Back to Journals » OncoTargets and Therapy » Volume 9
Are compression corsets beneficial for the treatment of breast cancer-related lymphedema? New opportunities in physiotherapy treatment – a preliminary report
Authors Hansdorfer-Korzon R, Teodorczyk J, Gruszecka A, Lass P
Received 9 November 2015
Accepted for publication 16 February 2016
Published 7 April 2016 Volume 2016:9 Pages 2089—2098
DOI https://doi.org/10.2147/OTT.S100120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Rita Hansdorfer-Korzon,1 Jacek Teodorczyk,2 Agnieszka Gruszecka,3 Piotr Lass2,4
1Department of Physiotherapy, 2Department of Nuclear Medicine, 3Department of Informatics and Statistics, 4Department of Molecular Spectroscopy, Institute of Experimental Physics, Gdansk, Poland
Introduction: Treatment of secondary lymphedema still remains an important medical issue. Treatment response is characterized by periodic remission rather than complete recovery. Compression methods currently used as part of complete decongestive therapy vary considerably in efficacy. Manual drainage, bandaging, and compression pumps are ineffective in everyday practice. Positive results have increasingly been reported where compression garments have been used as part of the treatment. This pilot study demonstrates a beneficial effect following the use of compression corsets in the treatment of edema in breast cancer-related lymphedema (BCRL).
Material: A total of 35 women with BCRL were enrolled. Of these, 29 patients completed the study.
Methods: Ultrasound (B-mode) was used to evaluate lymphedema in the side of the chest after mastectomy. This test was performed three times at a specific site on the operated side and symmetrically on the opposite side. Subsequently, patients were fit with an appropriate compression corset. The data were then statistically analyzed.
Conclusion: After the surgical treatment of breast cancer, lymphatic fluid reservoirs may form at the side of the chest. The use of carefully selected compression corsets is an effective treatment for BCRL. Corsets are an important item, which we recommend should be included in compression clothing sets. We anticipate this finding will form the foundation for further work on the use of modern compression garments for the treatment of BCRL as well as contribute to the limited number of published reports that exist on the subject.
Keywords: lymphedema, breast cancer, physical therapy
Introduction
One of the major important long-term complications of breast cancer treatment is secondary lymphedema, a condition associated with adverse physical and psychosocial consequences.1
Lymphedema in patients with breast cancer is caused by the interruption of the axillary lymphatic channels by surgery and/or radiation therapy.2,3 Axillary dissection and radiation therapy have a synergistic effect on lymphedema risk. Lymphedema may present immediately or years after axillary dissection. Onset of the disease has been reported as late as 30 years after treatment.
Risk factors for the development of edema include surgical invasiveness, severity of the cancer (metastasis), obesity (high body mass index), chemotherapy, radiotherapy, and individual factors.3–5
Treatment of secondary lymphedema is time consuming and expensive, and an early multidisciplinary approach is required to diagnose, treat, and prevent recurrence.6 The main purpose of therapy is to reduce swelling at the site of residual fluid, maintain positive postsurgical outcomes, and prevent disease progression. Modern treatment of secondary lymphedema is based on complete decongestive therapy (CDT), originally proposed by Foldi and Foldi34 in 1989, which aims to stimulate lymphatic drainage.7 The program consists of several elements: manual lymphatic drainage (MLD), multilayer compression bandaging, physical exercise, and skin care at the site of edema.5,8,9 According to the American Society of Oncology and Lymphology, CDT is now considered by experts to be the most effective treatment of secondary lymphedema and is already an international standard of care.7,10,11 Numerous published randomized controlled trials examining the effectiveness of CDT have reported beneficial effects of this treatment. These studies used large patient cohorts and had treatment durations of about 3 weeks.7,12
The removal of lymph nodes and lymph vessels in the affected area can compromise local integrity of the lymphatic system, thereby adversely impacting lymphatic drainage.13 Radiation therapy can also damage or destroy lymphatic components, thereby further contributing to lymphatic compromise. Impairment of the lymphatic system can change the way that lymph fluid flows in an affected quadrant of the human body. Over time, sometimes years later, the lymphatic system can fail and fluid can back up, thereby causing swelling in a localized area. Chronic swelling caused by the accumulation of lymph fluid in an area of the body is called lymphedema, a condition that is common among postmastectomy patients and other cancer survivors who have had lymph nodes or lymph vessels removed, or who have undergone radiation therapy. Lymphedema typically occurs in a limb (such as an arm), but it can also occur in the torso region, especially among breast cancer survivors. It may be characterized by swelling, heaviness, pain, pitting, tightness, or hardness of the tissues.14
