Back to Journals » Journal of Asthma and Allergy » Volume 12
Approaches to the assessment of severe asthma: barriers and strategies
Authors Majellano EC , Clark VL , Winter NA , Gibson PG, McDonald VM
Received 4 June 2019
Accepted for publication 25 July 2019
Published 23 August 2019 Volume 2019:12 Pages 235—251
DOI https://doi.org/10.2147/JAA.S178927
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Amrita Dosanjh
Eleanor C Majellano,1,2 Vanessa L Clark,1,2 Natasha A Winter,1,3 Peter G Gibson,1,4 Vanessa M McDonald1,2,4
1Faculty of Health and Medicine, National Health and Medical Research Council Centre for Research Excellence in Severe Asthma and the Priority Research Centre for Healthy Lungs, The University of Newcastle, Newcastle, NSW, Australia; 2Faculty of Health and Medicine, School of Nursing and Midwifery, The University of Newcastle, Newcastle, NSW, Australia; 3Faculty of Health and Medicine, School of Medicine and Public Health, The University of Newcastle, Newcastle, NSW, Australia; 4Department of Respiratory and Sleep Medicine, John Hunter Hospital, Hunter Medical Research Institute, Newcastle, NSW, Australia
Correspondence: Vanessa M McDonald
Level 2 West Wing, Hunter Medical Research Institute, Locked Bag 1000, New Lambton Heights, NSW 2305, Australia
Tel +61 24 042 0146
Fax +61 4 042 0046
Email [email protected]
Abstract: Asthma is a chronic condition with great variability. It is characterized by intermittent episodes of wheeze, cough, chest tightness, dyspnea and backed by variable airflow limitation, airway inflammation and airway hyper-responsiveness. Asthma severity varies uniquely between individuals and may change over time. Stratification of asthma severity is an integral part of asthma management linking appropriate treatment to establish asthma control. Precision assessment of severe asthma is crucial for monitoring the health of people with this disease. The literature suggests multiple factors that impede the assessment of severe asthma, these can be grouped into health care professional, patient and organizational related barriers. These barriers do not exist in isolation but interact and influence one another. Recognition of these barriers is necessary to promote precision in the assessment and management of severe asthma in the era of targeted therapy. In this review, we discuss the current knowledge of the barriers that impede assessment in severe asthma and recommend potential strategies for overcoming these barriers. We highlight the relevance of multidimensional assessment as an ideal approach to the assessment and management of severe asthma.
Keywords: asthma, severe asthma, severity, assessment, barriers, strategies
Introduction
Asthma is a significant public health threat, affecting more than 300 million individuals globally.1 Asthma is classified as a non-communicable disease and leads to reduced quality of life,2 poor physical functioning3 and reduced emotional well-being.4 The impact of this disease can be widespread and extends beyond the person living with the disease, affecting the lives of their family members, carers, communities and the health care system.5
Asthma is a variable chronic respiratory condition. It is characterized by symptoms of wheeze, cough, chest tightness, dyspnea and backed by variable airflow limitation, airway inflammation and airway hyper-responsiveness (AHR).1 The severity of asthma varies considerably, both between individuals and within individuals over time.1 Some people may have intermittent asthma and others may experience severe, potentially life-threatening disease. In mild-to-moderate asthma, inhaled corticosteroids (ICS), bronchodilators and self-management education are the cornerstone of effective treatment.5 However, 3%6 to 10%7 of the patients experience a severe form of asthma that fails to respond to standard therapy despite receiving maximal treatment. Thus, severe asthma is defined as “asthma which requires maximum controller therapy to prevent a patient from becoming uncontrolled or which, despite high dose therapy remains uncontrolled.”7 Patients diagnosed with severe asthma endure significant difficulties in daily living, a decrease in physical activity,3 work capacity or productivity8 and social exclusion.2 Furthermore, patients with severe asthma are faced with an increased comorbidity burden.2,7
There is a wide array of comorbidities present in severe asthma that may contribute to disease severity, mimic asthma symptoms, and therefore confound assessment and treatment.9 For example, chronic rhinosinusitis is a prevalent comorbidity of asthma and contributes to disease severity.7 Similarly, obstructive sleep apnea, obesity and psychological factors often co-exist and complicate management.7 These comorbidities mimic asthma symptoms and affect the intensity of the disease, management or diagnosis, leading to a much greater risk of asthma morbidity and mortality.7 Given the complexity and heterogeneity of the disease, assessment and management of severe asthma warrants advanced approaches.5,10
Guidelines for asthma management have proposed that evaluation of disease severity is necessary to initialize therapy and maintain treatment through a step-wise process.11 Misclassification of the levels of severity may contribute to the underuse or overuse of anti-inflammatory medications, resulting in either poor asthma control or adverse side-effects associated with overtreatment.11 With the advent of biological therapies,12 recognition of the level of asthma severity is imperative to facilitate treatment interventions to the right patients.12
The literature on diagnosing, treating and managing severe asthma indicates that significant barriers exist across health care settings and that these barriers relate to health care providers, patients and organizational systems.10 Overcoming these barriers is necessary in order to facilitate effective assessment and accelerate appropriate treatment for severe asthma patients.13 Therefore, identification of the barriers related to precision assessment of severe asthma is an important step. The purpose of this review is to discuss current knowledge of the barriers that impede assessment in severe asthma and to recommend potential strategies for overcoming these barriers. We highlight the importance of multidimensional assessment as an approach to the assessment and management of severe asthma.
Search strategy
The literature search is current as of March 2019 using the electronic databases CINAHL, Pub Med, Web of Science, Google Scholar, Wiley, and Medline. The search strategy includes the keywords of asthma, severe asthma, severity, assessment, barriers and strategies. English written articles between 2014 and 2019 were retrieved and included to reflect the current literature. However, we did not exclude seminal papers which were highly cited and judged to be relevant to answer our aims. We also checked reference lists to identify relevant studies significant for our review.
Measurement of asthma severity
Severity-based stratification of patients with asthma is an integral part of management, providing a useful blueprint for treatment decision making.11 Categorization of asthma severity reinforces the regulation, duration and calculation of the amount and type of therapy to establish asthma control.11 Patients with well-controlled asthma have minimal symptoms or functional impairment related to their disease.11
The general definition of severity implies “the intrinsic intensity of the disease process,”11 however, defining severity is often challenging because asthma is associated with a wide range of heterogeneity.14 In addition, genes and environmental exposures like allergens, cigarette smoke or air pollution play a crucial role that may change or influence disease progression over time.14 Therefore, periodic assessment is necessary to assist asthma management and treatment. To date, there is no gold standard for classifying asthma severity or robust data showing significant changes in disease severity in a longitudinal cohort.15 The Global Initiative for Asthma (GINA) strategy recommends that asthma severity should be determined according to the level of treatment required to control and reduce symptoms and exacerbations.1 A step-wise approach to treatment is recommended where each of the five steps constitutes five levels of increasing treatment recommended according to severity. Step 1 to 3 represents mild–moderate disease, with steps 4 and 5 depicting moderate-severe asthma, which requires high-dose ICS/Long-Acting Beta-Agonist (LABA) treatment to achieve and maintain asthma control.1 In some cases, severe asthma may remain uncontrolled despite high-dose therapy, suggesting the need for further multidimensional and systematic assessment and treatment (Figure 1).
