Back to Journals » Drug Design, Development and Therapy » Volume 10
Apremilast in the therapy of moderate-to-severe chronic plaque psoriasis
Authors Gisondi P , Girolomoni G
Received 9 March 2016
Accepted for publication 19 April 2016
Published 25 May 2016 Volume 2016:10 Pages 1763—1770
DOI https://doi.org/10.2147/DDDT.S108115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
Paolo Gisondi, Giampiero Girolomoni
Department of Medicine, Section of Dermatology and Venereology, University of Verona, Verona, Italy
Abstract: Chronic plaque psoriasis presents clinically as an inflammatory disease of the skin, which is often associated with comorbidities and responsible for a poor quality of life. It can widely vary among patients because of different age of onset, type of symptoms, areas of involvement, and disease severity. The choice of the treatment of psoriasis should be personalized according to the specific needs of the patients. Apremilast is a well-tolerated and effective phosphodiesterase type 4 inhibitor that is indicated for the treatment of moderate-to-severe plaque psoriasis and psoriatic arthritis. In this article, the pharmacological, clinical, and safety aspects of apremilast are reviewed. Based on these data, apremilast could be indicated for patients with a Psoriasis Area and Severity Index score <10 but with a significant impact on quality of life and seems to be an appropriate treatment for elderly patients also.
Keywords: psoriasis, apremilast, therapy, psoriasis severity
Psoriasis
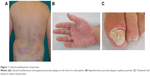
Chronic plaque psoriasis is a common inflammatory disease of the skin. Its prevalence ranges from 1% to 3% in the Western world.1,2 Genetic and enviromental factors are relevant in the pathogenesis of psoriasis.3 In psoriasis, the inflammatory cytokine network is deregulated, leading to the excessive release of proinflammatory mediators from immune cells and increase in the proliferation of keratinocytes.4 In particular, Th1 and Th17 cell populations produce different cytokines (interleukin [IL]-6, IL-17, and IL-22, interferon-γ, and tumor necrosis factor [TNF]-α), causing a change in differentiation and hyperproliferation of keratinocytes, dilatation of blood vessels, and infiltration of leukocytes into the dermis and epidermis.5 The hallmarks of psoriasis are raised and clearly delimited erythematous lesions covered by silver scales (Figure 1A). Psoriatic lesions are commonly localized on the elbows, knees, trunk, sacrum, and scalp; the involvement of the face, genitals, nails, palmoplantar regions is associated with higher impact on quality of life (Figure 1B and C).6 In most patients, the lesions cover <10% of the body surface area (BSA), but very rarely, psoriasis could involve the whole BSA, leading to erythroderma. Psoriatic lesions are frequently symptomatic with pruritus, followed by scaling and flaking.7 Psoriasis may affect many facets of life, including emotional, social, work, and leisure. Approximately one-third of patients present signs/symptoms of concomitant psoriatic arhtritis (PsA),8 besides, several metabolic diseases, such as obesity, diabetes, fatty liver disease, metabolic syndrome, and cardiovascular diseases (psoriasis itself could be an independent cardiovascular risk factor), are frequently associated with psoriasis.9–12
Measuring the severity of chronic plaque psoriasis
The concept of severity relates to many different aspects of psoriasis, including the extent of disease, location of lesions, degree of inflammation, responsiveness to treatment, and impact on patient quality of life. No international standard or validated categories of severity are recognized. Generally, several assessment tools are used to assess the severity of chronic plaque psoriasis, including the Psoriasis Area and Severity Index (PASI) score that is based on the intensity of redness, thickness, and scaling of the representative lesions, and it ranges from 0 to 72. The BSA estimates the percentage of body surface affected by psoriasis ranging from 0% to 100%. The Physician Global Assessment (PGA) grades disease severity in six categories including clear, almost clear, mild, moderate, severe, and very severe.13 The multifaceted nature of psoriasis burden drives the need for a specific focus on health-related quality of life and patient-reported outcome measures.14 Patient’s quality of life is commonly assessed by questionnaires, including Dermatology Life Quality Index (DLQI) and Short Form-36 health survey. DLQI is a dermatology-specific tool aimed at assessing itch, pain, embarrassment, and interference of skin disease in the patient’s daily activities, relationships, and sexual activity. Short Form-36 is a