Wnt–LRP–FZD complex increases the stability of β-catenin through inhibiting the β-catenin destruction complex (casein kinase1α, AXIN, adenomatous polyposis coli, glycogen synthase kinase-3β [GSK-3β]) that targets β-catenin for degradation, resulting in the accumulation of β-catenin in cytoplasm and its translocation to the nucleus. In the nucleus, β-catenin interacts with T-cell factor/lymphoid enhancer factor transcription factor and then stimulates the expression of downstream target genes, including several oncogenes, such as Cyclin D1, JUN, and c-MYC.12,13 Deregulation of the Wnt signaling pathway is closely associated with many human diseases including cancer.14
Secreted frizzled related protein-1 (SFRP1) is a well-known antagonist of the Wnt signaling pathway and belongs to the secreted glycoprotein SFRP family. SFRP1 can bind to Wnt proteins via its cysteine-rich domain that is homologous to the FZD receptors, and inhibits the interaction between the Wnt ligand and FZD receptor competitively, leading to the inhibition of signal transduction.15,16 Silencing of SFRP1 gene expression by aberrant cytosine-phosphate-guanine (CpG) methylation has been reported in many malignant tumors, including PCa,13 suggesting the possibility of its tumor-specific inactivation. SFRP1 is a candidate mediator of stromal-to-epithelial signaling in PCa.17 Moreover, SFRP1 inhibits the transcriptional activity of androgen receptor and the proliferation of androgen-dependent LNCaP cells.18 However, the effect of SFRP1 gene methylation in PCa cells is still inconclusive.
Arsenic trioxide (As2O3) is a well-studied chemotherapeutic agent that has been widely used to treat acute promyelocytic leukemia and multiple myeloma with good results.19,20 Preclinical studies have demonstrated that As2O3 can induce apoptosis and inhibit tumor cell growth in a wide range of malignant solid cancers including PCa.21–23 As2O3 may exert its antitumor function in PCa through p38 and/or Akt/mTOR signaling pathways.23–25 As2O3 also decreases the methylation level of CDKN2B/CDKN2A genes in human hematologic malignant cells.26 Aberrant activation of the Wnt signaling pathway is closely associated with the pathogenesis of PCa.27,28 However, the relationship between the Wnt signaling pathway and As2O3 has not been clarified. In this study, we examined whether the Wnt signaling pathway is involved in the mechanisms by which As2O3 inhibits the growth of PCa cells. In this study, androgen-dependent human PCa cell line LNCaP and androgen-independent human PCa cell line PC3 were used to investigate the anticancer effect of As2O3. We demonstrated that As2O3 inhibited viability and induced apoptosis in LNCaP and PC3 cells. In addition, As2O3 suppressed the Wnt signaling pathway and downregulated the expression of its target genes. We also showed that As2O3 increased the protein and mRNA levels of SFRP1, and induced the demethylation of the SFRP1 gene promoter. Our data suggest that As2O3 may inhibit the viability of PCa cells through the Wnt signaling pathway in addition to the p38 and Akt/mTOR signaling pathways.
Materials and methods
Cell culture and reagents
This study was approved by the Ethics Committee of Dalian Medical University, and conformed with the provisions of the Declaration of Helsinki. Human PCa cell lines LNCaP and PC3 were obtained from Shanghai Cell Bank (Chinese Academy of Sciences, Shanghai, People’s Republic of China). LNCaP cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% inactivated fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), 100 μg mL−1 penicillin, and 100 μg mL−1 streptomycin. PC3 cells were cultured in fresh Ham’s F12 medium (HyClone, Logan, UT, USA) plus 10% fetal bovine serum (Gibco), 100 μg mL−1 penicillin, and 100 μg mL−1 streptomycin. All cells were incubated at 37°C in a humidified incubator with 5% CO2.
Injectable As2O3 (SL Pharmaceutical Co., Ltd, Beijing, People’s Republic of China) was dissolved in 0.9% sodium chloride injection solution to prepare a 1.0 mmol L−1 stock solution, and stored at 4°C. The As2O3 solution was diluted in culture medium just before use. DAPI, sodium hydrogen sulfite, and hydroquinone were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Annexin V and propidium iodide (PI) were purchased from KeyGen Biotech (Nanjing, People’s Republic of China). Rabbit monoclonal anti-SFRP1 (ab126613) and mouse monoclonal anti-β-catenin (ab22656) were obtained from Abcam (Cambridge, UK). Mouse monoclonal anti-actin, goat antimouse IgG, and goat antirabbit IgG were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Protease inhibitor mixture was obtained from Roche Applied Science (Basel, Switzerland).