Severe asthma assessment
The assessment of severe asthma is complex. This is because the disease is heterogeneous in nature and associated with many comorbidities; therefore, a multidimensional evaluation is essential.16 Figure 1 shows a practical guide designed to critically diagnose, characterize and manage severe asthma. We elaborate on these steps further in the next section.
Diagnosis confirmed
Harm from asthma medications may arise from misdiagnosis.15,17 Hence, a comprehensive work-up is important to ascertain whether the patient has severe asthma.9 A detailed history is the first step to the assessment and diagnosis.9 The medical history should focus on characterizing the specific symptoms, their frequency and severity.7 Clarification of the onset of symptoms, determination of the severity of exacerbations and associated comorbidities are also essentials.9 Accordingly, clinical questionnaires can facilitate these assessments.9 Physical examination of the cardio-respiratory system is also important.9 When critical information of the patient’s symptoms is gathered, clinicians gain a better understanding of the patient’s problem.
There are a number of standard assessments included in lung function examinations in asthma. These include, but are not limited to, the measurement of airflow limitation through spirometry, assessment of bronchodilator responsiveness and assessment of AHR. Table 1 shows a summary of the lung function and volume assessments to consider in severe asthma.
 |
Table 1 Pulmonary function tests to consider in severe asthma |
Biomarkers are observable characteristics that are objectively measured as an indicator of normal or abnormal biologic processes.28 The clinical utility of biomarkers includes four main roles: 1) diagnosis, 2) disease staging, 3) ongoing assessment of disease progression and 4) assessment of treatment response. In addition to being a guide for clinicians, the process of biomarker investigation allows for a comprehensive understanding of the underlying molecular pathways behind disease pathogenesis as well as the discovery of new targets for novel therapies. At present, airway inflammatory biomarkers used in practice include serum immunoglobulin-E (IgE), peripheral blood eosinophils, sputum eosinophil counts and exhaled fraction of nitric oxide (FeNO).29 However, limitations of biomarkers do exist, impeding implementation in clinical practice. Barriers and strategies to overcome these limitations will be discussed in the sections below.
Is it severe?
If a diagnosis of asthma is confirmed and control has not been achieved, asking the question of whether it is actually severe asthma is required.9 Common features of uncontrolled asthma are presented in Table 2. Differentiating between difficult to treat asthma and severe treatment refractory asthma at this stage is integral. Key differences between these forms of asthma are highlighted in Table 3 and differentiation can be achieved by an assessment of asthma self-management skills, comorbidities, risk factors and triggers, and whether treatment is indeed optimized (Figure 1).
 |
Table 2 Criteria for uncontrolled asthma |
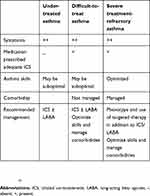 |
Table 3 Asthma definitions and characteristics |
 |
Figure 1 Checklist for the multidimensional assessment of severe asthma. Note: Figure reproduced with permission from the Centre of Excellence in Severe Asthma, originally developed as part of the Severe Asthma Toolkit © (http://www.severeasthma.org.au).30 |
The most common asthma management skills, comorbidities, triggers and risk factors in severe asthma are shown in Table 4.30 A careful assessment for each of these factors promotes optimal asthma control, therefore should be priority during clinic visits both in primary and specialist care.17
 |
Table 4 Checklist of important contributory factors in severe asthma |
Is treatment optimized?
It is essential to determine whether patients with uncontrolled asthma are receiving adequate treatment9 with high-doses of ICS and LABA or moderate dose ICS and two or more controllers. A trial of add-on therapies like tiotropium, anti-fungal agents, montelukast and low dose macrolide antibiotics is often warranted before initiation of biological therapies.31 However, if benefit of these therapies is not achieved, appropriate treatment re-evaluation is required. Apart from assessing asthma treatment, adherence and inhaler technique should also be evaluated.32 Suboptimal adherence and inhalation technique are two of the most prevalent factors resulting in poor asthma control.32 In severe asthma, adherence to treatment tends to be poor in both adults and children with prevalence estimated to be >50%.7 If left unaddressed, the consequences of non-adherence will lead to a greater risk of asthma exacerbations and increased health care costs.33 Ultimately, non-adherence may in turn lead to unnecessary treatment escalation, which can be both harmful and expensive.33 Adherence to medications and inhaler technique should be regularly assessed.32 Objective assessment should also be considered whenever possible (Table 5).17 Integrating the results of these tests identifies factors that impede medication adherence.
 |
Table 5 Useful tools for assessing adherence |
Multidimensional assessment
Diagnosis and management of severe asthma offer unique challenges because of the heterogeneity of the disease itself. The current guidelines7 recommend that people with severe asthma undergo a systematic assessment or multidimensional assessment. The multidimensional assessment includes a series of coordinated steps designed to assist in confirming the diagnosis, recognition and treatment of common comorbid conditions, and in determining risk-factors.42 Multidimensional assessment may cover domains related to pulmonary or airway assessments, extrapulmonary or comorbidity assessments as well as risk-factor and behavioral assessments (Figure 2).16,43,44 The results from a multidimensional assessment can be used to inform management decisions comprising identification of therapeutic strategies and specialist referrals.16,43,44
 |
Figure 2 Clinical domains in the phenotyping of severe refractory asthma. Note: Figure reproduced with permission from the Centre of Excellence in Severe Asthma, originally developed as part of the Severe Asthma Toolkit © (http://www.severeasthma.org.au).30 |
To demonstrate the possible benefits of multidimensional assessment, a meta-analysis of three observational studies has reported improvements in health-related quality of life (HRQoL), asthma control and reduction in exacerbations, up to a year after the implementation of multidimensional assessment.43 More recently, McDonald and colleagues completed the first randomized controlled trial (RCT) in severe asthma utilizing multidimensional assessment and individualized management targeting the traits identified in each individual and demonstrated improved outcomes for patients.45
Individualized management plan
Patients with severe asthma require tailored evidence-based interventions to meet their needs. Case-management is one approach that can assess, plan, facilitate and coordinate health care delivery through partnerships with clinicians, patients and their families.46 In a study of older patients with asthma and COPD, significant improvements in quality of life were sustained for 1 year in COPD patients who underwent multidimensional assessment and individualized management.47 More recently, this approach has been tested in patients with severe asthma as described earlier.45
Barriers to severe asthma assessment
Precise assessment of asthma severity is key to optimizing health and well-being of people with asthma.15 We have described severe asthma assessment in terms of objective tests used in diagnosis. However, it is recognized that there are barriers to undertaking a complete and comprehensive assessment of severe asthma in clinical practice.10 The barriers are multifactorial and relate to system challenges, health care professional (HCP) and patient barriers.13,32 Figure 3 summarizes the factors that influence the assessment of severe asthma.