The topic of chest lymphedema has been raised in the literature mainly in reference to the breast-conserving surgeries, which are always accompanied by irradiation of the axilla, the preserved breast, and the chest wall in the midaxillary line just below the mammary gland. This explains the increasing frequency of secondary lymphedema of the breast and the trunk wall in addition to the usual secondary lymphedema of the arm. Mild compression of the lymphedematous and occasionally hardened fibrotic breast and trunk wall is essential to prevent progression of secondary tissue changes.15
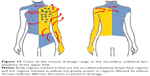
Most of the publications in the field of physiotherapy in women who underwent mastectomy describe the CDT method in relation to the limb on the operated side, whereas the chest area, which is where the surgery and radiotherapy had been performed, is completely ignored. According to Bringezu and Günther, what is important is the extent of the surgical procedure and the application of radiotherapy because they irreversibly damage the lymphatic system, causing disruption in lymph drainage on the affected side of the body.16 In light of these reports, it is understandable why in some medical centers sequential pneumatic compression (SPC) sleeves have been replaced by multichamber compression jackets (Figure 1). This instrument is equipped with a special algorithm designed for lymphatic drainage and its course is consistent with the technique of MLD established by E. Vodder.12 Compression begins with the stimulation of active vessels and lymph nodes (abdomen and chest, back, limb on the nonaffected side), and lymph from the upper limb on the operated side is drained only at the end of the procedure.
  | Figure 1 Pneumatic jacket for sequential compression drainage. |
Because of development of the production of innovative materials and medical instruments (elastomers), which can be used in the treatment of patients with lymphedema (special bandages, sleeves, underwear, and other compression garments), compression therapy is increasingly attractive.11,12 Elastic compression corsets can exert controlled pressure on body regions affected by edema for a long time and they can then be removed for personal hygiene practices. The compression force can be accurately adjusted within the defined parameters. Compression therapy initially involved application of compression bandages with low elasticity rate. However, their use proved difficult in daily treatment because their application required extensive experience. Many patients refused to cooperate mainly due to the requirement to frequently remove and reapply the bandages. Many kinds of compression garments, which not only increase the venous return and reduce edema but also protect the skin and tissue from injuries, are more efficient and more likely to be worn by patients. They may be worn throughout the day and even during sleep. Surveys show that currently 75% of women with edema caused by mastectomy use compression garments, 49% apply mechanical compression therapy (pumps), and 26% apply compression bandages.17 The effects of compression therapy are encouraging.6,7 The pressure applied on the swollen tissue not only improves the lymphatic and venous circulation but also stimulates muscles, which are a natural pumping mechanism, and removes excess protein-rich lymph fluid, thus preventing infections and fibrosis caused by edema.7
CDT has proven to be beneficial in patients with breast cancer-related lymphedema (BCRL); however, to date there is no fully effective treatment method. The development of microsurgery, to create lymphaticovenous anastomosis (bypass), raises the hope of a cure for secondary edema.18 Currently, the use of MLD followed by compression treatment (using appropriate garments) in conjunction with physical exercise seems to be the therapy of choice to provide optimal and sustained therapeutic effects.19–22 Treatment of lymphedema can be divided into two successive phases. The first phase shortly after the surgery aims to reduce the size of edema. The second phase consists of long-term treatment at home, aimed at maintaining and stabilizing postsurgical outcomes. Appropriately selected compression clothing is very important. In the course of practice with patients who have BCRL, we often come across cases where edema causes considerable discomfort (physical and psychological). Included in the study group were only those patients with edema located in the side of the chest, which appeared at various times after surgery despite a history of CDT using MLD (Figure 2). Previously, compression therapy had been used by our patients, and it usually consisted of compression sleeves or limb bandaging and, in some patients, SPC.