Cell viability assay
Cell viability was measured with cholecystokinin-8 (CCK8) kit (Dojindo, Kumamoto, Japan). Cells in a 96-well plate were treated with various concentrations of As2O3 (0, 1, 2.5, 5, 10, and 20 μmol L−1). After incubation for 24 hours, CCK-8 stock solution (20 μL) was added to each well and incubated for 3 hours at 37°C. The absorbance of each well was measured by a microplate reader (Spectramax190, Molecular Devices LLC, Sunnyvale, CA, USA) at 450 nm. The inhibition rates of cell proliferation were calculated according to the following formula: inhibition rate (%)={1 − (mean absorbance of experimental group – mean absorbance of blank group)}/(mean absorbance of control group – mean absorbance of blank group)×100%. All experiments were repeated three times independently.
Cell apoptosis assay
Cell apoptosis was examined by flow cytometry. Cells were seeded into a six-well plate. When the cell density reached 8×105 mL−1, the medium was replaced with new medium containing various concentrations of As2O3 (0, 2.5, 5, and 10 μmol L−1) for 24 hours. Cells were harvested and fixed in 70% ethanol at −20°C overnight. The ethanol was removed and the samples were stained with Annexin V binding buffer (500 μL), Annexin V–FITC (5 μL), and PI (5 μL), and were kept in the dark at room temperature for 10 minutes. Cells in early apoptosis were assayed by using a flow cytometer (FACS Calibur, BD Bioscience, Franklin Lakes, NJ, USA). All experiments were repeated three times independently.
Western blot assay
Proteins from cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%–12%). After electrophoresis, the separated protein bands in the gel were transferred to a polyvinylidene fluoride membrane (Merck Millipore, Billerica, MA, USA). The PVDF membrane was blocked in 5% nonfat milk at room temperature for 1 hour, and then incubated with the specific primary antibodies, followed by the appropriate secondary antibodies. The results were analyzed by chemiluminescence detection. Immunoblot data were quantified by scanning the appropriate bands of interest and the relative density was plotted in gray scale. All experiments were repeated three times independently.
RNA extract and quantitative real-time PCR assay
Total RNA from cells was extracted by using RNAiso Plus reagent (Takara Biotechnology [Dalian] Co., Ltd, Dalian, People’s Republic of China). Reverse transcription was carried out by using a reverse transcription kit (Takara). Real-time polymerase chain reaction (PCR) was conducted with a real-time PCR System (LightCycler, Roche Diagnostics, Basel, Switzerland) by using the following primers: 5′-CCCGAGATGCTTAAGTGTGACAA-3′ (sense) and 5′-ACTCGCTGGCACAGAGATGTTC-3′ (antisense) for SFRP1; 5′-CACCACCAGCAGCGACTCT-3′ (sense) and 5′-CAGACTCTGACCTTTTGCCAGG-3′ (antisense) for c-MYC; 5′-GTATGGGACATTCCTCTGATCC-3′ (sense) and 5′-CCAATGAATGAATGAATG GATG-3′ (antisense) for MMP-7; 5′-AGCTATCTGTAACCAAGATGGATG-3′ (sense) and 5′-TGTCTTATTAGGACACTATGGTT-3′ (antisense) for COX-2; and 5′-CATTGCCGACAGGATGCA-3′ (sense) and 5′-CATCTGCTGGAAGGTGGACAG-3′ (antisense) for GAPDH. The samples were subjected to the following reaction: initial denaturation step of 95°C for 10 minutes; 40 cycles of 95°C for 30 seconds, 55°C for 15 seconds, and 72°C for 20 seconds; and a final step at 65°C for 15 seconds. The 2−ΔΔCT method was used for data analysis. The mRNA levels of SFRP1, c-MYC, MMP-7, and COX-2 were normalized to GAPDH, which was used as an endogenous control.