 |
Figure 3 Barriers to assessment of severe asthma by stakeholders. |
Barriers related to health care practitioners (HCP)
A number of barriers that relate to HCPs have been identified (Figure 3). These include a lack of knowledge about guideline-recommended therapies, inconsistent diagnosis and management, referral pathway issues and communication gaps.10,13,32
The role of guidelines and checklists
Although evidence suggests that the use of clinical guidelines and checklists are beneficial and are prime components in asthma management, unfortunately there is also evidence showing HCPs’ low adherence to guidelines.32,48,49 Findings from a national survey of severe asthma experts in Australia report that 60% of the clinicians managing patients with severe asthma do not use checklists, but believed they would find one useful.10 In an observational study that assessed the barriers to improving the management of patients with severe asthma, the underuse of guidelines suggested suboptimal asthma control.48
There are several proposed reasons as to why HCP adherence to guidelines and checklists may be suboptimal.50 These include professional issues relating to clarity of roles,51 lack of familiarity with guidelines,15,52 lack of training and skills,53 distrust about guidelines in general,54 complex and lengthy guidelines,55 poor teamwork, lack of self-efficacy,51 poor communication,56 limited access to spirometry57 or lack of belief that a guideline or checklist will produce positive outcomes.10,49 Other HCPs viewed guidelines as inflexible15 or identified that asthma guidelines do not thoroughly focus on severe asthma.10 Moreover, external barriers influencing HCPs’ adherence to guidelines include lack of time,51 organizational constraints10 and inability to reconcile patient preferences with guideline recommendations.52 Several approaches have been undertaken to address these issues in severe asthma. GINA published practical recommendations about how to identify, assess and manage difficult to treat and severe asthma in adolescent and adult patients.1 Practice guidelines are designed to provide recommendations to assist and guide HCPs in making clinical decisions.58 When used by an HCP in practice, asthma guidelines could minimize diagnosis and assessment inconsistencies, reducing avoidable emergency department visits and hospitalizations.58 Furthermore, to overcome the barriers related to the lack of training and resources available for severe asthma clinicians, the Australian Centre of Excellence in Severe Asthma produced an online toolkit to provide evidence-based guidance to clinicians globally (http://toolkit.severeasthma.org.au). The main goal of providing the toolkit is to equip HCPs with clinical resources essential in the management of severe asthma.59 Thus, better meeting the needs of HCPs across health care settings irrespective of geographical locations.59 The Severe Asthma Toolkit offers the latest treatment options to optimize care in severe asthma patients, covering various modules ranging from medications, diagnosis, assessment, management and comorbidities among others.59 The toolkit was created by a world-class multidisciplinary team with clinical expertise in severe asthma.59
Inconsistent approach for diagnosis or management
Despite the significance of spirometry in respiratory function testing for assessing severity, the test is widely underutilized for asthma in primary care.32 Furthermore, biomarkes used in severe asthma management are also underutilized, in an online survey to clinicians involved in severe asthma management, 53% of the respondents indicated that they never used FeNO testing,10 a surrogate marker of eosinophilic airway inflammation.
Similarly, this survey also reported that assessments for some comorbidities such as naso-endoscopy, functional exercise test or bone mineral densitometry were never initiated, by 60%, 46% and 32% of the respondents, respectively.10 Health status, asthma control and comorbidities questionnaires were also inconsistently used.10 Of note, determining whether or not a treatment effect is clinically meaningful is a great challenge in severe asthma.60 Barriers to routine use of self-reported questionnaires include logistical, technical and lengthy administration inhibiting successful adoption of some patient-reported outcome measures (PROMs) in clinical practice.2 PROMs that have been developed specifically for severe asthma provide a valid assessment of the patient’s health status, level of control and experience of severe asthma and are useful in the clinic.2 Variability in diagnosis, assessment and management could lead to serious consequences for patients with severe asthma.15 Diagnostic uncertainty in asthma still exists, with severe asthma being underdiagnosed or overdiagnosed,61 indicating that diagnostic precision remains a serious issue in the era of targeted therapy.
Referral pathways
Referral systems offer patients access to expert HCPs in the field of severe asthma.32,52 A timely referral to specialized care should be actioned when patients in primary health care continue to experience suboptimal control, with increased severity, despite guideline-based treatment.62 Notwithstanding the importance of a linkage pathway, variations in referral patterns from primary health care to secondary health care have been a constant concern both at the international48 and national scene.10 The lack of clear referral criteria limits success in optimal management. McDonald et al10 highlighted that for a referral system to work in severe asthma, a referral at all levels of care should be defined. One example of a referral intervention from primary health care to secondary health care is the SIMPLES model.63 The SIMPLES model is a structured framework used in primary health care to evaluate patients with difficult to control asthma.62,63 If patients have not achieved control despite structured reviews, a referral to severe asthma specialist clinic is warranted for further evaluation and management.62 The SIMPLES approach suggests a good interface between primary and specialist care, integrating clinical assessment and management, whilst avoiding inappropriate escalation of treatments.62,63
Orozco-Beltran et al48 conducted a study using a modified Delphi method on the management and referral of severe and poorly controlled asthma where they found physicians dissatisfaction in the referral process. The majority of non-severe asthma patients are treated in secondary health care when they can be appropriately managed in primary health care.48 The lack of clarity and consensus of the referral criteria between primary health care and secondary health care drives up costs and diversion of resources.48 A retrospective observational study64 has also reported findings that are consistent with the outcomes of Beltran et al.48 HCPs have identified the need for a well-defined and extensive criteria to guide referral decision making and to ensure equitable access to available health services.10,48
Barriers related to patients and clinicians
A number of factors have been identified as contributing to suboptimal severe asthma assessment: patient–provider communication, personal perceptions or beliefs65 and managing symptoms and risk factors.60
Poor patient–physician communication
Effective patient–provider relationships are important for patients with asthma and can lead to improved outcomes.66 Communication is a cardinal component and foundation for a positive patient–provider relationship, increasing patient satisfaction and better adherence to treatment.66 As a result, satisfied patients are more likely to share critical health information with their physicians.66