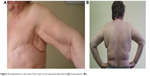  | Figure 2 Lymphedema in the side of the chest on the operated side (lateral [A] and posterior [B]). |
Assumptions and aims of study
In this study, we examined whether the use of compression corset, including compression of the chest, would be efficacious for the treatment of chest lymphedema (Figure 2) in BCRL patients.
Patients
The study consisted of a total of 35 patients who had undergone surgical treatment for breast cancer. During the course of the study, six patients were excluded: in one patient cancer relapsed, two were excluded due to erysipelas, one patient withdrew because of the difficulties associated with access to the site of our research, and in one patient, the garment was not the right size. Consequently, 29 patients completed the study: 27 after a radical mastectomy, and two after breast-conserving therapy. Axillary lymph nodes were removed from all patients. The mean age of the patients was 65.24±10.22 years (range: 44.00–81.00 years), and the mean elapsed time after surgery was 5 years. Patient treatments are listed in Table 1.
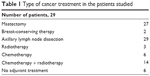  | Table 1 Type of cancer treatment in the patients studied |
At the beginning, before recruiting patients, and in view of the state-of-the-art research procedures, we reported our study to the Independent Bioethical Committee for Scientific Research at the Medical University of Gdansk, Poland. The Bioethical Committee agreed to perform the research study in the range shown in the application. During recruiting patients, oncologist informed patients extensively about the study and obtained written consent.
Methods
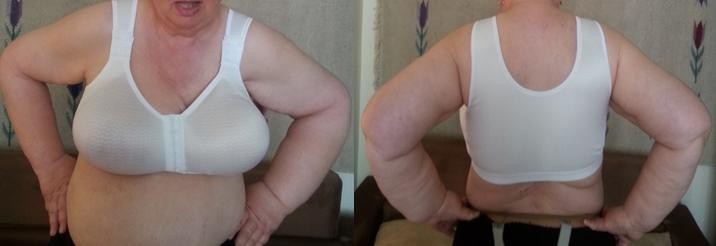
On the first day of the study, initial ultrasound measurements were taken to determine subcutaneous fat thickness on the operated side of the chest at the midaxillary line at the level of the lower sternum, and also symmetrically on the opposite side. Subsequently, patients received a properly fit compression garment (Figure 3), which was worn throughout the whole study period. Compression corsets were made in standard sizes (not custom-made). No other physiotherapeutic treatments were used during this period.
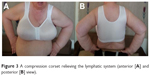  | Figure 3 A compression corset relieving the lymphatic system (anterior [A] and posterior [B] view). |
The compression garment was made of flexible material with projections, and the cups were formed seamlessly. Lateral support breast/breast implants (bielastic) applied a controlled pressure on the breast/chest area, supporting the outflow of lymph and accelerating the healing process. The built-up back and cuts under the arms provided good support for promoting drainage of lymph in the area of the armpits and upper back. Maximum extensibility of the most elastic area of the fabric was 105%. At 35%–50% extensibility, the material displays dynamic behavior toward both points of the scale. After calculating the results of three trials, an average value of 2.535 kPa was obtained. The value of compression corresponds to 18.987 mmHg (I class compression). Applied compression class is designed for long-term treatment, including at night.23 Corsets were selected individually for each patient, taking into account the circumference under the bust and cup size. All corsets have seamless, breathable, bilateral pockets for the prosthesis.
Ultrasound imaging was performed in B-mode (Voluson E8, the probe ML6-15; GE Healthcare, Piscataway NJ, USA). Measurements were made symmetrically on both sides of the chest at the midaxillary line at the level of the lower sternum (measuring point 1). The obtained values were referenced to the equivalent position on the nonoperated side (measuring point 2; Figure 4). To reduce the influence of the compression by ultrasound probe on the tissue, recordings were taken through a thick gel layer. Symmetric measurements of the thickness of subcutaneous tissue of the operated and nonoperated side were used to obtain values of asymmetry, which were independent of patient hydration, ambient temperature, time of year, and hormonal influences. The use of the coefficient of asymmetry also eliminated the influence of variations in thickness of body fat. The patients were examined three times: at baseline on the first day of observation; after 2 months; and after an additional 4 months (total follow-up of 6 months). Data obtained at the first and 6-month follow-up time points were analyzed.