Bisulfite sequencing PCR assay
Total DNA from cells was extracted using a DNA purification kit (Tiangen Biotech, Beijing, People’s Republic of China), and then subjected to bisulfite conversion by using the CpGenome Fast DNA Modification Kit (Millipore). The bisulfite-treated DNA was amplified by a PCR system (GeneAmp 9600, Thermo Fisher Scientific, Waltham, MA, USA). The primer sequences were as follows: CpG island 1 of SFRP1 promoter: 5′-GGTTAAGGTAGGAGTATTATTTGAGGT-3′ (sense) and 5′-AACCTAAATCATACTTA CAAACCCAT-3′ (antisense); and CpG island 2 of SFRP1 promoter: 5′-TTGTTTTTTAAGGGGTGTTGAGT-3′ (sense) and 5′-TACCACAAACTTCCAAAAACCTC-3′ (antisense). The following reaction conditions were used: ten cycles of 95°C for 5 minutes, 95°C for 30 seconds, then 60°C–50°C for 45 seconds beginning at 60°C for the first cycle, and then decreasing by 1°C for each subsequent cycle, and 72°C for 45 seconds; 33 cycles of 95°C for 30 seconds, 50°C for 45 seconds, and 72°C for 45 seconds; and finally 60°C for 30 minutes. The PCR products were inserted into the pMD-19T vector (Takara). After transformation, ten clones were selected and sequenced from each sample.
Statistical analysis
Data were analyzed by using ANOVA followed by multiple comparison Student–Neuman–Keuls tests and bi-variant relationship analyses. Data are presented as mean ± standard deviation. P<0.05 was considered statistically significant.
Results
As2O3 inhibits viability and induces apoptosis in PCa cells
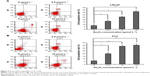
As2O3 has a cytotoxic effect on a wide range of cancer cells derived from solid tumors and hematopoietic malignancies.19–23 To investigate the inhibitory effect of As2O3 on the viability of PCa cells, LNCaP and PC3 cells were incubated with increasing concentrations of As2O3 (0, 1, 2.5, 5, 10, and 20 μmol L−1) for 24 hours. The inhibition rates were evaluated by CCK8 assay. As2O3 reduced the viability of LNCaP and PC3 cells in a dose-dependent manner (Figure 1).
We also assessed the effect of As2O3 on the apoptosis of PCa cells by a flow cytometry assay. LNCaP and PC3 cells were treated with As2O3 (0, 2.5, 5, and 10 μmol L−1) for 24 hours, and then stained with Annexin V/PI. As2O3 enhanced the proportion of cells undergoing apoptosis in a dose-dependent manner (Figure 2). These results demonstrated that As2O3 inhibited PCa cell viability and induced apoptosis in a dose-dependent manner.
As2O3 inhibits the Wnt signaling pathway in PCa cells
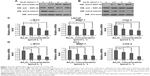
Deregulation of the Wnt signaling pathway plays important roles in the initiation and development of PCa. To examine whether the Wnt signaling pathway is involved in the treatment of PCa by As2O3, LNCaP and PC3 cells were treated with different concentrations of As2O3 (0, 2.5, 5, and 10 μmol L−1) for 24 hours. Western blot assay showed that the expression of phospho-GSK-3β increased, whereas the expression of β-catenin decreased in LNCaP and PC3 cells treated with As2O3 in a dose-dependent manner, and the expression of GSK-3β was not affected by As2O3 (Figure 3A and B). Moreover, we examined the effect of As2O3 on the expression of downstream target genes on the Wnt signaling pathway. Real-time PCR assay showed that the mRNA level of c-MYC, MMP-7, and COX-2 decreased in LNCaP (Figure 3C) and PC3 (Figure 3D) cells treated with As2O3 in a dose-dependent manner, respectively. These results showed that As2O3 inhibited the Wnt signaling pathway and downregulated the expression of its target genes.
As2O3 increases the protein and mRNA levels of SFRP1 in PCa cells
SFRP1 is a well-studied competitive inhibitor of the FZD receptor, which binds Wnt ligands and inhibits Wnt signal transduction. To investigate whether SFRP1 is involved in the inhibition of As2O3 on the Wnt signaling pathway, we examined the expression of SFRP1 in LNCaP and PC3 cells treated with different concentrations of As2O3. Figure 4A and B shows that the protein and mRNA levels of SFRP1 in LNCaP cells were increased by As2O3 in a dose-dependent manner in the experimental group compared with the control group. Similar results were obtained in PC3 cells (Figure 4C and D). These results showed that the expression of SFRP1 was upregulated substantially by As2O3, indicating the involvement of SFRP1 in the inhibition of the Wnt signaling pathway by As2O3.