However, conflicting perception between the patient and the HCP can negatively impact patients’ health.67 The observational study of discordance in patients and HCPs’ views on asthma control by Crespo-Lessmann et al67 concluded that patient–provider discordance is likely to contribute to an increased risk of poor asthma control. Communication is a fundamental element to developing a patient-centered treatment plan but authoritarianism or paternalism in clinical settings disempowers patients, limiting a two-way conversation.68 The level of respect, time constraint, cultural and language diversity also impede stronger relationships and communication between patients and physicians.69
Patient personal perceptions
The patient’s perspective is essential to both assessment and management of severe asthma, but when patients overestimate or underestimate disease severity or the level of asthma control required, then it becomes a significant barrier.70 A qualitative study by Bidad et al71 that sought to determine patients’ perception of asthma control identified five themes: (a) personal meaning of control, (b) intermittent prevention, (c) compromising control to avoid medication, (d) pharmacological agents overemphasized in control and (e) the role of asthma review in control.71 In one of the themes, patients described having their own “internal barometer” of the level of symptoms in determining when their level of control was decreasing and this was determined to be much higher than the asthma control test (ACT) cut points.71 Moreover, primary care patients did not perceive asthma review appointments as an advantage to asthma control, but rather as an additional burden.71 This perception was compounded by clinical practice variations and conflicting advice provided by HCPs.71
Managing symptoms and risk factors
The complexity of severe asthma explains why patients seek a comprehensive understanding about their disease.60 When patients acquire greater understanding of their illness, a sense of control and empowerment is achieved enabling them to make positive choices and set goals.68 Unfortunately, the patients’ journey toward acquiring the desired knowledge is not straightforward. When faced with ambiguity, patients have reported that they receive little information from their HCPs.68 As a result, patients will seek information from elsewhere, including potentially unreliable sources.68 Individuals with limited knowledge about asthma symptoms, common triggers and risk factors are at a heightened risk for poor asthma control. Limited health literacy in patients and an absence of patient-centered care can lead to non-adherence to medications and suboptimal self-management.68 In an explorative study by Lingner et al72 on patient and HCPs’ concept of good asthma treatment, patients desired to share the decision-making process with their HCPs. However, several patients discussed their reluctance to discuss their asthma with their HCP due to their perception that their HCPs had preconceived notions, for example, “they always blame your weight”,68 implicating a barrier to the patient–clinician relationship.60
Barriers related to system factors and equitable access to specialized service
There are numerous factors influencing delivery of care. Barriers arising from the structural level include longer waiting lists due to lack of specially trained HCPs, high service demand32 coupled with insufficient resources.10 In addition, there is a complex procedure required to access novel therapies12 and there is limited access to some biomarkers in many facilities.73
Geographic barriers disproportionately burden patients with financial incapacities or even severe asthma patients who require closer attention from the specialist and multidisciplinary team (MDT).10 MDTs are a group of HCPs who coordinate, manage and organize care for people with severe asthma.31 The core staff necessary to run a severe asthma clinic includes respiratory physician, specialist nurse, pulmonary function scientist and speech pathologist.31
Often rural patients have to travel long distances to specialized services and therefore, places severe asthma patients in a difficult position, weighing up travel costs and benefits.10
Waitlist and appointment delays
Longer waiting times suggest underlying issues or unresolved conflicts in resources, stakeholders, policies or systems in the delivery of services.74 This means that prolonged waiting periods are significant barriers for patients. Not being able to obtain the right services and appropriate treatments at the right time impacts patient expectations and satisfactions.75 Some studies have demonstrated that appointment delay causes stress for both patients and HCPs reducing positive outcomes.74 Fielden et al76 demonstrated that prolonged waiting times of >6 months result in greater economic costs and deterioration in physical function and in HRQoL.
Complexities of accessing novel biological therapies
With the use of novel biological therapies on the rise, patients deserve timely access to these targeted treatments.62 Access to novel therapies can vary significantly across international health care settings and requires different prerequisites before these drugs can be prescribed. In Australia, the long waiting periods of usually 6–12 months deter access to biologics.10,12 During those months, specialists, for their part, are trying one or more add-on therapies before initiating biological therapies.12
Lack of accessible biomarkers
In order to improve outcomes for severe asthma patients, it is critical that we devise a more definitive approach to assessing patients with asthma. Biomarkers represent a solution to characterizing patients in order to predict prognosis and treatment response.77 Airway inflammation is an acceptable starting point for discovering biomarkers as inflammation plays a critical role in underlying pathologies.78–80 The current gold standard of assessing airway inflammation is sputum cell counts but this has limitations in the clinical setting. Techniques involved in collecting and processing specimens are time-consuming, require skill and there are issues with reproducibility and inconsistent cut-off values, restricting sputum cell counting to specialized- or research-based centers.81 In response, surrogates of airway inflammation have been developed, including blood eosinophils,82 FeNO83,84 and periostin.85 However, none are free from contradicting results81,86,87 and confounding factors.88,89 As a result, the use of these markers in diagnosis and assessment, prediction and prognosis has had slow uptake but is a priority for future research and current practice. In addition, although biomarkers indicative of eosinophilic inflammation have been developed and validated, there are currently no biomarker surrogates for non-eosinophilic inflammation.90,91 This is of critical importance as many severe asthma patients present with non-eosinophilic inflammation and some may have persistent neutrophilic inflammation.92
So how do we develop the “ideal biomarker”? Many factors determine the ideal biomarker and can act as hindrances to biomarker development and its clinical utility.93 Important in the clinical setting are that biomarkers are accessible and non-invasive and the techniques to measure are rapid, straightforward and relatively inexpensive.93 Whereas, it is completely valid to use invasive techniques, such as bronchial biopsies and bronchial lavage, to detect new molecular pathways and mechanisms, accessible biomarker sources such as blood, urine, sputum and exhaled breath are preferred. Additionally, due to the complexity of asthma, a single biomarker will not be sufficient to capture the full disease process. Therefore, we need further research generating composite panels of biomarkers from varying non-invasive sources.73,94
Commendations must be made to the surrogate inflammatory biomarkers that do exist. But unfortunately, biomarkers must also be reliable and reproducible and despite the great amount of research that has been invested in biomarker discovery and testing, the ideal biomarker does not yet exist in asthma and conflicting results and limitations still remain a reality.
Overcoming barriers
To facilitate optimal evaluation and assessment of severe asthma, the following key recommendations should be taken into consideration.