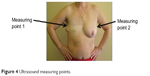  | Figure 4 Ultrasound measuring points. |
The Lilliefors test for normality revealed that the data were not normally distributed. Therefore, data were analyzed using the nonparametric Wilcoxon test of matched pairs.
After 6 months of follow-up, patients were given a survey and were questioned about the corset worn. They were asked in detail about fitting the corset (straps, width, thickness, size of bowls, etc), breathability and moisture drainage, resistance to sweat, appearance, quality after washing, subjective feelings of stimulation of lymph outflow, improvement of skin, and scars appearance. Patients had to choose the answer: I have no opinion – 0 points, unsatisfactory – 1 point, satisfactory – 2 points, good – 3 points, and very good – 4 points. For each of the questions, the results were presented in the form of a histogram. Because of the volume of this work, only results concerning the fit and comfort of wearing are presented.
Results
At examination I (baseline), the highest value of the quotient of the operated side to the nonoperated was 2.57, the lowest was 0.62, the median value was 1.026, and the mean was 1.159. At examination II (6-month follow-up), the corresponding values were as follows: maximum 1.36, minimum 0.60, median 0.987, and mean 1.025. (Table 2 and Figures 5 and 6). From the above values, it appears that wearing a compression corset for 6 months reduced the thickness of the subcutaneous tissue, and thus the size of the edema reduced on average by 12%. An example of clinical improvement is presented in Figures 7 and 8.
  | Table 2 Quotient of the thickness of subcutaneous tissue on the operated side of the chest versus the nonoperated side |
  | Figure 5 The median thickness ratio of subcutaneous tissue on the side of the chest on the operated side versus nonoperated side at the beginning and after 6 months of treatment. |
  | Figure 6 Mean quotient of the thickness of subcutaneous tissue on the side of the chest on the operated side versus nonoperated side at the beginning and after 6 months of treatment. |
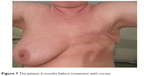  | Figure 7 The patient 6 months before treatment with corset. |
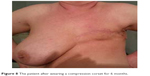  | Figure 8 The patient after wearing a compression corset for 6 months. |
Figures 9–12 show histograms created on the basis of a subjective assessment of compression corsets by patients after a 6-month period of use during day and night. For each question, 100% response rate was recorded. As many as 70% of respondents marked “a very good comfort wearing the corset”, and none of them evaluated it negatively. Breathability of corset material was rated as good by almost 52% of respondents, and 41% rated it as very good; none of the patients identified it as unsatisfactory. The feeling that the material causes on the skin was defined as very good (55%) and good (38%). Again, no patient described it as unsatisfactory. Important for the evaluation of the therapy results was also a question about a corset effect on stimulating the outflow of lymph. Only one of the patients was not satisfied with the impact of the corset to stimulate lymph drainage and three had no opinion on the subject; in contrast, 44% commented as good and 27% as very good.
  | Figure 9 Wearing comfort of compression corsets by patients after a 6-month period. |
  | Figure 10 Breathability of compression corsets by patients after a 6-month period. |
  | Figure 11 Feeling on the skin of compression corsets by patients after a 6-month period. |
  | Figure 12 Subjective stimulation of lymph outflow after a 6-month period of therapy. |
Discussion
Lymphedema after breast cancer treatment is an important, long-term, and persistent complication that affects the patient’s quality of life.24 If it is not diagnosed and treated in the early period, treatment may be difficult and the disease becomes chronic. Once developed, lymphedema cannot be completely cured; therefore, it is important to prevent this condition.1 Although risk factors for lymphedema after breast cancer treatment relate primarily to the axillary dissection or radiation therapy, other risk factors may play a role in lymphedema development, given that even sentinel node biopsy has been associated with a 0%–6% risk of lymphedema.25 In the study group, all 29 patients had axillary lymphadenectomy performed, of whom 23 had additional adjuvant therapy.