As2O3 promotes the demethylation of the SFRP1 gene promoter
The methylation of the SFRP1 gene downregulates SFRP1 expression in a wide range of malignant tumors.13 To determine whether As2O3 downregulated the expression of SFRP1 through promoting demethylation of the SFRP1 gene, we examined the methylation level of the SFRP1 gene promoter in LNCaP and PC3 cells. Methylation usually occurs in CpG-rich promoter regions, known as CpG islands. Sequence analysis revealed that two CpG islands might be methylated in the SFRP1 gene promoter (Figure 5A, blue regions). A bisulfite sequencing PCR assay showed that the two CpG islands were methylated in LNCaP and PC3 cells. The methylation rates of CpG islands 1 and 2 in LNCaP cells not treated with As2O3 were 43.3% and 3.6%, respectively, whereas the methylation rates dropped to 12.5% and 1.3%, respectively, after As2O3 treatment (Figure 5B). In PC3 cells, the methylation rates of CpG islands 1 and 2 decreased from 86.7% and 71.4% to 48.3% and 34.1%, respectively, after As2O3 treatment (Figure 5C). These results were consistent with the data from Western blot and RT-PCR assays (Figure 4A–D), indicating that the As2O3-induced upregulation of SFRP1 is probably caused by inducing demethylation of the SFRP1 gene.
Discussion
PCa is the most commonly diagnosed nonskin cancer in men in industrialized countries.29 Despite continuous biomedical research, PCa is still a major public health problem. Currently, common treatment strategies for PCa include endocrine therapy, radical surgical resection, radiotherapy, and chemotherapy.4 However, the side effects of these therapies are severe. Therefore, much research is focusing on identifying new chemotherapeutic agents with lower toxicity and stronger antitumor effects. In this study, we showed that As2O3 could inhibit cell viability and induce apoptosis in both androgen-dependent and -independent human PCa cells. As2O3 exerted its antitumor effect, at least partly, through downregulating the Wnt signaling pathway by promoting demethylation of the SFRP1 promoter.
As2O3 is a safe, effective treatment for patients with acute promyelocytic leukemia, and it has also shown promising results in patients with relapsed multiple myeloma.30 In addition, a wide range of malignancies, including hematologic cancer and solid tumors derived from several tissue types, are susceptible to As2O3 treatment.31 Here, we found that As2O3 inhibited the viability (Figure 1) and induced apoptosis (Figure 2) of LNCaP and PC3 cells in a dose-dependent manner. These results were consistent with previous studies,24,25,29 suggesting that As2O3 possessed excellent antitumor effects in both androgen-dependent and -independent PCa cells.
The mechanism of As2O3 in treating acute promyelocytic leukemia is mediated by targeting disease-specific PML–RAR fusion proteins.32,33 However, the detailed mechanism of As2O3 in treating PCa is still inconclusive. In our previous studies, As2O3 inhibited the growth of PC3 cells and induced apoptosis through the p38 signaling pathway.23,24 As2O3 combined with ionizing radiation increases reactive oxygen species generation, and induces autophagy and apoptosis of LNCaP and PC3 cells. As2O3 also can induce the cell death through inhibition of the Akt/mTOR signaling pathways in LNCaP and PC3 cells.25 Increasing evidence suggests that aberrant activation of the Wnt signaling pathway contributes to tumorigenesis by upregulation of downstream target genes, such as Livin, cyclin D1, and c-MYC.34 However, it has not been reported whether As2O3 exerts its antitumor function through regulating the Wnt signaling pathway in PCa. In this study, we demonstrated that As2O3 inhibits the Wnt signaling pathway with a corresponding reduction in the mRNA levels of its downstream target genes (Figure 3A–D), indicating that the inhibition of Wnt signaling by As2O3 might be a molecular mechanism by which As2O3 exerts its antitumor effects in PCa. These results support the use of As2O3 as an anticancer drug for treating PCa.
SFRP1 is recognized as a tumor suppressor in several human cancers.35,36 Silencing of SFRP1 expression has been observed in a wide range of malignancies,13 which prevents SFRP1 from performing its physiological role of inhibiting the Wnt signaling pathway. In our previous study, the expression of SFRP1 decreased significantly with increasing PCa malignancy.34 Here, we found that As2O3 could induce the re-expression of the SFRP1 gene, and upregulate the protein and mRNA levels of SFRP1 in a dose-dependent manner in PCa cells (Figure 4A–D), indicating that As2O3 probably inhibits the Wnt signaling pathway through reactivating the Wnt inhibitor, SFRP1. In addition, SFRP1 is a negative regulator of androgen receptor activity in PCa.18 Therefore, the role of SFRP1 in prostate carcinogenesis may not be limited to inhibiting the Wnt signaling pathway. As2O3 may also inhibit the transcriptional activity of androgen receptor via SFRP1.