Strategies related to health professional factors
Optimization of referral avenues and guidelines
A centralized web-based database system can facilitate the transmission of valuable information.10 When necessary, treating physicians have the capacity to access details including asthma symptom severity, exacerbation frequency, past and current therapies and spirometry outcomes.32 The accessibility of this information can shorten the patients’ journey through the referral pathways, which can lead to improved HRQoL for severe asthma patients.62 In addition, establishing a universal referral pathway to help select patients who will likely benefit from specialist evaluation can also streamline care.10,13 To reinforce suitable referrals, regular monitoring should be implemented especially to patients with moderate or severe asthma.95 For example, pulmonary specialists should follow-up patients admitted with severe asthma exacerbations for at least 1 year after the admission.52
Provision for telehealth consultations from primary health care to secondary health care strengthens referral pathway as well.48 It should be noted that primary care implements a gatekeeping system, whereby the general practitioner (GP) is in the principal position to recognize poorly controlled asthma.32 The need to establish and develop a mechanism for involving GPs in the management of asthma should be consistent from the assessment all the way to the administration of biological therapies.32 Systems in which patients can have their novel biological therapies administered within primary care, similar to rheumatoid arthritis, have been proposed to substantially reduce the burden of specialists in asthma services, paving the way to conveniently accommodate more patients.10,32
Use of assessment tools
The use of assessment tools, specifically subjective and objective tools in severe asthma assists in viewing patients from a multidimensional perspective.9 Furthermore, outcomes from the assessment form the basis of the care plan. The Centre of Excellence in Severe Asthma has developed valuable resources for HCPs, packaged in an online toolkit https://toolkit.severeasthma.org.au/.96 The Severe Asthma Toolkit is comprehensive and provides a convenient, easy-to-use resource to support and equip clinicians on how to achieve optimal severe asthma management.96 Furthermore, to enhance diagnostic and assessments proficiency, knowledge dissemination within the workplace either through video conferencing, seminars and webinars can aid in practice.97 Decision support tools like computerized systems, treatment guides, and standardized prescriptions assist HCPs in treatment decision making.97
Strategies related to patient factors
Patient–physician communication
The patient–provider relationship is dependent on good communication skills.66 Apart from clinical competence, HCPs are required to master and demonstrate empathy, compassion, caring, non-judgment, open and concern during patient encounters.68 There is a wealth of evidence in the literature that supports the benefit of efficient and effective communication resulting in increased patient satisfaction, better health outcomes, and decreased health care utilization98 even without lengthening appointment times.99 Providing a patient-centered care (PCC) approach is essential in asthma management. Under a PCC model, partnerships in health between HCPs, patients and carers are highlighted.100 Consideration of patients’ preferences and values is demonstrated through patients’ active participation in clinical decision making.100 A PCC model acts as a springboard in promoting flexible provision of health care and is moving beyond the traditional paternalistic approach.100 Additionally, the role of patient advocates help patients navigate the health care system and bridge between patient and their treating clinicians reducing communication gaps.101 Furthermore, communication failures between HCPs can be fostered through regular meetings and joint clinical sessions.48
Strategies related to organizational factors
Assuring appropriate resourcing
The severe asthma health workforce depends on the size of the service. In a conservative setting, at least one pulmonary physician and nurse specialist are needed; a second physician is necessary to overcome patient load or absences.31 When workforce shortages exist, partnerships with nearby asthma network services may mitigate short-term periods of absence.13 Benefits of a shared-care model, wherein special arrangements between public and private providers offer opportunity for expanding services and resources.13,102
Improve access to MDT
Specialist MDTs are core for confirming diagnosis of severe asthma.13 To optimize phenotyping and targeted therapy, providing continuous education and trainings for MDT members in severe asthma management is warranted.10 Onsite availability and participation of nursing and allied health offer efficient services.13 Funding for MDT could be achieved through a clinical re-design approach.31 This method seeks to balance the costs and benefits, by reducing health care utilization and justifying the utilization of expensive therapies and patient outcomes. The use of teleconferencing or video-linked MDT discussion to its full advantage allows other practitioners to collaborate in decision-making process.13 Telehealth can break down the wall of geographical barriers for patient treatment, follow up or initial in-person consultation.10,13
Accessible biomarkers
A two-step approach can be taken to develop strategies to overcome barriers obstructing biomarker development and use in primary care. The first step is to discover novel biomarkers. Followed by the investigation and validation of newly discovered biomarkers from non-invasive sources, using easy-to-measure techniques.
“Omics” technology epitomizes the advancements that have been made in medicine and science. This new generation of exploratory science refers to the study of the biological system. They include, but are not limited to, genomics, proteomics, transcriptomics and metabolomics. The use of computational networking, bioinformatics and systems biology seeks to interpret the “big data” generated from the extensive exploration of the human organism.103,104 In asthma, unbiased “omics” screening studies have been used to discover novel biomarkers, such as protein measurements in proteomics studies105 and differential gene expression in transcriptomic studies.106 With methods used to obtain data becoming less expensive and databases becoming larger and more secure to store the deluge of data, “omics” studies are becoming more prevalent and the combination of “omics” data, known as “multiomics”, is expanding,107 deepening our understanding of the molecular and genetic pathways underlying disease.
In tying in with the characteristics of the “ideal biomarker”, new biomarkers must also be easy to obtain from non-invasive sources, using feasible techniques. Biomarkers from easily accessible tissues and fluids such as blood, urine, sputum and exhaled breath are ideal. Serum provides one of the most ideal sources for biomarkers as blood collection, serum preparation and analysis are highly standardized techniques and collection is minimally invasive.73 However, recent studies in asthma have found that urine73 and exhaled breath88 are also promising sources of novel biomarkers, especially for children.108 Although, these will require further research to validate. An example of ongoing research into non-invasive alternatives to sputum induction and more feasible measurements of airway biomarkers are through the recently developed absorptive nasal strip technology. Technology within absorptive strips permits the sampling of mucosal fluid within the upper respiratory tract109 and measurement of airway inflammatory biomarkers, successfully shown to reflect sputum eosinophilia in a recent small study in asthma.110 As a result, the powerful use of new technologies that convert ongoing advances of biomedical research into user-friendly tools that supplements and enhances current clinical tools can facilitate inter-professional communication between basic scientists, medical researchers and clinicians.111
Point-of-care testing will also allow for fast and on-site assessment of multiple biomarkers.112 In addition to biosensors and wearable monitoring devices, technology that continuously measures analytes in body fluids are currently being developed that could give real-time data on the measurement of specific molecules or biomarkers.113 These point-of-care tests empower clinicians and allow patients to participate in the clinical decision-making process; an enabler for patient–clinician dialogue regarding treatment and management options.
Conclusion
Barriers to severe asthma assessment are influenced by multiple factors and can be grouped according to HCP-, patient- and systems-related factors. We have identified the barriers to assessing severe asthma and presented strategies to overcome these barriers. The highlighted barriers relate to inconsistent approaches to diagnosis and assessment, under referral, gaps in communication, poor perception on asthma control and organizational delimitations. Facilitators to overcome barriers to severe asthma assessment are standardized approaches and referrals, use of assessment tools and guidelines, implementation of a patient-centered care approach and better resources. Important opportunities of utilizing multidimensional assessment as an approach for implementation of care needs to be pursued where possible, to help overcome barriers in the assessment of severe asthma. Multidimensional assessment requires systematic assessment across three key domains (pulmonary/airway, extra-pulmonary/comorbidity and risk factor/behavioral domains) and can help identify important and clinically relevant traits, and help guide treatment decisions. Recognizing that multidimensional assessment can be time consuming and requires specialist teams, we propose that the benefits of this approach outweigh these barriers. This review highlights the need for further research into determining HCPs’ views of a feasible and acceptable approach to implement effective severe asthma management and generating composite panels of biomarkers from various non-invasive resources. These barriers are worthy of our attention if we desire a precision assessment in severe asthma.
Abbreviations
AHR, airway hyper-responsiveness; ICS, inhaled corticosteroids; GINA, Global Initiative for Asthma; LABA, long-acting beta agonist; IgE, immunoglobulin-E; FeNO, exhaled fraction of nitric oxide; ACQ, Asthma Control Questionnaire; ACT, asthma control test; FEV1, forced expiratory volume in one second; FVA, forced vital capacity; RCT, randomized controlled trial; HCP, health care professional; MDT, multidisciplinary team; GP, general practitioner; PCC, patient-centered care; HRQoL, health-related quality of life; PROMs, patient-reported outcome measures.
