Back to Journals » Journal of Hepatocellular Carcinoma » Volume 7
Appraisal of Long-Term Outcomes of Liver-Directed Concurrent Chemoradiotherapy for Hepatocellular Carcinoma with Major Portal Vein Invasion
Authors Han S, Lee HW, Park JY, Kim SU , Kim DY , Ahn SH, Han KH, Seong J , Won JY , Han DH, Kim BK
Received 16 September 2020
Accepted for publication 3 December 2020
Published 17 December 2020 Volume 2020:7 Pages 403—412
DOI https://doi.org/10.2147/JHC.S276528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ahmed Kaseb
Sojung Han,1,2 Hye Won Lee,1– 3 Jun Yong Park,1– 3 Seung Up Kim,1– 3 Do Young Kim,1– 3 Sang Hoon Ahn,1– 3 Kwang-Hyub Han,1 Jinsil Seong,4 Jong Yun Won,5 Dai Hoon Han,2,6,7,* Beom Kyung Kim1– 3,*
1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea; 2Yonsei Liver Center, Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea; 3Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Republic of Korea; 4Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, Republic of Korea; 5Department of Radiology, Yonsei University College of Medicine, Seoul, Republic of Korea; 6Liver Cancer Center, Yonsei Cancer Center, Yonsei University Health System, Seoul, Republic of Korea; 7Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Yonsei University College of Medicine, Seoul, Republic of Korea
*These authors contributed equally to this work.
Correspondence: Dai Hoon Han
Department of Surgery, Yonsei University College of Medicine, 50-1 Yonsei-Ro, Seodaemun–Gu, Seoul 03722, Republic of Korea
Tel +82-2-2228-2139
Fax +82-2-313-8289
Email [email protected]
Beom Kyung Kim
Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun–gu, Seoul 03722, Republic of Korea
Tel +82-2-2228-1930
Fax +82-2-393-6884
Email [email protected]
Backgrounds and Aims: Molecular-targeted agents are acceptable standards to treat advanced-stage hepatocellular carcinoma (HCC), however, their therapeutic benefit, ie, sorafenib, was significantly offset in case of major vessel invasion. Liver-directed concurrent chemo-radiotherapy (LD-CCRT) provided favorable outcomes in terms of survivals and tumor shrinkage, so, we appraised its long-term therapeutic efficacy.
Patients and Methods: Advanced HCC patients with portal vein invasion (main trunk or the 1st order branch) were enrolled. During a 5-week radiotherapy course, concurrent hepatic arterial infusion chemotherapy (HAIC) with 5-fluorouracil and leucovorin was administered through an implanted port on the first and last 5 days. Four weeks after LD-CCRT, a maintenance HAIC using 5-fluorouracil and cisplatin was administered every 4 weeks.
Results: Among 152 patients, the objective response rates as the best response by modified Response Evaluation Criteria In Solid Tumors were 48.0% after LD-CCRT and 55.3% during subsequent HAIC maintenance. After LD-CCRT, biological responses in alpha-fetoprotein and protein induced by the absence of vitamin K or antagonist-II levels were achieved in 46.2% and 52.6%, respectively. Sixteen patients (10.5%) underwent curative resection or liver transplantation after down-staging. Median overall survival and progression-free survival were 13.5 and 6.9 months, respectively.
Conclusion: LD-CCRT followed by maintenance HAIC yielded favorable survival outcomes in advanced HCC patients with major portal vein invasion. Through initial tumor reduction, LD-CCRT induced down-staging with subsequent curative treatment feasible in 10.5% of patients, resulting in long-term survival. Further prospective trials are warranted to confirm these results.
Keywords: hepatocellular carcinoma, portal vein invasion, concurrent chemoradiotherapy, prognosis, response
Plain Language Summary
-Treatment options other than molecular-targeted agents are still limited for advanced stage-hepatocellular carcinoma (HCC) with major portal vein invasion.
-We assessed their survival outcomes undergoing liver-directed concurrent chemoradiotherapy (LD-CCRT) followed by maintenance hepatic arterial infusion chemotherapy (HAIC).
-Among 152 patients, median overall survival and progression-free survival were 13.5 and 6.9 months, respectively. The objective response rates as the best response by modified Response Evaluation Criteria in Solid Tumors were 48.0% after LD-CCRT and 55.3% during the planned treatment course. After LD-CCRT, biological responses in alpha-fetoprotein and protein induced by the absence of vitamin K or antagonist-II levels were achieved in 46.2% and 52.6%, respectively. Sixteen patients (10.5%) underwent curative resection or liver transplantation after down-staging.
