Back to Journals » International Journal of Women's Health » Volume 11
Are cold extremities an issue in women’s health? Epidemiological evaluation of cold extremities among Japanese women
Authors Tsuboi S, Mine T , Tomioka Y, Shiraishi S, Fukushima F, Ikaga T
Received 10 October 2018
Accepted for publication 19 December 2018
Published 11 January 2019 Volume 2019:11 Pages 31—39
DOI https://doi.org/10.2147/IJWH.S190414
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Satoshi Tsuboi,1 Tomosa Mine,2 Yumi Tomioka,3 Saeka Shiraishi,4 Fujiko Fukushima,3 Toshiharu Ikaga4
1Department of Epidemiology, Fukushima Medical University, Fukushima, Japan; 2Department of the Scientific Study of Children, Shokei Gakuin University, Natori, Japan; 3Department of Family and Reproductive Health Nursing, Toho University, Tokyo, Japan; 4Department of System Design Engineering, Faculty of Science and Technology, Keio University, Yokohama, Japan
Background: Unlike traditional East Asian medicine, the necessity of health care services for cold extremities is yet to be acknowledged in Western medicine. In this study, we aimed to conduct an epidemiological evaluation of this unremarkable symptom among women in Japan.
Materials and methods: A cross-sectional study was conducted from February 2016 to April 2017, and data of 238 women throughout Japan were analyzed. Questionnaires were used to examine participants’ demographics, health-related behaviors, health status, and frequency of subjective symptoms over the past 1 year. The association between cold extremities and other subjective symptoms was examined by the multiple logistic regression analysis.
Results: The prevalences of mild and severe cold extremities were 49.6% and 35.3%, respectively. Temperature and utilization of health care services were not significantly different by the severity of cold extremities. The accompanying symptoms that were significantly associated with the cold extremities were shoulder stiffness, fatigue, low back pain, headache, nasal congestion, itching, injury, and difficulty hearing. After multiple logistic regression analysis, low back pain (OR: 4.91) and difficulty hearing (OR: 4.84) kept the significance. Factors related to cold extremities including mental quality of life, sleep quality, and habitual drinking were significantly associated with other accompanying symptoms.
Conclusion: Women with cold extremities have various accompanying symptoms and health-risk behaviors. Symptomatic treatment for cold extremities may not be sufficient, and comprehensive care would be required.
Keywords: cold extremities, cold hands, Flammer syndrome, vascular dysregulation, patient stratification, risk factors
Introduction
Cold extremities are perceived as an unremarkable symptom in Western medicine, and the necessity of health care services for them is not generally considered unless induced by a specific disease, such as connective tissue disease, peripheral neuropathy, and hypothyroidism.1,2 On the contrary, cold extremities are regarded as a trigger for various diseases in traditional East Asian medicine.1,3 Women with cold extremities are known to often have abnormal temperature perception or prolonged skin surface temperature recovery after mild cold stress.4–6 Arai et al reported that 31.4% of people living in a Japanese village complained of cold extremities in 2010.7 The characteristics associated with cold extremities are considered to include female gender, younger age, and low body mass index (BMI).7,8 According to several qualitative studies, other symptoms including fatigue, headache, insomnia, and low mental health status are also assumed to be accompanied with cold extremities.3,9 Although these findings suggest that cold extremities are associated with various health problems, a degree of burden of cold extremities is uncertain. Population-based epidemiological studies are required.10
Although the necessity of health care services for cold extremities is yet to be acknowledged, a new concept dealing with cold extremities was found in Western medicine, that is, Flammer syndrome.11–13 Flammer syndrome describes the phenotype of people with a predisposition for an altered reaction of the blood vessels to stimuli like coldness, emotional stress, or hypoxia.12 Flammer syndrome often occurs in people who are slim, active, and have a low blood pressure but also experience cold extremities, increased pain sensitivity, prolonged sleep onset time, and shifted circadian rhythm.12,13 According to Dr Flammer himself, cold extremities are often striking symptom when shaking hands with patients who are assumed to have Flammer syndrome.12 Symptoms of patients with Flammer syndrome seem to be consistent with those of women with cold extremities in Japan mentioned above. Nevertheless, uncertainty surrounds the necessity and appropriate health care services for women with cold extremities.