nondermatological questionnaire that investigates the physical functioning, bodily pain, general health perceptions, vitality, social functioning, and mental health. Although none of the mentioned severity scores meets all the validation criteria required for an ideal score, PASI is the most commonly used, being the gold standard tool in clinical trials as well as in daily practice. According to the European S3 guidelines on the systemic treatment of psoriasis vulgaris, moderate-to-severe disease is defined as a PASI score >10.15 PASI 75 and PASI 90 responses are dynamic parameters that indicate the percentage of patients who have achieved at least a 75% or 90% improvement, respectively, in their baseline PASI score during treatment. The Scottish Intercollegiate Guidelines for the diagnosis and management of psoriasis in adults differentiate between mild and severe psoriasis for the purposes of referral and selection of treatments. Mild psoriasis is defined as DLQI ≤5, while severe psoriasis, for which systemic or biological therapy may be appropriate, is defined as PASI and DLQI scores ≥10.16 The guidelines on clinical investigation of medicinal products indicated for the treatment of psoriasis, edited by the European Medicines Agency, proposes the following “operational” definition of psoriasis severity, which can be used to describe patient population in clinical trials.17
Mild-to-moderate psoriasis: Good control of lesions with topical therapy alone. BSA involvement <10% or PASI <10. Category “mild-to-moderate” on PGA.
Moderate psoriasis: Topical therapy still possible to control the disease. BSA involvement >10% or PASI 10 or more. Category “moderate” on PGA.
Moderate-to-severe psoriasis: Topical therapies fail to control the disease. BSA involvement >10% or PASI 10–20. Very thick lesions located in “difficult to treat” regions (eg, palmoplantar) with BSA involvement <10% may also be considered. Category “moderate-to-severe” on PGA.
Severe psoriasis: A justified need for systemic treatment to control the disease. BSA involvement >20% or PASI >20. Very important local signs with very thick lesions with BSA involvement >10% may also be considered. Category “severe” on PGA.
Finally, a European consensus was achieved to define goals for treatment of plaque psoriasis with systemic therapy and improve patient care.18 Severity of plaque psoriasis was graded into mild and moderate-to-severe disease. Mild disease was defined as BSA ≤10%, and PASI and DLQI scores ≤10; and moderate-to-severe psoriasis as BSA >10%, and PASI and DLQI scores >10. In accordance with existing guidelines, it is recommended to treat mild psoriasis with topical agents. In refractory cases, the addition of phototherapy should be considered. However, psoriasis could be graded as moderate-to-severe also in cases of BSA ≤10% and/or PASI ≤10 but DLQI >10 because of a significant impact on the quality of life and systemic therapy could be indicated. However, patients with mild psoriasis, as indicated by the BSA and PASI scores, may present with disease manifestations not adequately controlled by topical therapy alone, which may lead to a significantly impaired quality of life. These manifestations can include the involvement of visible areas (ie, face, scalps, and hands), genitals, palms and/or soles, nails, intense pruritus, and presence of single recalcitrant plaques. The Consensus Group recognized that the presence of disease manifestations listed earlier may alter the classification of mild disease (PASI ≤10, BSA ≤10, DLQI ≤10) to moderate-to-severe disease that warrants phototherapy, systemic treatment, and/or combination therapy.18
Therapy of chronic plaque psoriasis
The main outcome of psoriasis therapy is to safely achieve remission of psoriasis, that is, complete or almost complete clearance of skin lesions with no impairment of the disease on the quality of life.19 Treatments of psoriasis are numerous and they can be topical, systemic, or phototherapy (Table 1). The topical therapies include keratolytics, corticosteroids, vitamin D analogs, retinoids, dithranol, and topical calcineurin inhibitors. First-line treatments are vitamin D derivatives and corticosteroids, usually given in combination schedules. For topical treatments, the selection of the most appropriate vehicle is of major importance, thus improving adherence to the treatment, which is frequently impaired by the complexities of topical therapeutic choices. Evidence for efficacy and safety of topical treatments is readily available for vitamin D and short-term treatments with corticosteroids. Data on long-term use of topical therapies are scarce. New small molecules, including Janus kinase-signal transducer and