Disclosure
Dr Vanessa L Clark reports personal fees from Astra Zeneca and grants from National Health and Medical Research Council, outside the submitted work. Professor Peter G Gibson reports grants and personal fees from AstraZeneca, GlaxoSmithKline, Sanofi and Novartis, outside the submitted work. Professor Vanessa McDonald reports grants and personal fees from AstraZeneca, GSK and personal fees from Menarini, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, [Updated 2018]. Available from: https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed December 2, 2018.
2. McDonald VM, Hiles SA, Jones KA, Clark VL, Yorke J. Health-related quality of life burden in severe asthma. Med J Aust. 2018;209(2 Suppl):S28–S33.
3. Cordova-Rivera L, Gibson PG, Gardiner PA, Powell H, McDonald VM. Physical activity and exercise capacity in severe asthma: key clinical associations. J Allergy Clin Immunol Pract. 2018;6(3):814–822. doi:10.1016/j.jaip.2017.09.022
4. Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J. 2017;50:3. doi:10.1183/13993003.00711-2017
5. Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46(3):622–639. doi:10.1183/13993003.00853-2015
6. Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042
7. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
8. Hiles SA, Harvey ES, McDonald VM, et al. Working while unwell: workplace impairment in people with severe asthma. Clin Exp Allergy. 2018;48(6):650–662. doi:10.1111/cea.13153
9. Wark PA, Hew M, Maltby S, McDonald VM, Gibson PG. Diagnosis and investigation in the severe asthma clinic. Expert Rev Respir Med. 2016;10(5):491–503. doi:10.1586/17476348.2016.1165096
10. McDonald VM, Maltby S, Reddel HK, et al. Severe asthma: current management, targeted therapies and future directions-A roundtable report. Respirology. 2017;22(1):53–60. doi:10.1111/resp.12957
11. National AE. Prevention P. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94. doi:10.1016/j.jaci.2007.09.029
12. Upham JW, Chung LP. Optimising treatment for severe asthma. Med J Aust. 2018;209(S2):S22–S27.
13. Chung LP, Hew M, Bardin P, McDonald VM, Upham JW. Managing patients with severe asthma in Australia: current challenges with the existing models of care. Intern Med J. 2018;48(12):1536–1541. doi:10.1111/imj.14103
14. Carr TF, Bleecker E. Asthma heterogeneity and severity. World Allergy Organ J. 2016;9(1):41. doi:10.1186/s40413-016-0118-z
15. Papaioannou AI, Kostikas K, Zervas E, et al. Control of asthma in real life: still a valuable goal? Eur Respir Rev. 2015;24(136):361. doi:10.1183/16000617.00002215
16. Gibson P, McDonald VM. Management of severe asthma: targeting the airways, comorbidities and risk factors. Intern Med J. 2017;47(6):623–631. doi:10.1111/imj.13441
17. Tay TR, Lee JW-Y, Hew M. Diagnosis of severe asthma. Med J Aust. 2018;209(2 Suppl):S3–S10.
18. Moore VC. Spirometry: step by step. Breathe. 2012;8(3):232. doi:10.1183/20734735.0021711
19. Ayuk AC, Uwaezuoke SN, Ndukwu CI, et al. Spirometry in asthma care: a review of the trends and challenges in pediatric practice. Clin Med Insights Pediatr. 2017;11:1179556517720675. doi:10.1177/1179556517720675
20. Brannan JD, Lougheed MD. Airway hyperresponsiveness in asthma: mechanisms, clinical significance, and treatment. Front Physiol. 2012;3:460. doi:10.3389/fphys.2012.00460
21. Sanguinetti CM. When to perform a bronchial challenge with mannitol? Multidiscip Respir Med. 2011;6(2):76–78. doi:10.1186/2049-6958-6-2-76
22. Taylor DR, Pijnenburg MW, Smith AD, De Jongste JC. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61(9):817–827. doi:10.1136/thx.2005.056093
23. Pijnenburg MW. The role of FeNO in predicting asthma. Front Pediatr. 2019;7:41. doi:10.3389/fped.2019.00041
24. Ruppel GL. What is the clinical value of lung volumes? Respir Care. 2012;57(1):26–38. doi:10.4187/respcare.01374
25. Ritz T, Dahme B, Dubois AB, et al. Guidelines for mechanical lung function measurements in psychophysiology. Psychophysiology. 2002;39(5):546–567.
26. Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. doi:10.1183/13993003.00016-2016
27. Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am. 2012;96(4):745–752. doi:10.1016/j.mcna.2012.04.011
28. McDonald VM, Fingleton J, Agusti A. et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: treatable traits down under international workshop report. Eur Respir J. 2019;1802058. doi:10.1183/13993003.02058-2018
29. Fricker M, Heaney LG, Upham JW. Can biomarkers help us hit targets in difficult-to-treat asthma? Respirology. 2017;22(3):430–442. doi:10.1111/resp.13014
30. Centre of Excellence in Severe Asthma. The severe asthma toolkit. Available from: https://toolkit.severeasthma.org.au/.
31. McDonald VM, Vertigan AE, Gibson PG. How to set up a severe asthma service. Respirology. 2011;16(6):900–911. doi:10.1111/j.1440-1843.2011.02012.x
32. Chung LP, Johnson P, Summers Q. Models of care for severe asthma: the role of primary care. Med J Aust. 2018;209(2 Suppl):S34–S40.
33. McDonald VM, Yorke J. Adherence in severe asthma: time to get it right. Eur Respir J. 2017;50:6. doi:10.1183/13993003.00711-2017
34. Chuenjit W, Engchuan V, Yuenyongviwat A, Sangsupawanich P. Achieving good adherence to inhaled corticosteroids after weighing canisters of asthmatic children. F1000Res. 2017;6:266. doi:10.12688/f1000research.10710.1
35. McNicholl DM, Stevenson M, McGarvey LP, Heaney LG. The utility of fractional exhaled nitric oxide suppression in the identification of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2012;186(11):1102–1108. doi:10.1164/rccm.201204-0587OC
36. Lindsay JT, Heaney LG. Nonadherence in difficult asthma - facts, myths, and a time to act. Patient Prefer Adherence. 2013;7:329–336. doi:10.2147/PPA.S38208
37. Taylor TE, Zigel Y, Egan C, et al. Objective assessment of patient inhaler user technique using an audio-based classification approach. Sci Rep. 2018;8(1):2164. doi:10.1038/s41598-018-20523-w
38. Lavorini F, Fontana GA, Usmani OS. New inhaler devices-the good, the bad and the ugly. Respiration. 2014;88(1):3–15. doi:10.1159/000363390
39. Faruqi S, Thompson J, Robinson T, et al. Fractional exhaled nitric oxide (FeNO) suppression with directly observed inhaled corticosteroid therapy: does it make a difference to patient outcomes? Eur Respir J. 2018;52(suppl62):PA4453. doi:10.1183/13993003.01675-2018
40. Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180(9):817–822. doi:10.1164/rccm.200902-0166OC
41. Murphy AC, Proeschal A, Brightling CE, et al. The relationship between clinical outcomes and medication adherence in difficult-to-control asthma. Thorax. 2012;67(8):751–753. doi:10.1136/thoraxjnl-2011-201096
42. Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010;376(9743):803–813. doi:10.1016/S0140-6736(10)61087-2
43. Clark VL, Gibson PG, Genn G, et al. Multidimensional assessment of severe asthma: a systematic review and meta-analysis. Respirology. 2017;22(7):1262–1275. doi:10.1111/resp.13134