In this study, we aimed to conduct an epidemiological evaluation of women with cold extremities in Japan. The degree of burden on health is crucial to whether cold extremities are considered an issue in women’s health, and the insufficiency of such information would lead health care providers and researchers to possibly ignore the importance of this matter. We hypothesize that a considerable number of women complain of cold extremities with various accompanying symptoms and that they do not receive sufficient health care services. This study helps understand the burden of cold extremities in women’s health and explores the content of appropriate health care services for women with cold extremities.
Materials and methods
Study design
A cross-sectional study was conducted in Japan from February 2016 to April 2017.
Data collection
We enrolled 283 women throughout Japan by public invitation as a part of a governmental project supported by Ministry of Land, Infrastructure, Transport and Tourism, Japan. The project offered incentive pay for constructing carbon dioxide reduction houses with detailed study of the participants’ living environment and their health status. Questionnaires were sent with instructions for answering the questionnaires, along with enclosed equipment to measure blood pressure, pulse rate, and body temperature. An explanatory leaflet and a consent form of this study were also enclosed, and we confirmed the participants’ consent with the returned signed consent forms. An automated blood pressure monitor and ear thermometer were used.14 Participants were asked to take all measurements in the morning and evening each day for 2 weeks at home. The questionnaire was answered only once during that period. Seven women refused to participate in the study, and we also could not make contact with three other women. Therefore, 272 women participated in the study (96% participation rate). Of these 272 participants, 34 were eliminated due to having participated in the study twice during the study period, making errors in more than half of the measurements of blood pressure, pulse, or temperature, and being over 70 years of age. Therefore, we analyzed 238 participants. The questionnaire included participants’ demographics (age, educational status, marital status, job status, and annual household income), health-related behaviors and health status (utilization of health care services, habitual smoking, habitual drinking, weight, height, quality of life, and sleep quality), and frequency of subjective symptoms (cold extremities, shoulder stiffness, fatigue, low back pain, headache, nasal congestion, itching, cough and sputum, arthralgia, injury, difficulty hearing, hives, anorexia, and common cold) over the past 12 months. (The questionnaire of frequency of subjective symptoms over the past 12 months was translated into English from Japanese original version, and it is shown in Table S1.) Quality of life was measured by the 8-item short-form health survey (SF-8).15 Sleep quality was assessed using the Pittsburgh sleep quality index (Japanese version).16 The frequency of subjective symptoms was answered with five options: more than once a week, more than once a month (but less than twice a week), more than once a year (but less than twice a month), once a year, and never.
Definition
The severity of cold extremities was categorized into three groups: severe, mild, and none. Severe was defined by feeling cold extremities more than once a week, mild was more than once a year (but less than twice a week), and none was less than twice a year or never feeling cold extremities. The other symptoms were categorized into two groups: the frequency of the symptoms occurring more than once a month as presence, and less than twice a month as absence. Utilization of health care services was categorized into two groups: with and without regular use of traditional oriental medical services such as acupuncture, moxibustion, massage, or judo therapy, as well as those of Western medicine. Annual household income was categorized into two groups: more and less than 4,000,000 yen (around 40,000 US dollars). Educational status was categorized into two groups: higher and lower degree than university graduate. Marital status was categorized into two groups: married and the others. Occupational status was categorized into two groups: unemployed and the others. Smoking was categorized into two groups: habitual smoking and the others. Habitual smoking was defined as smoking in the previous month and having smoked for >6 months or over 100 cigarettes. Drinking was categorized into two groups: habitual drinking and the others. We defined habitual drinking as drinking sometimes or every day. BMI was calculated by the height and weight reported by the participants in the questionnaires. Quality of life and sleep quality were evaluated following previous studies:17,18 physical component summary (PCS), mental component summary (MCS), and global score of sleep quality were calculated for physical quality of life, mental quality of life, and sleep quality, respectively. PCS and MCS were measured by the eight domains of SF-8: general health, physical functioning, role-physical, bodily pain, vitality, social functioning, role-emotional, and mental health. PCS was calculated by the domains weighted to emphasize general health, physical functioning, role-physical, and bodily pain. Similarly, MCS was calculated by the domains weighted to emphasize vitality, social functioning, role-emotional, and mental health.17 The global score of sleep quality was yielded by the sum of scores of seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction.18 Higher scores of PCS and MCS indicate better quality of life. Higher global score of sleep quality, on the contrary, means poorer sleep quality.