DNA methylation is one major epigenetic modification that plays crucial roles in the control of gene activity and nuclear architecture. Aberrant hypermethylation of gene promoter regions induces the inactivation of tumor suppressor genes, leading to the occurrence of human malignancies.37 Methylation is the main mechanism of SFRP1 gene silencing, which has been observed in multiple malignant tumors including PCa.13 Moreover, SFRP1 is a good predictive and prognostic biomarker of PCa.34 Therefore, detecting the methylation status of the SFRP1 gene could provide a basis for monitoring and early diagnosis of cancers. As2O3 decreases the methylation level of CDKN2B/CDKN2A genes in human hematologic malignant cells,26 indicating its role in demethylation. In this study, we found that the methylation level of the SFRP1 gene promoter was also downregulated by As2O3 in PCa cells (Figure 5B and C). This is consistent with its role in the demethylation of CDKN2B/CDKN2A genes.26 These data suggest that As2O3 increased the protein and mRNA levels of SFRP1 by promoting demethylation of the SFRP1 gene promoter. However, the detailed mechanism through which As2O3 promotes demethylation of the SFRP1 gene is still unclear.
In summary, we showed that As2O3 inhibited viability and induced apoptosis in both the androgen-dependent human PCa cell line LNCaP and the androgen-independent human PCa cell line PC3. The mechanism of the antitumor function of As2O3 in PCa cells at least partly involved its regulation of the Wnt signaling pathway. As2O3 increased SFRP1 expression through inducing demethylation of the SFRP1 gene promoter, thereby inhibiting Wnt signal transduction. Moreover, As2O3 has emerged as a potential treatment for PCa patients owing to its lower toxicity and stronger antitumor effects. Further research is required to address the mechanism through which As2O3 promotes the demethylation of the SFRP1 gene and evaluate the safety of As2O3 in clinical treatment of PCa.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (no 21272032).
Disclosure
Tao Jiang and Hui Jiang are full brothers. The authors report no other conflicts of interest in this work.
References
Nelson AW, Tilley WD, Neal DE, Carroll JS. Estrogen receptor beta in prostate cancer: friend or foe? Endocr Relat Cancer. 2014;21(4):T219–T234. | ||
Cooperberg MR, Park S, Carroll PR. Prostate cancer 2004: insights from national disease registries. Oncology (Williston Park). 2004;18(10):1239–1247. | ||
Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. | ||
Hara I. Advancement of treatment for prostate cancer. Gan To Kagaku Ryoho. 2006;33(2):178–182. | ||
Mahler C, Denis L. Management of relapsing disease in prostate cancer. Cancer. 1992;70(1 Suppl):329–334. | ||
Fowler FJ Jr, Barry MJ, Lu-Yao G, Wasson J, Roman A, Wennberg J. Effect of radical prostatectomy for prostate cancer on patient quality of life: results from a Medicare survey. Urology. 1995;45(6): 1007–1013. | ||
Crandley EF, Hegarty SE, Hyslop T, Wilson DD, Dicker AP, Showalter TN. Treatment-related complications of radiation therapy after radical prostatectomy: comparative effectiveness of intensity-modulated versus conformal radiation therapy. Cancer Med. 2014;3(2):397–405. | ||
Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26(3):570–579. | ||
Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129(4):199–221. | ||
Inestrosa NC, Varela-Nallar L. Wnt signaling in the nervous system and in Alzheimer’s disease. J Mol Cell Biol. 2014;6(1):64–74. | ||
Dickinson ME, McMahon AP. The role of Wnt genes in vertebrate development. Curr Opin Genet Dev. 1992;2(4):562–566. | ||
Hubaux R, Thu KL, Lam WL. Re: the Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106(8). | ||
Kang P, Wan M, Huang P, et al. The Wnt antagonist sFRP1 as a favorable prognosticator in human biliary tract carcinoma. PLoS One. 2014;9(3):e90308. | ||
Gao C, Xiao G, Hu J. Regulation of Wnt/beta-catenin signaling by posttranslational modifications. Cell Biosci. 2014;4(1):13. | ||