- LD-CCRT followed by maintenance HAIC yielded favorable survival outcomes in advanced stage-HCC patients with major portal vein invasion. Through initial tumor reduction, LD-CCRT induced down-staging with subsequent curative treatment feasible in 10.5% of patients. Further prospective trials are warranted to confirm these results.
Introduction
Approximately 10–40% of hepatocellular carcinoma (HCC) patients are diagnosed at an advanced stage with major portal vein (PV) tumor invasion. The expected prognosis in such cases is typically poor, despite the administration of optimal systemic therapy according to the best practice guidelines.1–3 Sorafenib is the first approved oral multi-kinase inhibitor for prolonging the overall survival (OS) of patients with advanced-stage HCC. Along with lenvatinib, it is currently regarded as the standard of care.1,3,4 However, data supporting the survival benefits from molecular-targeted agents among patients with advanced-stage HCC with major PV invasion are still limited. For example, among patients treated with sorafenib,5 the OS markedly decreased from 14.3 to 5.7 months in case of extrahepatic spread and/or macrovascular invasion.6–8 Furthermore, although the median OS reached approximately 13 months in the Phase III trial comparing lenvatinib and sorafenib as the first-line treatment for advanced-stage HCC, patients with major PV invasion at baseline were primarily excluded in that phase III trial. Thus, there is a pressing need for more effective treatment strategies in these patients. Recently, we could observe many literatures indicating that alternative strategies including loco-regional treatment (LRT) could improve survival outcomes compared to molecular-targeted agents alone.1,9,10
Among various LRTs, external beam radiation therapy (EBRT) has become widely popular primarily due to technological advances, including the introduction of 3-dimensional conformal radiation therapy (3D-CRT) and more recently, intensity-modulated radiotherapy (IMRT).11,12 These technologies allow the delivery of high tumoricidal radiation doses with minimal risk of damage to adjacent non-tumorous tissues. As a result, a high-dose EBRT for advanced HCC can lead to a long-lasting local tumor control as well as a higher probability of down-staging which allows curative resection or OLT with improved survival, in comparison with historical controls.12–14 According to several observational studies, liver-directed concurrent chemoradiotherapy (LD-CCRT) for advanced-stage HCC was associated with the favorable clinical outcomes.15–17 In particular, Kim et al16 reported that the objective response rates (ORR) during the LD-CCRT-based treatment course was up to 53.2% and that 19.1% underwent curative resection or transplantation after down-staging, in contrast to only a minimal tumor shrinkage effect by sorafenib, ie at least about 2 ~5%.18,19 Another studies also supported anti-tumor effect by an intra-arterial infusion of 5-fluorouracil with or without EBRT.20–25
Based on the available evidence that effective initial tumor shrinkage by LD-CCRT may facilitate subsequent treatments with curative intent among advanced-stage HCC patients with major PV invasion, we aimed to assess clinical outcomes of LD-CCRT, in terms of not only survival outcomes but ORR and the proportion of conversion to curative treatment, of LD-CCRT.
Patients and Methods
Participants and Treatments
Patients who underwent LD-CCRT for advanced-stage HCC between 2011 and 2016 at Severance Hospital, Seoul, Republic of Korea, were retrospectively analyzed. HCC was diagnosed by histological or radiological evaluation with reference to the international guidelines.1,3,26,27 The eligibility criteria for LD-CCRT are described in Table 1. For example, LD-CCRT was not performed for patients with diffuse or multifocal bi-lobal tumors which cannot be included in a technically feasible RT field from the viewpoint of safety based upon liver function and radiation dosage to organs. In addition, the exclusion criteria were also described in Table 1.
 |
Table 1 Patients’ Enrollment |
LD-CCRT and subsequent maintenance HAIC were performed according to the previous studies by Kim et al16 and Park et al28 respectively. Provided that patients have intrahepatic lesions eligible for transarterial chemo-embolization (TACE),29–31 they could be included in the study at physicians’ discretion. The overall process of the treatment delivery was described in the Supplementary Table 1.
This study protocol was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the institutional review board (IRB) of Severance Hospital. The informed consent was waived because of the retrospective nature of the study. All patient identifiers were removed once the data from the patient medical record is collected.