Statistical analysis
With regard to continuous variables (age, BMI, blood pressure, pulse, temperature, physical quality of life, mental quality of life, and sleep quality), the arithmetical means were calculated and a trend analysis was conducted on the severity of cold extremities. Blood pressure, pulse, and body temperature were divided by the number of days the data could be obtained. For nominal variables (utilization of health care services, educational status, marital status, job status, annual household income, habitual smoking, and habitual drinking), the proportions of them were calculated and trend analysis was conducted on the severity of cold extremities. As a nonparametric test for trend across the severity of cold extremities, an extension of the Wilcoxon rank sum test was conducted to explore the association between the cold extremities and the other symptoms.19 Then, each associated symptom was put into a multiple logistic regression model one by one as an objective variable. The severity of cold extremities was entered into the model as an explanatory variable, and age and a series of variables that were significantly associated with cold extremities were used to show potential confounding associations. Mean systolic blood pressure was calculated and put into the model. (Diastolic blood pressure was not included.) ORs were calculated for the presence of each associated symptom. The reference of the OR of the severity of cold extremities was mainly the odds of none group, but the odds of mild group was used as another reference when there was no participant in none group.
All statistical analyses were conducted using STATA (version 13.1 for Mac; StataCorp, College Station, Texas, USA). A P-value of <0.05 was considered as statistically significant.
Results
Of the 238 participants, mild and severe cold extremities were found in 118 (49.6%) and 84 (35.3%), respectively. Thirty-six (15.1%) participants did not complain of cold extremities.
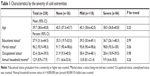
The demographics by the severity of the cold extremities (none, mild, and severe) are shown in Table 1. There were no significant differences in age (P=0.20), educational status (P=0.99), marital status (P=0.06), occupational status (P=0.98), and annual household income (P=0.26). The age range was from 22 to 68 years old.
Health-related behaviors and health statuses are shown in Table 2. Variables significantly related to cold extremities were habitual drinking (P=0.04), BMI (P=0.048), mental quality of life (P<0.01), sleep quality (P=0.03), and systolic and diastolic blood pressure in the morning (P<0.01 for both) and in the evening (P<0.01 and P=0.02, respectively). Higher rates of habitual drinking and poorer sleep quality were associated with worsened severity of the cold extremities. In contrast, blood pressure, BMI, and mental quality of life decreased as cold extremities worsened. Utilization of health care services was not significantly different by the severity of the cold extremities (P=0.23).
The accompanying symptoms are shown in Table 3. The significantly associated symptoms with the cold extremities are as follows: shoulder stiffness (P<0.01), fatigue (P=0.02), low back pain (P<0.01), headache (P<0.01), nasal congestion (P<0.01), itching (P=0.047), injury (P<0.01), and difficulty hearing (P<0.01). The presence of these symptoms tended to increase as cold extremities worsened.
  | Table 3 Accompanying symptoms by the severity of cold extremities |
The results of each multiple logistic regression model are shown in Table 4. The abovementioned eight significantly associated symptoms were used as objective variables. Explanatory variables were the severity of the cold extremities and a series of other variables (age, BMI, mental quality of life, sleep quality, systolic blood pressure, and habitual drinking). These models showed the significant associations: cold extremities was associated with low back pain (OR: 4.91 in severe cold extremities, none as the reference) and difficulty hearing (OR: 4.84 in severe cold extremities, mild as the reference); age was associated with difficulty hearing (OR: 1.11); BMI was associated with shoulder stiffness (OR: 1.16), low back pain (OR: 1.23), and itching (OR: 1.21); mental quality of life was associated with fatigue (OR: 0.90); sleep quality was associated with low back pain (OR: 1.18) and headache (OR: 1.30); habitual drinking was associated with headache (OR: 3.02).