activator of transcription and phosphodiesterase type 4 (PDE4) inhibitors, are under clinical development.20 A major issue with topical therapy is adherence, which may reduce dramatically in the long term, rendering topical treatments poorly accepted and ineffective. Phototherapy, which includes either narrow-band ultraviolet B light or photochemotherapy (ie, psoralen plus ultraviolet A light), and systemic agents, such as cyclosporine, methotrexate, fumaric acid esters, and acitretin, are indicated for moderate-to-severe patients. According to the European guidelines, in case of intolerance, inefficacy, or contraindication to phototherapy or conventional systemic treatments, the patients are eligible for biological agents, which include TNF-α antagonists (adalimumab, etanercept, and infliximab), anti-IL12/23 monoclonal antibody ustekinumab, and anti-17 monoclonal antibodies secukinumab and ixekizumab.15 The efficacy of biologic drugs for the treatment of skin signs of psoriasis and PsA is well known. The retention rate of biological agents is higher than conventional drugs because they are better tolerated in the long term.21 Treatment decisions are based on clinical status, patient treatment history, disease-related psychosocial burden, comorbidities, safety considerations, and patient’s preferences. In particular, disease severity is the leading factor for the choice of a systemic therapy. According to the rule of tens proposed by Finlay,22 a systemic treatment is indicated when the PASI score and/or BSA involvement and/or DLQI >10. Indeed, a systemic therapy could also be appropriate in the case of involvement of high impact areas (ie, scalp, genitals, palms and/or soles and nails), high intensity of symptoms such as pruritus, or presence of lesions not responsive to topical therapy. Treatment choice is also influenced by the concomitance of comorbidities, because methotrexate, cyclosporine, and acitretin may be harmful in some cases. For example, cyclosporine could cause arterial hypertension, alter glucose tolerance, and/or favor dyslipidemia.23 Consequently, cyclosporine is contraindicated in patients with metabolic syndrome and reduced kidney function. Similarly, acitretin could induce or worsen hypercholesterolemia and hypertriglyceridemia.23 Methotrexate and acitretin have a documented teratogenic effect and cannot be administered to females with child-bearing potential or those who wish to become pregnant. Methotrexate is also contraindicated in patients with a history of alcohol abuse or significant liver impairment. Females who have undergone acitretin treatment must avoid getting pregnant for at least 3 years following its withdrawal.15 The major safety concern of biologics emerging from registries or long-term studies is an increased risk of infections.24 In a large study based on cumulative incidence, the rate of serious infections was 1.45 per 100 patient-years (n=323) across treatment cohorts; 0.83, 1.47, 1.97, and 2.49 per 100 patient-years in the ustekinumab, etanercept, adalimumab, and infliximab cohorts, respectively; and 1.05 and 1.28 per 100 patient-years in the nonmethotrexate/nonbiologics and methotrexate/nonbiologics cohorts, respectively. The most commonly reported types of serious infections across the registry were pneumonia and cellulitis. Increasing age, diabetes mellitus, smoking, significant infection history, infliximab and adalimumab exposure were each associated with an increased risk of serious infection.25 More importantly, a significant proportion of patients (10%–20% every year) develop treatment resistance, which may impair the long-term effectiveness of biologic agents. In treatment decision, it is also very important to take into account several patient-related factors, including the age and sex, likelihood of adherence, expectation of remission, and fear of side effects. Recent large surveys reported that many patients with psoriasis discontinue their treatment because of lack/loss of the therapeutic efficacy, intolerance, and perceived safety concerns.7,26 Undertreatment of moderate-to-severe psoriasis is quite common. A recent population-based, multinational survey of 3,426 patients from 139,948 screened households in North America and Europe found that only 11% of patients with BSA >10% were receiving a systemic agent for psoriasis, with 52% receiving a topical treatment alone, and 37% being untreated.7 Indeed, despite all the medications available, new drugs for psoriasis and PsA are urgently needed to guarantee better disease control. In particular, oral drugs would be very much appreciated by some patients because they overcome the injection-related issues that may be observed with biologic therapies.