Thereafter, maintenance CDT is required, which is mostly home-based (self-management) compression therapy. In our patients, the oncological treatment varied (Table 1), and the length of time from the operation to the implementation of the compression garment was also different. Each of the patients enrolled, in the past repeatedly had comprehensive MLD physiotherapy, however, limited only to rehabilitation of the extremity on the surgery side (without elaboration of the chest).
In all 29 cases, however, at baseline, distinct edema located at the side of the chest was observed, causing significant ailments. Since numerous physiological and clinical factors influence the development of edema, after surgical treatment for breast cancer, there is no single recommended procedure. Eighty percent of the upper limb lymphatic circulation drains to the axillary lymph nodes, so the size of the edema depends on how radical the surgery of axillary dissection was22 and on the subsequent radiation and chemotherapy.
Because of the progressive nature of lymphedema, the effects of the treatment depend on the detection time, promptness of implementation, and type of treatment. Although some clinical examination methods such as metric measurements are commonly used, they are not sufficient because they identify the disease when symptoms are clearly visible.26,27 Development of lymphedema following breast cancer surgery involves numerous physiological and clinical factors, which impairs efforts to define the most appropriate treatment. Gurdal et al28 pointed out that the combination treatment modalities including intermittent pneumatic compression with self-lymphatic drainage, and MLD with compression bandage, are both effective and tolerable modalities in the treatment of arm lymphedema.
As mentioned above, unfortunately, most of the publications in the field of physiotherapy in women who underwent mastectomy describe the CDT method in relation to the limb on the operated side, whereas the chest area, which is where the surgery and radiotherapy had been performed, is completely ignored. So it was with all of our patients who underwent physiotherapy repeatedly without elaborating the chest on the surgery side despite the apparent lymphedema.
The chest region on the nonoperated side is the region where lymph fluid flows from the dysfunctional side after mastectomy. In addition to basic techniques, more techniques can be performed on median sagittal watershed, with the gravity center on axillo-axillary anastomoses to improve lymphangiomotoricity of the healthy vessels. This procedure is based on the assumption that to drain the lymph fluid from the chest region on the operated side after the surgery, and then from the upper limb with axillary lymphadenectomy, first it is necessary to stimulate lymphangiomotoricity of superficial lymphatic system on the healthy side of the chest. To obtain a more profound effect, additional manual techniques can be performed on the intercostal space, on the edge of the chest, and in the epigastric region.
In irradiated patients the stimulation of lymph vessels, manual techniques to improve the lymph flow from the upper limb, should be performed on the dorsal side16 (Figure 13). Analyzing the figure, it can be seen that in this study, the applied compression corsets (18.987 mmHg) activate with their compression the same areas of lymph outflow.
Compression garments of I compression class are designed to apply external pressure to an affected area, thereby helping to maintain the therapy results. A compression garment can also improve the pumping of lymphatics and veins as well by creating a firm abutment for muscles to work against. Another benefit of a compression garment is that it reduces local blood volume in the veins, which in turn results in increased velocity in the vein and greater fluid throughput.14 To achieve this goal, compression treatment should preferably be applied round the clock. Permanent maintenance of pressure at the site of edema stimulates drainage of lymphatic fluid. Most of our patients in a questionnaire positively assessed wearing comfort of compression corsets (also during the nighttime). They also emphasize that the material from which the corset is sewn has excellent moisture draining capacity, preventing sweat accumulation, especially during the night.
Badger et al29 compared the effects of bandaging and compression sleeves, which were applied for 24 weeks in patients, and confirmed the effectiveness of both methods. Our observation shows that compliance is much better if the patient can use comfortable compression clothes. Overall, studies in the literature indicate that the use of compression garments significantly improves treatment results.30,31 Compression sleeves and gloves are most frequently used in patients with lymphedema. However, no clinical studies can be found on the use of compression corsets. Research by Vignes et al showed that after 1 year of treatment, therapy is not continued by 38.1%–53.1% of patients, and after 4 years, up to 64.8% of patients discontinue therapy.32 On the basis of the literature, there appears to be a slow retreat from time consuming and costly MLD treatment in favor of specially designed compression garments.32,33 Our prospective pilot study of patients, in whom edema occurred at the side of the chest despite previous treatment, showed the high efficacy of compression corsets. The cause of lymphatic fluid reservoirs in the side of the chest in our patients is not clear. We hypothesize that the cause may be related to insufficient patency of collateral lymphatic vessels of the chest. When performing therapy, attention should be focused on moving the high protein fluid, which fills the interstitial space, to reach areas of the skin where physiological lymphatic drainage is properly functioning.