Discussion
In this study, we described the relationships between the cold extremities and health-related behaviors, health status, and accompanying symptoms. Women with cold extremities tended to have lower blood pressure, lower BMI, lower mental quality of life, lower sleep quality, and more habitual drinking. They also had various accompanying symptoms including shoulder stiffness, fatigue, low back pain, headache, nasal congestion, itching, difficulty hearing, and injury. Of these signs and symptoms, lower blood pressure, lower BMI, shoulder stiffness, low back pain, headache, difficulty hearing, and injury have already been mentioned in previous studies regarding Flammer syndrome,11,12 and these symptoms were more likely to occur as cold extremities worsened. Although prolonged sleep onset and shifted circadian rhythm have been mentioned before, mental quality of life, sleep quality, and drinking habit in women with cold extremities have not been well investigated. Lower mental quality of life, poor sleep quality, and alcohol consumption are thought to be the signs of mental illness including depression.20–24 The studies for understanding mental health in women with cold extremities should be warranted. The utilization of health care services, on the contrary, was around 20% and not significantly different by the severity of the cold extremities. In addition, due to the high specialization in the currently organized medical care, individual specialists do not always obtain the entire patient history of those assumed to have Flammer syndrome.21 Consequently, the patients are often left feeling that the different complaints represent different diseases or at least different predispositions.21,25 Therefore, women with cold extremities may have unmet needs regarding treatment for cold extremities as well as the accompanying symptoms. More research for understanding such women’s perception to current health care services and developing the effective care to them should not be disregarded.
To improve the quality of such services, symptomatic treatment for cold extremities may not be sufficient. As reported in this study, women with cold extremities may have many accompanying symptoms that were in line with previous studies.26,27 Therefore, medical history taking into consideration other potential accompanying symptoms would lead to improve their satisfaction with personalized health care services. In addition, checking underlying diseases and health-risk behaviors should not be omitted. Many suspicious latent diseases have been pointed out in previous studies, including chronic rhinitis, gastroduodenal ulcer, chronic gastritis, sudden hearing loss, thyroid dysfunction, and ocular diseases such as normal tension glaucoma.1,8,20 As health-risk behaviors, we showed that sleep quality of women with cold extremities was poor and they have more habitual drinking. Sleep disturbance is known as a risk factor for infectious diseases, several major medical illnesses including cardiovascular disease and cancer, and the incidence of depression.28 The harmful use of alcohol is a causal factor in >200 diseases and injury conditions, including mental and behavioral disorders.29 Screening for potential accompanying symptoms, underlying diseases, and health-risk behaviors is recommended in the clinical setting.
To acknowledge and promote health in women with cold extremities effectively, employing prospective studies to understand the causal relationships around cold extremities are necessary. A multiple logistic regression analysis showed that cold extremities were significantly associated with symptoms including low back pain and difficulty hearing. One possibility is that there is fundamental common disorder in cold extremities and these symptoms. Well-known assumed pathologies of Flammer syndrome are local and systemic hypoxia, as well as oxidative stress caused by vascular dysregulation.21,25 The functional link between these effects, chronic inflammation, and delayed wound healing might play a role in developing these symptoms, though the studies are needed.25,30,31 In this case, drastic treatment for vascular dysregulation would be effective. Another possibility is that there is causal relationship between cold extremities and these symptoms. In this case, treatment for the cause (either cold extremities or these symptoms) would improve the patients’ health. In addition, fatigue was significantly associated with lower mental quality of life, which was negatively related to cold extremities (P for trend: <0.01). Similarly, headache was significantly associated with low sleep quality and habitual drinking, which were positively related to cold extremities ([P for trend: 0.03] and [P for trend: 0.04], respectively). Although the relationships among these factors are complicated, understanding the relationships among these factors is essential to determine whether managements of mental health, sleep quality, or alcohol consumption are effective interventions to improve cold extremities or improving cold extremities ameliorates fatigue and headache indirectly. Further studies are required.