  | Table 1 Treatments of chronic plaque psoriasis |
Apremilast
Apremilast, an oral, small molecule PDE4 inhibitor, was approved for the management of psoriasis and PsA in 2014 by the US Food and Drug Administration and in 2015 by the European Commission. In EU, apremilast is indicated for adult patients with moderate-to-severe plaque psoriasis who have failed to respond or have a contraindication to, or are intolerant to other systemic therapy. In active PsA, apremilast is administered alone or in combination with conventional drugs to adult patients who have had an inadequate response or have been intolerant to a prior conventional therapy. Apremilast is the first small-molecule inhibitor of PDE4, an enzyme involved in the chronic inflammatory pathways, including those associated with psoriasis.27 PDE4 is the main cAMP-specific PDE in inflammatory cells, including macrophages, monocytes, mast cells, dendritic cells, eosinophils, and T cells. PDE4 inhibitors block the degradation of intracellular cAMP. The main mechanism of action of apremilast is the inhibition of PDE, which consequently increases the intracellular levels of cAMP and modulates the signaling pathways, by the activation of protein kinase A and phosphorylation of cAMP-response element binding protein, that inhibit the secretion of inflammatory cytokines (eg, TNF-α, interferon-γ, IL-2, IL-12, and IL-23) and stimulate the production of anti-inflammatory cytokines, such as IL-6 and IL-10 (Figure 2).28
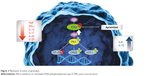  | Figure 2 Mechanism of action of apremilast. |
Preliminary studies on patients with severe psoriasis showed that apremilast is able to effectively modulate the inflammatory outcomes in psoriatic lesions.29 A Phase II study on 30 patients with recalcitrant psoriasis confirmed this activity of apremilast, as it showed a significant reduction in the number of immune cells (myeloid dendritic cells, T-cells, NK-cells) infiltrating the psoriatic lesions of the dermis and epidermis.7 After 4 and 12 weeks, other inflammatory mediators (IL-8, DEFB4, MX-1, K16, inducible nitric oxide synthase, IL-12/23 p40, IL-17A) also showed reduced levels. Furthermore, a relationship between the median change from the baseline PASI scores at week 12 and decrease in inducible nitric oxide synthase, IL-17A, defensin beta 4 and keratin 16 levels was observed. These results highlight that the wider modulation of the inflammatory response by apremilast versus other drugs targeting a single proinflammatory mediator is potentially responsible for its biologic effects.29
Apremilast in chronic plaque psoriasis
Efficacy
Apremilast in the treatment of chronic plaque psoriasis has been investigated in the Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) Phase III clinical trial program. ESTEEM 1 and 2 are two multi-center, randomized, double-blind, placebo-controlled studies aimed at analyzing the efficacy and safety of apremilast in the management of patients with moderate-to-severe plaque psoriasis (n=844 in ESTEEM 1 and n=411 in ESTEEM 2).30,31 Both trials had similar design. First, the patients were randomized in two groups: apremilast 30 mg twice daily or placebo for 16 weeks; in the second phase of the study, all the patients received apremilast for another 16 weeks (until week 32). Then, patients initially randomized to apremilast with a 75% (ESTEEM 1) or 50% (ESTEEM 2) reduction in PASI score (PASI 75 or 50 response) were rerandomized to continuation of apremilast or placebo (in this group, apremilast was restarted in case of loss of response). In both the ESTEEM trials, the primary end point was the proportion of patients who achieved PASI 75 at week 16. In ESTEEM 1, significantly more patients taking apremilast than placebo achieved PASI 75 at week 16, that is, 33.1% versus 5.3%, P<0.0001. PGA score of 0 or 1 at week 16, the major secondary end point of the study, was achieved by significantly more patients receiving apremilast than placebo, that is, 21.7% versus 3.9%, P<0.01. After 16 weeks, the mean change in PASI score from baseline was -52.1% versus -16.7% in apremilast and placebo groups, respectively (P<0.0001). The improvement in PASI was retained over 52 weeks in those responders.30 Apremilast reduced the severity of pruritus. Indeed, the severity of pruritus was reduced in patients receiving apremilast compared with placebo (-33.8 mm vs -7.7 mm in ESTEEM 1; -36.4 mm vs -12.9 