44. Gibson P, McDonald V. Phenotyping asthma and COPD. BRN Rev. 2016;2:239–252.
45. McDonald V, Clark V, Wark P, Baines K, Gibson P. Multidimensional assessment and targeted therapy of severe asthma: a randomised controlled trial (RCT). Eur Respir J. 2017;50(suppl 61):OA1482. doi:10.1183/13993003.00711-2017
46. Hudon C, Chouinard M-C, Lambert M, Dufour I, Krieg C. Effectiveness of case management interventions for frequent users of healthcare services: a scoping review. BMJ Open. 2016;6(9):e012353. doi:10.1136/bmjopen-2016-012353
47. McDonald VM, Higgins I, Wood LG, Gibson PG. Multidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense? Thorax. 2013;68(7):691–694. doi:10.1136/thoraxjnl-2012-202646
48. Orozco-Beltrán D, Carratalá-Munuera C, Arriero JM, et al. Management and referral of patients with severe and poorly controlled asthma in primary care. Fam Pract. 2016;33(6):678–683. doi:10.1093/fampra/cmw081
49. Braido F, Baiardini I, Stagi E, et al. Unsatisfactory asthma control: astonishing evidence from general practitioners and respiratory medicine specialists. J Investig Allergol Clin Immunol. 2010;20(1):9–12.
50. Cousins JL, Wark PA, McDonald VM. Acute oxygen therapy: a review of prescribing and delivery practices. Int J Chron Obstruct Pulmon Dis. 2016;11:1067–1075. doi:10.2147/COPD.S103607
51. Morrow S, Daines L, Wiener-Ogilvie S, et al. Exploring the perspectives of clinical professionals and support staff on implementing supported self-management for asthma in UK general practice: an IMP(2)ART qualitative study. NPJ Prim Care Respir Med. 2017;27(1):45. doi:10.1038/s41533-017-0041-y
52. Price D, Bjermer L, Bergin DA, Martinez R. Asthma referrals: a key component of asthma management that needs to be addressed. J Asthma Allergy. 2017;10:209–223. doi:10.2147/JAA.S134300
53. Baiardini I, Braido F, Bonini M, Compalati E, Canonica GW. Why do doctors and patients not follow guidelines? Curr Opin Allergy Clin Immunol. 2009;9(3):228–233. doi:10.1097/ACI.0b013e32832b4651
54. Lugtenberg M, Zegers-van Schaick JM, Westert GP, Burgers JS. Why don’t physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54. doi:10.1186/1748-5908-4-54
55. Lenney W, Bush A, Fitzgerald DA, et al. Improving the global diagnosis and management of asthma in children. Thorax. 2018;73(7):662. doi:10.1136/thoraxjnl-2018-211626
56. Moffat M, Cleland J, van der Molen T, Price D. Poor communication may impair optimal asthma care: a qualitative study. Fam Pract. 2007;24(1):65–70. doi:10.1093/fampra/cml062
57. Dombkowski KJ, Hassan F, Wasilevich EA, Clark SJ. Spirometry use among pediatric primary care physicians. Pediatrics. 2010;126(4):682–687. doi:10.1542/peds.2010-0362
58. Mazrou SHA. Expected benefits of clinical practice guidelines: factors affecting their adherence and methods of implementation and dissemination. JHS. 2013;1(3):141.
59. McDonald VM, Gibson PG. The severe asthma toolkit: a new online resource for clinicians. Respir Med Today. 2018;3(1):26–27.
60. McDonald V, Kennington E, Hyland M. Understanding the experience of people living with severe asthma. In: Chung KF, Israel E, Gibson P, editors. Severe Asthma (ERS Monograph). Sheffield: European Respiratory Society; 2019:16–29.
61. Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. 2018;198(8):1012–1020. doi:10.1164/rccm.201804-0682CI
62. Menzies-Gow A, Canonica GW, Winders TA, et al. A charter to improve patient care in severe asthma. Adv Ther. 2018;35(10):1485–1496. doi:10.1007/s12325-018-0777-y
63. Ryan D, Murphy A, Stallberg B, Baxter N, Heaney LG. ‘SIMPLES’: a structured primary care approach to adults with difficult asthma. Prim Care Respir J. 2013;22(3):365–373. doi:10.4104/pcrj.2013.00075
64. Laforest L, Van Ganse E, Devouassoux G, et al. Management of asthma in patients supervised by primary care physicians or by specialists. Eur Respir J. 2006;27(1):42–50. doi:10.1183/09031936.06.00035805
65. Dennis SM, Zwar NA, Marks GB. Diagnosing asthma in adults in primary care: a qualitative study of Australian GPs’ experiences. Prim Care Respir J. 2010;19(1):52–56. doi:10.4104/pcrj.2009.00046
66. Young HN, Len-Rios ME, Brown R, Moreno MM, Cox E. How does patient-provider communication influence adherence to asthma medications? Patient Educ Couns. 2017;100(4):696–702. doi:10.1016/j.pec.2016.11.022
67. Crespo-Lessmann A, Plaza V, González-Barcala F-J, Fernández-Sánchez T, Sastre J. Concordance of opinions between patients and physicians and their relationship with symptomatic control and future risk in patients with moderate–severe asthma. BMJ Open Respir Res. 2017;4(1):e000189. doi:10.1136/bmjresp-2017-000189
68. Eassey D, Reddel HK, Foster JM, et al. “…I've said I wish I was dead, you'd be better off without me”: A systematic review of people's experiences of living with severe asthma. J Asthma. 2019;56(3):311-322.