There are several strengths in this study. First, this epidemiological evaluation was conducted in a general field unlike most previous studies that were carried out in clinical settings.1,26,27 In these previous studies, the prevalence of cold extremities was from 48% to 66%. In this study, in contrast, the prevalence of mild or severe cold extremities was 85%. Although more studies are needed, it is reasonable to state that the difference in study fields (clinical setting or general field) contributes to the different prevalence. As previously mentioned, women with cold extremities in a general field may have unmet needs by the current health care services, which is supposed to keep themselves away from clinical setting. This study could add a value highlighting the burden of cold extremities on women’s health in a general field. Second, we investigated health-related behaviors, sleep quality, mental health, and common symptoms. This information helps in understanding the epidemiological features of women with cold extremities in detail. Third, consistency with previous studies has reinforced the validity of our findings. Women who have cold extremities are known to have lower levels of blood pressure, BMI, and sleep quality than healthy people.3,7–9 These tendencies were also found in this study.
This study contains several limitations. We have examined only women but not men with cold extremities. Although Flammer syndrome occurs in both men and women,12 the prevalence of cold extremities in men was from 22% to 39% in previous studies.1,26 Considering the huge difference between the prevalence in men and women, different findings would be found in men. In addition, low statistical power due to small sample size made the interpretation of results difficult. For example, the multiple logistic regression analysis showed no significant association between the explanatory variables and symptoms such as nasal congestion and injury. Shoulder stiffness and itching were significantly associated with BMI only; however, women with cold extremities tended to have a lower BMI. Investigation of relationship among these variables in detail requires more statistical power. In addition, because we conducted a cross-sectional study, causal relationships were not examined. The influence of sampling bias could not be denied.
Conclusion
In this study, we described the epidemiological features of women with cold extremities in Japan. Women with cold extremities have various accompanying symptoms and health-risk behaviors. Symptomatic treatment for cold extremities may not be sufficient, and comprehensive care including medical history taking into consideration other potential accompanying symptoms, and screening for potential accompanying symptoms, underlying diseases, and health-risk behaviors are required to improve the overall quality of women’s health care. In addition, the studies for understanding mental health in women with cold extremities, the perception of the current health care services, and developing effective care are warranted.
Ethics statement
This study protocol was approved by the Keio University Ethics Review Board on 9 March 2016 (28-20) and 1 April 2017 (29-33).
Acknowledgment
The authors would like to thank the participants who made this investigation possible.
Disclosure
The authors disclosed the receipt of the following financial support for the research, authorship, and/or publication of this article: Grant-in-Aid for Scientific Research (A) (No 26249083) and Scientific Research (S) (No 17H06151; Principal Investigator: TI). The authors report no other conflicts of interest in this work.
References
Bae KH, Go HY, Park KH, Ahn I, Yoon Y, Lee S. The association between cold hypersensitivity in the hands and feet and chronic disease: results of a multicentre study. BMC Complement Altern Med. 2018;18(1):40. | ||
Nishida S, Eguchi E, Ohira T, et al. Effects of a traditional herbal medicine on peripheral blood flow in women experiencing peripheral coldness: a randomized controlled trial. BMC Complement Altern Med. 2015;15(1):105. | ||
Iio Y, Mizuno-Matsumoto Y, Suzui E. A literature review on cold sensitivity among women of reproductive age. J Hyogo Univ of Health Sci. 2015;3(1):1–12. | ||
Sadakata M, Yamada Y. Perception of foot temperature in young women with cold constitution: analysis of skin temperature and warm and cold sensation thresholds. J Physiol Anthropol. 2007;26(4):449–457. | ||