mm in ESTEEM 2; P<0.0001 for both studies) at week 16.32 Improvement in the severity of pruritus with apremilast was correlated with improvement in the quality of life as measured by DLQI change from baseline.30 In the ESTEEM 1 study, a reduction in itching >20% (ie, minimal clinically important difference) was more frequently observed in subjects treated with apremilast than with placebo (70.6% vs 33.7%, P<0.0001).33 Apremilast was effective in treating patients with psoriasis located in nail, scalp, and palmoplantar areas. In particular, at week 16, a NAPSI 50 response (eg, ≥50% improvement from baseline in target nail NAPSI score) was observed in 33% and 45% of patients with nail psoriasis in ESTEEM 1 and 2, respectively.34 A greater proportion of patients treated with apremilast than placebo achieved a scalp PGA score of 0–1 (clear/almost clear) at week 16 (46.5% vs 17.5% and 40.9% vs 17.2%) in ESTEEM 1 and 2, respectively.34 Among patients with moderate-to-severe palmoplantar psoriasis at baseline, palmoplantar PGA score of 0 or 1 achievement at week 16 was significantly greater in patients who received apremilast versus placebo (65.4% vs 31.3%, P=0.03) and it was maintained at week 32 in 53.8% of patients.34 Finally, apremilast was able to significantly improve patient-reported outcomes versus placebo, as assessed by the DLQI, SF-36 item health survey version 2, and the Work Limitations Questionnaire-25 index.35 A pooled analysis of ESTEEM 1 and 2 trials showed that apremilast significantly improved work limitations, as well as increased work productivity compared with placebo.35
Safety
ESTEEM 1 and 2 trials confirmed the well-known and manageable safety and tolerability profile of apremilast. In these studies, adverse reactions were mostly mild-to-moderate in severity in all the study periods (short- and long-term). The most frequent adverse events were diarrhea (17.8%), nausea (16.6%), and upper respiratory tract infections (8.4%). The incidence of diarrhea and nausea was higher during the first 2 weeks of treatment compared with other study periods, and commonly resolved within 4 weeks.36 Long-term safety data (52 weeks of exposure) did not show a higher incidence of adverse events (including serious adverse events) based on exposure-adjusted incidence rates per 100 patient-years. During the ESTEEM program, only three deaths were observed: one in the placebo group for suicide and two in the apremilast group, both for cardiovascular accidents. Discontinuations due to adverse events occurred in 6.1% and 4.1% of patients receiving apremilast and placebo, respectively, during weeks 0–16 (mainly due to diarrhea 0.9% and nausea 1.4%) and did not increase with longer apremilast exposure. Incidence of major adverse cardiac events, serious infections, opportunistic infections, or malignancies in ESTEEM 1 and 2 (pooled analysis) trials were comparable to placebo.36 No reactivations of tuberculosis were noted, even if eight patients (n=5 in ESTEEM 1 and n=3 in ESTEEM 2) treated with apremilast had a positive anamnesis for tuberculosis. Abnormal laboratory test results in apremilast-treated patients were rare, transient, and not clinically significant. Furthermore, patients treated with apremilast in ESTEEM studies showed a weight loss. During the period from week 0 to 16, weight loss of >5% was experienced by 13.7% of patients receiving apremilast and 5.5% of patients receiving placebo; and from week 0 to 52 by 19.2% of patients receiving apremilast. At week 52, the mean (median) change from baseline weight was -1.99 (-1.40) kg in patients taking apremilast. Weight loss was not associated with any overt medical consequences, including diarrhea or nausea/vomiting.37 According to the analysis of clinical trials and published literature, there is no evidence of an increase in the risk of psychiatric events, including suicidality, with the use of apremilast.38 Finally, apremilast showed an acceptable safety profile and was well tolerated for up to 104 weeks of exposure.39
Expert opinion on the position of apremilast in the treatment for psoriasis
The clinical presentation of psoriasis could widely vary among patients because of different ages of onset, types of symptoms, areas of involvement, comorbidities, and disease severity. The choice of the treatment of psoriasis should be personalized according to the specific needs of the patients in order to optimize the outcome.