69. Moffat M, Cleland J, van der Molen T, Price D. Sub-optimal patient and physician communication in primary care consultations: its relation to severe and difficult asthma. Prim Care Respir J. 2006;15(3):159–165. doi:10.1016/j.pcrj.2006.02.006
70. Taylor DR, Bateman ED, Boulet LP, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008;32(3):545–554. doi:10.1183/09031936.00155307
71. Bidad N, Barnes N, Griffiths C, Horne R. Understanding patients’ perceptions of asthma control: a qualitative study. Eur Respir J. 2018;51(6):1701346. doi:10.1183/13993003.01346-2017
72. Lingner H, Burger B, Kardos P, et al. What patients really think about asthma guidelines: barriers to guideline implementation from the patients’ perspective. BMC Pulm Med. 2017;17(1):13. doi:10.1186/s12890-016-0346-6
73. Fitzpatrick AM. Biomarkers of asthma and allergic airway diseases. Ann Allergy Asthma Immunol. 2015;115(5):335–340. doi:10.1016/j.anai.2015.09.003
74. Ward PR, Rokkas P, Cenko C, et al. ‘Waiting for’ and ‘waiting in’ public and private hospitals: a qualitative study of patient trust in South Australia. BMC Health Serv Res. 2017;17(1):333. doi:10.1186/s12913-017-2281-5
75. Normansell R, Welsh E. “Asthma can take over your life but having the right support makes that easier to deal with.” Informing research priorities by exploring the barriers and facilitators to asthma control: a qualitative analysis of survey data. Asthma Res Pract. 2015;1(1):11. doi:10.1186/s40733-015-0011-5
76. Fielden JM, Cumming JM, Horne JG, et al. Waiting for hip arthroplasty: economic costs and health outcomes. J Arthroplasty. 2005;20(8):990–997. doi:10.1016/j.arth.2004.12.060
77. Eguiluz-Gracia I, Tay TR, Hew M, et al. Recent developments and highlights in biomarkers in allergic diseases and asthma. Allergy. 2018;73(12):2290–2305. doi:10.1111/all.13628
78. Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62(12):1043–1049. doi:10.1136/thx.2006.073429
79. Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–1721. doi:10.1016/S0140-6736(02)11679-5
80. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–984. doi:10.1056/NEJMoa0808991
81. Pavlidis S, Takahashi K, Ng Kee Kwong F, et al. “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J. 2019;53:1. doi:10.1183/13993003.01184-2018
82. Zhang XY, Simpson JL, Powell H, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy. 2014;44(9):1137–1145. doi:10.1111/cea.12345
83. Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352(21):2163–2173. doi:10.1056/NEJMoa043596
84. Schleich FN, Seidel L, Sele J, et al. Exhaled nitric oxide thresholds associated with a sputum eosinophil count ≥3% in a cohort of unselected patients with asthma. Thorax. 2010;65(12):1039–1044. doi:10.1136/thx.2009.124925
85. Jia G, Erickson RW, Choy DF, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130(3):647-654.e10.
86. Simpson JL, Yang IA, Upham JW, et al. Periostin levels and eosinophilic inflammation in poorly-controlled asthma. BMC Pulm Med. 2016;16(1):67. doi:10.1186/s12890-016-0276-3
87. Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FENO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. doi:10.1136/thoraxjnl-2014-205634
88. Medrek SK, Parulekar AD, Hanania NA. Predictive biomarkers for asthma therapy. Curr Allergy Asthma Rep. 2017;17(10):69. doi:10.1007/s11882-017-0739-5
89. Gibson PG. Variability of blood eosinophils as a biomarker in asthma and COPD. Respirology. 2018;23(1):12–13. doi:10.1111/resp.13200
90. Schleich FN, Manise M, Sele J, et al. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013;13:11. doi:10.1186/1471-2466-13-11
91. Hastie AT, Moore WC, Li H, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013;132(1):72–80. doi:10.1016/j.jaci.2013.03.044
92. Gibson PG. Inflammatory phenotypes in adult asthma: clinical applications. Clin Respir J. 2009;3(4):198–206. doi:10.1111/j.1752-699X.2009.00162.x
93. Wadsworth S, Sin D, Dorscheid D. Clinical update on the use of biomarkers of airway inflammation in the management of asthma. J Asthma Allergy. 2011;4:77–86. doi:10.2147/JAA.S15081
94. Korevaar DA, Westerhof GA, Wang J, et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(4):290–300. doi:10.1016/S2213-2600(15)00050-8
95. Trevor JL, Chipps BE. Severe asthma in primary care: identification and management. Am J Med. 2018;131(5):484–491. doi:10.1016/j.amjmed.2017.12.034
96. Mcdonald VM, Maltby S, Clark VL, et al. Development of the Severe Asthma Toolkit: A Clinical Website Resource for the Management of Severe Treatment-refractory Asthma. Respirology. 2018;23(S2):28-28.
97. Alexandrine JL, Sandra P, Roland G, et al. Facilitators and solutions for practicing optimal guided asthma self-management: the physician perspective. Can Respir J. 2013;20(4):285. doi:10.1155/2013/562104
98. Cabana MD, Slish KK, Evans D, et al. Impact of physician asthma care education on patient outcomes. Pediatrics. 2006;117(6):2149–2157. doi:10.1542/peds.2005-1055
99. Clark NM, Cabana MD, Nan B, et al. The clinician-patient partnership paradigm: outcomes associated with physician communication behavior. Clin Pediatr (Phila). 2008;47(1):49–57. doi:10.1177/0009922807305650
100. Wolf A, Moore L, Lydahl D, et al. The realities of partnership in person-centred care: a qualitative interview study with patients and professionals. BMJ Open. 2017;7(7):e016491. doi:10.1136/bmjopen-2017-016491
101. Apter AJ, Morales KH, Han X, et al. A patient advocate to facilitate access and improve communication, care, and outcomes in adults with moderate or severe asthma: rationale, design, and methods of a randomized controlled trial. Contemp Clin Trials. 2017;56:34–45. doi:10.1016/j.cct.2017.03.004
102. Kane B, Cramb S, Hudson V, et al. Specialised commissioning for severe asthma: oxymoron or opportunity? Thorax. 2016;71(2):196–198. doi:10.1136/thoraxjnl-2015-207380
103. Loscalzo J, Kohane I, Barabasi AL. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. doi:10.1038/msb4100163
104. Ritchie MD, de Andrade M, Kuivaniemi H. The foundation of precision medicine: integration of electronic health records with genomics through basic, clinical, and translational research. Front Genet. 2015;6:104. doi:10.3389/fgene.2015.00104
105. Verrills NM, Irwin JA, He XY, et al. Identification of novel diagnostic biomarkers for asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(12):1633–1643. doi:10.1164/rccm.201010-1623OC
106. Baines KJ, Simpson JL, Wood LG, et al. Sputum gene expression signature of 6 biomarkers discriminates asthma inflammatory phenotypes. J Allergy Clin Immunol. 2014;133(4):997–1007. doi:10.1016/j.jaci.2013.12.1091
107. Kim S, Oesterreich S, Kim S, Park Y, Tseng GC. Integrative clustering of multi-level omics data for disease subtype discovery using sequential double regularization. Biostatistics. 2017;18(1):165–179. doi:10.1093/biostatistics/kxw039
108. Dallinga JW, Robroeks CM, van Berkel JJ, et al. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin Exp Allergy. 2010;40(1):68–76. doi:10.1111/j.1365-2222.2009.03343.x
109. Thwaites RS, Jarvis HC, Singh N, et al. Absorption of nasal and bronchial fluids: precision sampling of the human respiratory mucosa and laboratory processing of samples. J Vis Exp. 2018(131):e56413.
110. Melo JT
111. Restifo LL, Phelan GR. The cultural divide: exploring communication barriers between scientists and clinicians. Dis Model Mech. 2011;4(4):423–426. doi:10.1242/dmm.008177
112. St John A. The evidence to support point-of-care testing. Clin Biochem Rev. 2010;31(3):111–119.
113. Ferguson BS, Hoggarth DA, Maliniak D, et al. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci Transl Med. 2013;5(213):213ra165–213ra165. doi:10.1126/scitranslmed.3007095
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