Moore WJ, Kern JC, Bhat R, et al. Modulation of Wnt signaling through inhibition of secreted frizzled-related protein I (sFRP-1) with N-substituted piperidinyl diphenylsulfonyl sulfonamides: part II. Bioorg Med Chem. 2010;18(1):190–201. | ||
Gopalsamy A, Shi M, Stauffer B, et al. Identification of diarylsulfone sulfonamides as secreted frizzled related protein-1 (sFRP-1) inhibitors. J Med Chem. 2008;51(24):7670–7672. | ||
Joesting MS, Perrin S, Elenbaas B, et al. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005;65(22):10423–10430. | ||
Kawano Y, Diez S, Uysal-Onganer P, Darrington RS, Waxman J, Kypta RM. Secreted Frizzled-related protein-1 is a negative regulator of androgen receptor activity in prostate cancer. Br J Cancer. 2009;100(7):1165–1174. | ||
Mathews V, Chendamarai E, George B, Viswabandya A, Srivastava A. Treatment of acute promyelocytic leukemia with single-agent arsenic trioxide. Mediterr J Hematol Infect Diss. 2011;3(1):e2011056. | ||
Sanaat Z, Rezazadeh M, Gharamaleki JV, Ziae JE, Esfahani A. Arsenic trioxide in patients with refractory multiple myeloma: a prospective, phase II, single-arm study. Acta Med Iran. 2011;49(8):504–508. | ||
Jutooru I, Chadalapaka G, Sreevalsan S, et al. Arsenic trioxide downregulates specificity protein (Sp) transcription factors and inhibits bladder cancer cell and tumor growth. Exp Cell Res. 2010;316(13):2174–2188. | ||
Oketani M, Kohara K, Tuvdendorj D, et al. Inhibition by arsenic trioxide of human hepatoma cell growth. Cancer Lett. 2002;183(2):147–153. | ||
Jiang T, Jiang H, Wang Y. Experimental research on the effect of arsenic trioxide on the growth of prostate cancer PC-3 cell lines. Zhonghua Nan Ke Xue. 2004;10(8):578–581. | ||
Su XM, Jiang T, Zheng L, et al. Arsenic trioxide induces the apoptosis of prostate cancer PC-3 cells via the P38 signaling pathway. Zhonghua Nan Ke Xue. 2013;19(7):583–587. | ||
Chiu HW, Chen YA, Ho SY, Wang YJ. Arsenic trioxide enhances the radiation sensitivity of androgen-dependent and -independent human prostate cancer cells. PLoS One. 2012;7(2):e31579. | ||
Fu HY, Shen JZ, Wu Y, Shen SF, Zhou HR, Fan LP. Arsenic trioxide inhibits DNA methyltransferase and restores expression of methylation-silenced CDKN2B/CDKN2A genes in human hematologic malignant cells. Oncol Rep. 2010;24(2):335–343. | ||
Zong Y, Huang J, Sankarasharma D, et al. Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling. Proc Natl Acad Sci U S A. 2012;109(50):E3395–E3404. | ||
Rabbani SA, Arakelian A, Farookhi R. LRP5 knockdown: effect on prostate cancer invasion growth and skeletal metastasis in vitro and in vivo. Cancer Med. 2013;2(5):625–635. | ||
Gauthier-Landry L, Belanger A, Barbier O. Multiple roles for UDP-glucuronosyltransferase (UGT)2B15 and UGT2B17 enzymes in androgen metabolism and prostate cancer evolution. J Steroid Biochem Mol Biol. 2015;145:187–192. | ||
Hu J, Huang X, Hong X, Lu Q, Zhu X. Arsenic trioxide inhibits the proliferation of myeloma cell line through notch signaling pathway. Cancer Cell Int. 2013;13(1):25. | ||
Thomas-Schoemann A, Batteux F, Mongaret C, et al. Arsenic trioxide exerts antitumor activity through regulatory T cell depletion mediated by oxidative stress in a murine model of colon cancer. J Immunol. 2012;189(11):5171–5177. | ||
Chen GQ, Zhu J, Shi XG, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88(3):1052–1061. | ||
Shao W, Fanelli M, Ferrara FF, et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90(2):124–133. | ||
Zheng L, Sun D, Fan W, Zhang Z, Li Q, Jiang T. Diagnostic value of SFRP1 as a favorable predictive and prognostic biomarker in patients with prostate cancer. PLoS One. 2015;10(2):e0118276. | ||
Kongkham PN, Northcott PA, Croul SE, Smith CA, Taylor MD, Rutka JT. The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene. 2010;29(20):3017–3024. | ||
Ugolini F, Charafe-Jauffret E, Bardou VJ, et al. WNT pathway and mammary carcinogenesis: loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene. 2001;20(41):5810–5817. | ||
Ozdemir F, Altinisik J, Karateke A, Coksuer H, Buyru N. Methylation of tumor suppressor genes in ovarian cancer. Exp Ther Med. 2012;4(6):1092–1096. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.