In 1989, Foldi and Foldi34 described that these substitute drainage areas for lymphedema of the extremities (axillary barrier post-lymphadenectomy) are usually just the trunk of the body. A review of the literature leads to the conclusion that the use of compression garments significantly improves the results in lymphedema treatment.31 The most commonly used compression garments are compression sleeves and gloves. Unfortunately, the available literature does not provide any clinical publications associated with the use of truncal compression corsets. It is known that the effect of treatment depends not only on the aforementioned risk factors of edema in patients after surgical treatment of breast cancer, but also on the number of lymphatic sinuses,5 which may be stimulated by correct treatment during anticongestive manual therapy,4,34 supplemented with compression corset therapy which is highly accepted and well tolerated by patients.
Conclusion
After surgical treatment of breast cancer, lymphatic fluid reservoirs may form in the side of the chest. The most likely cause, in addition to individual factors, is the lack of chest physiotherapy treatment on the side of the surgery. The use of carefully selected compression corsets is an effective, simple, and cheap treatment in these conditions. Corsets should be included as an integral part of compression clothing sets. In the absence of many reports in the literature, further studies are necessary to objectify and confirm the positive effects of compression corsets after axillary lymphadenectomy.
Disclosure
The authors report no conflicts of interest in this work.
References
Ugur S, Arici C, Yaprak M, et al. Risk factors of breast cancer-related lymphedema. Lymphat Res Biol. 2013;11(2):72–75. | ||
Brennan MJ, Weitz J. Lymphedema thirty years after radical mastectomy. Am J Phys Med Rehabil. 1992;71:12–14. | ||
Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. J Clin Oncol. 2012;30:3726–3733. | ||
Newman B, Lose F, Kedda M-A, et al. Possible genetic predisposition to lymphedema after breast cancer. Lymphat Res Biol. 2012;10:2–13. | ||
Dominick SA, Madlensky L, Natarajan L, Pierce JP. Risk factors associated with breast cancer-related lymphedema in the WHEL study. J Cancer Surviv Res Pract. 2013;7:115–123. | ||
Meneses KD, McNees MP. Upper extremity lymphedema after treatment for breast cancer: a review of the literature. Ostomy Wound Manage. 2007;53:16–29. | ||
Vignes S, Porcher R, Arrault M, Dupuy A. Long-term management of breast cancer-related lymphedema after intensive decongestive physiotherapy. Breast Cancer Res Treat. 2007;101:285–290. | ||
Horning KM, Guhde J. Lymphedema: an under-treated problem. Medsurg Nurs. 2007;16:221–227. | ||
Fu MR, Ridner SH, Armer J. Post-breast cancer. Lymphedema: part 1. Am J Nurs. 2009;109:48–54. | ||
Vignes S, Porcher R, Arrault M, Dupuy A. Factors influencing breast cancer-related lymphedema volume after intensive decongestive physiotherapy. Support Care Cancer. 2011;19:935–940. | ||
Williams AF, Vadgama A, Franks PK, Mortimer PS. A randomized controlled crossover study of manual lymphatic drainage therapy in women with breast cancer-related lymphoedema. Eur J Cancer Care. 2002;11:254–261. | ||
Türk G, Khorshid L. The complete decongestive therapy in lymphedema management developing in relation with mastectomy. The Journal of Breast Health. 2011;7:96–100. | ||
DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics. CA Cancer J Clin. 2011;61:409–418. | ||
Lesli RB, Eugenie Z, Inventors; Lightening2 LLC, assignee. Lindahl compression garment. European Patent Specification (45) EP 1536705 B1. May 26, 2010. | ||