Apremilast is a well-tolerated and effective PDE4 inhibitor, indicated in the treatment of moderate-to-severe plaque psoriasis and PsA. The clinical development program of apremilast includes other potential indications, including ankylosing spondylitis, Behçet’s disease, ulcerative colitis, and atopic dermatitis. Apremilast will have an important place in the treatment of chronic plaque psoriasis because of oral administration, very favorable safety profile, lack of label-required screening or ongoing laboratory monitoring, efficacy in difficult-to-treat areas including nails, scalp, and palmoplantar regions, and rapid improvement in pruritus. Safety is a major unmet need in the therapy of psoriasis.40 In the long term, conventional systemic treatments could cause adverse effects, including renal and liver toxicity, myelosuppression, impairment of metabolic comorbidities, and necessitate periodic laboratory monitoring. The major concerns of biologics are the onset of infections and malignancies, and immunogenicity, which can cause a progressive loss or reduction of clinical efficacy. Apremilast does not formally require any screening to assess latent tuberculosis and chronic viral infections, or laboratory monitoring, and thus is perceived as extremely safe. To what extent the transient gastrointestinal side effects (nausea and vomiting) will limit its use will be addressed only with its use in clinical practice. PASI 75 response observed with apremilast is lower compared with biological treatments, including the novel IL-17 inhibitors.41 However, it should be considered that patients enrolled in ESTEEM studies had a high degree of severity of psoriasis according to the baseline PASI score of 19.4 ± 7.4 (mean ± standard deviation), and a significant proportion of them had been pretreated with phototherapy (30%) and standard systemic and/or biologic therapy (54%). Moreover, clinical efficacy of apremilast was maintained over 52 weeks with continued treatment. Apremilast could also be adopted as intermittent treatment, as most patients who lost PASI 75 response after discontinuing it because of rerandomization to placebo regained it after reinitiation of apremilast. As patients with psoriasis are frequently overweight or obese, it is of interest that ~20% of patients treated with apremilast showed a decrease in weight >5%. The relevance of this effect of apremilast on metabolic parameters associated with obesity needs to be investigated in further clinical studies.
In conclusion, psoriasis has a complex and substantial impact on a patient’s health-related quality of life across physical, psychological, social, and economic domains. Many of the currently available systemic treatments for psoriasis have limitations with respect to efficacy and/or safety. New biologic drugs (including IL-17 or -23 inhibitors and new small molecules) are currently under investigation in Phase III studies. Apremilast is a novel and valuable option for its safety profile and for refractory psoriatic lesions in difficult-to-treat regions such as scalp, nails, and palmoplantar areas. Moreover, apremilast could be indicated for those patients with a PASI score <10 but with a significant impact on quality of life (ie, DLQI >10) as well as in those with concomitant PsA. Given the favorable safety profile and the low risk of drug interactions, apremilast seems an appropriate treatment also for elderly patients. The long-term safety and efficacy of apremilast must be further investigated, and more data are needed to guide clinicians in the use of this drug in clinical practice.
Disclosure
This work was supported by an unconditional grant from Celgene, Italy. PG has been a consultant and/or speaker for AbbVie, Celgene, Janssen, Leo-pharma, Eli-Lilly, Merck Sharp & Dohme, Novartis, Pfizer, and UCB. GG has received personal fees from AbbVie, Almirall, Biogen, Boehringer-Ingelheim, Celgene, Hospira, Janssen, Leo-pharma, Eli-Lilly, Merck Sharp & Dohme, Mundipharma, Novartis, and Pfizer. The authors report no other conflicts of interest in this work.