Nagashima K, Yoda T, Yagishita T, Taniguchi A, Hosono T, Kanosue K. Thermal regulation and comfort during a mild-cold exposure in young Japanese women complaining of unusual coldness. J Appl Physiol. 2002;92(3):1029–1035. | ||
Yamada N, Bekku N, Yoshimura H. Determinants for diagnosis of young women with and without chilliness. Nihon Shinkei Seishin Yakurigaku Zasshi. 2007;27(5–6):191–199. | ||
Arai M, Okabe R, Ookishima S, et al. Epidemiologic survey of subjective symptoms based on kampo medicine in Hase Village, Nagano. Kampo Medicine. 2010;61(2):154–168. | ||
Furuya Y, Watanabe T, Nagata Y, Obi R, Hikiami H, Shimada Y. Risk factors for excessive sensitivity to cold and physical characteristics: a prospective cohort study. Kampo Medicine. 2011;62(5):609–614. | ||
Nakamura S. “Sensitivity to cold”: a concept analysis. J Jpn Acad Nurs Sci. 2010;30(1):62–71. | ||
Tomooka K, Tanigawa T. A descriptive epidemiological study of subjective symptoms and patterns in oriental medicine: The Toon Health Study. JJSAM. 2015;65(3):178–188. | ||
Konieczka K, Ritch R, Traverso CE, et al. Flammer syndrome. EPMA J. 2014;5(1):11. | ||
Flammer J, Konieczka K. The discovery of the Flammer syndrome: a historical and personal perspective. EPMA J. 2017;8(2):75–97. | ||
Pache M, Kräuchi K, Cajochen C, et al. Cold feet and prolonged sleep-onset latency in vasospastic syndrome. Lancet. 2001;358(9276):125–126. | ||
Brown MA, Roberts L, Davis G, Mangos G. Can we use the Omron T9P automated blood pressure monitor in pregnancy? Hypertens Pregnancy. 2011;30(2):188–193. | ||
Tokuda Y, Okubo T, Ohde S, et al. Assessing items on the SF-8 Japanese version for health-related quality of life: a psychometric analysis based on the nominal categories model of item response theory. Value Health. 2009;12(4):568–573. | ||
Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97(2–3):165–172. | ||
Fukuhara S, Suzukamo Y. Manual of the SF-8 Japanese Version. Kyoto: Institute for Health Outcomes and Process Evaluation Research; 2004. | ||
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. | ||
Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. | ||
Konieczka K, Erb C. Diseases potentially related to Flammer syndrome. EPMA J. 2017;8(4):327–332. | ||
Golubnitschaja O, Flammer J. Individualised patient profile: clinical utility of Flammer syndrome phenotype and general lessons for predictive, preventive and personalised medicine. EPMA J. 2018;9(1):15–20. | ||
Bentley SM, Pagalilauan GL, Simpson SA. Major depression. Med Clin North Am. 2014;98(5):981–1005. | ||
Levola J, Aalto M, Holopainen A, Cieza A, Pitkänen T. Health-related quality of life in alcohol dependence: a systematic literature review with a specific focus on the role of depression and other psychopathology. Nord J Psychiatry. 2014;68(6):369–384. | ||
Spiegelhalder K, Regen W, Nanovska S, Baglioni C, Riemann D. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Curr Psychiatry Rep. 2013;15(6):364. | ||
Kunin A, Polivka J, Moiseeva N, Golubnitschaja O. “Dry mouth” and “Flammer” syndromes-neglected risks in adolescents and new concepts by predictive, preventive and personalised approach. EPMA J. 2018;9(3):307–317. | ||
Yoshino T, Katayama K, Munakata K, et al. Statistical Analysis of Hie (Cold Sensation) and Hiesho (Cold Disorder) in Kampo Clinic. Evid Based Complement Alternat Med. 2013;2013(3):1–8. | ||
Tokunaga H, Munakata K, Katayama K, et al. Clinical data mining related to the Japanese Kampo concept “Hie” (Oversensitivity to Coldness) in men and pre- and postmenopausal women. Evid Based Complement Alternat Med. 2014;2014(2):1–9. | ||
Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66(1):143–172. | ||
Alcohol. World Health Organization. [cited 2018 Sep 21]. Available from: http://www.who.int/mediacentre/factsheets/fs349/en/. Accessed October 10, 2018. | ||
Bubnov R, Polivka J, Zubor P, Konieczka K, Golubnitschaja O. “Pre-metastatic niches” in breast cancer: are they created by or prior to the tumour onset? “Flammer Syndrome” relevance to address the question. EPMA J. 2017;8(2):141–157. | ||
Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017;8(1):23–33. |
Supplementary material
  | Table S1 The questionnaire on the frequency of subjective symptoms over the past 12 months (Translated into English from Japanese original version) |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.