Pritschow H, Schuchhardt C, editors. Lymphedema Management and Complete Physical Decongestive Therapy. A Manual for Treatment. Köln: Viavital-Verl; 2010:183–185. | ||
Bringezu G, Günther O. Kompleksowa terapia przeciwzastoinowa: podręcznik limfologiczny: podstawy, opis i ocena metod. [T. 2] [Complete decongestive therapy]. Chorzów, Katowice: Polskie Towarzystwo Limfologiczne; 2009:125–139. Polish. | ||
Paskett ED, Stark N. Lymphedema: knowledge, treatment, and impact among breast cancer survivors. Breast J. 2000;6:373–378. | ||
Turk G, Khorshid L. The complete decongestive therapy in management developing in relation with mastectomy. J Breast Health. 2011;7:96–100. | ||
Campisi C, Davini D, Bellini C, et al. Lymphatic microsurgery for the treatment of lymphedema. Microsurgery. 2006;26:65–69. | ||
McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. | ||
Hayes SC, Reul-Hirche H, Turner J. Exercise and secondary lymphedema: safety, potential benefits, and research issues. Med Sci Sports Exerc. 2009;41:483–489. | ||
Uzkeser H, Karatay S, Erdemci B, et al. Efficacy of manual lymphatic drainage and intermittent pneumatic compression pump use in the treatment of lymphedema after mastectomy: a randomized controlled trial. Breast Cancer. 2015;22:300–307. | ||
Rabe E. Compression Guide. Bonn, Germany: Rabe Med. Publ.; 2009:87–89. | ||
Hayes S, Di Sipio T, Rye S, et al. Prevalence and prognostic significance of secondary lymphedema following breast cancer. Lymphat Res Biol. 2011;9:135–141. | ||
Barranger E, Dubernard G, Fleurence J, et al. Subjective morbidity and quality of life after sentinel node biopsy and axillary lymph node dissection for breast cancer. J Surg Oncol. 2005;92:17–22. | ||
Stanton AB, Badger C, Sitzia J. Non-invasive assessment of the lymphedematous limb. Lymphology. 2000;33:122–135. | ||
Hansdorfer-Korzon R, Bakuła S, Nowakowski M. Znaczenie przezpowłokowej ultrasonografii w prezentacji B w porównaniu z metodą pomiaru obwodów kończyn w rozpoznawaniu wczesnych stadiów obrzęku limfatycznego u kobiet po mastektomii. [The role of B-mode ultrasound examination compared to limb circumference measurement in the diagnosis of early of lymphatic edema in women after mastectomy]. Fizjoterapia Polska. 2005;5(1):31–40. Polish. | ||
Gurdal SO, Kostanoglu A, Cavdar I, et al. Comparison of intermittent pneumatic compression with manual lymphatic drainage for treatment of breast cancer-related lymphedema. Lymphat Res Biol. 2012;10:129–135. | ||
Badger CM, Peacock JL, Mortimer PS. A randomized, controlled, parallel-group clinical trial comparing multilayer bandaging followed by hosiery versus hosiery alone in the treatment of patients with lymphedema of the limb. Cancer. 2000;15(88):2832–2837. | ||
Devoogdt N, Van Kampen M, et al. Different physical treatment modalities for lymphoedema developing after axillary lymph node dissection for breast cancer: a review. Eur J Obstet Gynecol Reprod Biol. 2010;149:3–9. | ||
McNeely ML, Peddle CJ, et al. Conservative and dietary interventions for cancer-related lymphedema: a systematic review and meta-analysis. Cancer. 2011;117:1136–1148. | ||
Vignes S, Blanchard M, Arrault M, Porcher R. Intensive complete decongestive physiotherapy for cancer-related upper-limb lymphedema: 11 days achieved greater volume reduction than 4. Gynecol Oncol. 2013;131:127–130. | ||
Huang TW, Tseng SH, Lin CC, et al. Effects of manual lymphatic drainage on breast cancer-related lymphedema: a systematic review and meta-analysis of randomized controlled trials. World J Surg Oncol. 2013;24:11–15. | ||
Foldi E, Foldi M. The lymphoedema chaos: a lancet. Ann Plast Surg. 1989;22:505–515. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.