References
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. | ||
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. | ||
Chandra A, Ray A, Senapati S, Chatterjee R. Genetic and epigenetic basis of psoriasis pathogenesis. Mol Immunol. 2015;64:313–323. | ||
Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–255. | ||
Durham LE, Kirkham BW, Taams LS. Contribution of the IL-17 pathway to psoriasis and psoriatic arthritis. Curr Rheumatol Rep. 2015;17:55. | ||
Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PLoS One. 2012;7:e52935. | ||
Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871–881. | ||
Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: A review of available evidence. Curr Rheumatol Rep. 2015;17:540. | ||
Gisondi P, Targher G, Zoppini G, Girolomoni G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. 2009;51:758–764. | ||
Gisondi P, Tessari G, Conti A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol. 2007;157:68–73. | ||
Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2:e000062. | ||
Davidovici B, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785–1796. | ||
Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol. 2012;66:369–375. | ||
Finlay AY. Patient-reported outcome measures in psoriasis: assessing the assessments. Br J Dermatol. 2015;172:1178–1179. | ||
Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23 Suppl 2:1–70. | ||
Scottish Intercollegiate Guidelines Network (Scottish 2010). Diagnosis and management of psoriasis and psoriatic arthritis in adults. Available from: http://sign.ac.uk/guidelines/fulltext/121/index.html. Accessed May 11, 2016. | ||
Guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis; 2004. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003329.pdf. Accessed May 11, 2016. | ||
Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1–10. | ||
Mrowietz U, de Jong EM, Kragballe K, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014;28:438–453. | ||
van de Kerkhof PC. An update on topical therapies for mild-moderate psoriasis. Dermatol Clin. 2015;33:73–77. | ||
Gisondi P, Tessari G, Di Mercurio M, Del Giglio M, Girolomoni G. The retention rate of systemic drugs in patients with chronic plaque psoriasis. Clin Dermatol. 2013;1:8–14. | ||
Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861–867. | ||
Gisondi P, Cazzaniga S, Chimenti S, et al. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the Italian Psocare Registry. J Eur Acad Dermatol Venereol. 2013;27:e30–e41. | ||
Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441–1448. | ||
Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: Results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151(9):961–969. | ||
Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and 568 psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149:1180–1185. | ||
Baumer W, Hoppmann J, Rundfeldt C, Kietzmann M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm Allergy Drug Targets. 2007;6:17–26. | ||
Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26:2016–2029. | ||
Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12:888–897. | ||
Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (ESTEEM 1). J Am Acad Dermatol. 2015;73:37–49. | ||
Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–1399. | ||
Feldman S, Thaci D, Ling M, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis: pruritus and DLQI correlations at week 16 (ESTEEM 1 and 2). J Am Acad Dermatol. 2015;72(5 Suppl 1):226. | ||
Yosipovitch G, Papp K, Bagel J, et al. Effects of apremilast on pruritus in patients with moderate to severe plaque psoriasis: results from the ESTEEM 1 and 2 trials. Presented at: Annual Congress of the European Academy of Dermatology and Venereology; Amsterdam, The Netherlands; October 8–12; 638; 2014. | ||
Crowley J, Gooderham M, Wasel N, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with nail, scalp, and palmoplantar psoriasis 52-week results from the ESTEEM 2 trial. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20–24, 2015; San Francisco, CA. | ||
Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49. | ||
Reich K, Papp K, Gordon K, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis of two phase 3, randomized, controlled trials (ESTEEM 1 and 2). Presented at: Annual Congress of the European Academy of Dermatology and Venereology; October 8–12; 2014; Amsterdam, The Netherlands. | ||
Reich K, Sobell J, Stevens R, Day R. Change in weight with apremilast, an oral phosphodiesterase 4 inhibitor: pooled analysis of the ESTEEM 1 and ESTEEM 2 trials. Presented at: Annual Congress of the European Academy of Dermatology and Venereology; October 8–12, 2014; Amsterdam, The Netherlands. | ||
Kragballe K, van de Kerkhof PC, Gordon KB. Unmet needs in the treatment of psoriasis. Eur J Dermatol. 2014;24:523–532. | ||
Menter A, Paul C, Stevens RM, Day RM, Saha K. Psychiatric disorders and depression incidence with apremilast: pooled analysis of ESTEEM 1 and ESTEEM 2 trials. Presented at: the 23rd European Academy of Dermatology and Venereology; October 8–12; 2014, Amsterdam, The Netherlands. | ||
Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400–409. | ||
Papp K, Reich K, Sobell J, et al. Two years safety of apremilast in patients with moderate to severe psoriasis: results from randomised controlled trial ESTEEM 1. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20–24, 2015; San Francisco, CA. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.