Follow-Up
Treatment responses were assessed approximately 1 month after the completion of LD-CCRT and then every 8 ~ 12 weeks, using modified Response Evaluation Criteria in Solid Tumors (mRECIST).32 During follow up, patients who successfully achieved down-staging underwent curative surgical resection or orthotropic liver transplantation (OLT) under a multi-disciplinary approach.33 Such approaches were considered, when complete response (CR) or partial response (PR) by mRECIST32 was achieved and serum alpha-fetoprotein (AFP) and protein induced by the absence of vitamin K or antagonist-II (PIVKA-II) decreased to <20 ng/mL and <40 mAU/mL, respectively, through the treatment.15,34 Tumor resectability was determined in a multi-disciplinary approach through reviewing CT scans before and during the treatment.35 All gross lesions should be resected with a clear margin based on radiological image. The resection type was determined based on functional reserve of the liver (FRL) and patient’s performance status. For major liver resection defined as the resection of ≥3 anatomical segments, at least 40% of the total liver volume should be required as a future FRL. OLT was considered for patient with deteriorating liver function; those with pre-operative total bilirubin ≥2mg/dL or platelet counts <100,000/μL, or with future remnant liver volume/total liver volume <30% were considered for OLT.
The primary endpoint was the OS, and the secondary endpoints were progression-free survival (PFS), ORR (defined as the rate of CR or PR), and the proportion of patients undergoing curative surgical resection or OLT after down-staging. Patients were evaluated for any treatment-related adverse event throughout the planned treatment period. Adverse events were noted as per the standards and terminology set by the Cancer Therapy Evaluation Program’s Common Terminology Criteria for Adverse Events version 4.03.
Statistical Analysis
Data are expressed as mean ± standard deviation, median (interquartile range [IQR]) or number (%). Differences among categorical variables were analyzed for statistical significance with chi-square test (or Fisher’s exact test, if appropriate), respectively. Survival outcome was estimated using Kaplan–Meier analysis with a comparison by Log rank test. Hazard ratios (HR) were calculated using Cox regression analysis. OS was calculated as the time interval from the date of treatment initiation to the date of death, whereas PFS was calculated as the time interval from the date of treatment initiation to the date of progression, or any kind of death in the absence of confirmed progression. The Statistical analyses were performed using IBM® SPSS® Statistics Version 25.0 (IBM Corporation in Armonk, NY, US). All statistical tests with p-value of <0.05 were considered as significant.
Results
Patient Characteristics
In total, 152 patients with major PV invasion who underwent LD-CCRT were analyzed (Supplementary Figure 1). Baseline characteristics are summarized in Table 2. The median age was 56 years (IQR 50–63 years), and the patients were predominantly male (90.1%). Chronic hepatitis B virus (HBV) infection was the most common etiology (84.9%), and 127 (83.6%) patients had cirrhosis at enrollment. The majority of patients (128 [84.2%]) belonged to Child-Pugh A, but 15.8% had Child-Pugh B.
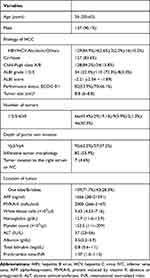 |
Table 2 Characteristics of the Study Patients (n=152) |
The median size of the largest HCC was 8.8 cm (IQR 6–8.8 cm), and 46 patients (30.3%) had ≥5 tumors. There were 57 (37.5%) patients with tumor invasion into Vp4 and 95 (62.5%) with tumor invasion into Vp3. Eighty-two (53.9%) patients had infiltrative tumor morphology.36–38 Only 7 (4.6%) patients had direct tumor invasion into the inferior vena cava (IVC) and/or right atrium. The median AFP and PIVKA-II levels were 1655.5 (IQR 38–21,591) ng/mL and 2000 (IQR 266–2167) mAU/mL, respectively.
Treatment Delivery and Responses
Four weeks after LD-CCRT, the ORR was 48.0%. Subsequently, patients underwent a median of 4 (IQR 2–6) cycles of HAIC. During the planned treatment courses, the ORR as their best responses increased to 55.3% (Table 3). Among a subgroup (n=56) undergoing TACE before the start of LD-CCRT, the ORRs 4 weeks after LD-CCRT and during the planned treatment courses were 55.4% and 62.5%, respectively. Radiological responses of malignant portal vein thrombosis as a non-measurable lesion by mRECIST32 4 weeks after LD-CCRT were as follows; CR (n=2, 1.3%), PR (n=52, 34.2%), stable disease (n=85, 55.9%), and progressive disease (n=13, 8.6%). During the planned treatment courses, they were 2.0% (n=3), 37.5% (n=57), 52.0% (n=79), and 8.6% (n=13), respectively.
 |
Table 3 Treatment Response |
Four weeks after LD-CCRT, 46.1% and 52.6% had favorable biological response (defined as a ≥ 50% decrease in tumor marker from the baseline) by AFP and PIVKA-II, respectively. During the planned treatment courses, they were 65.1% and 65.8%, respectively. In detail, from the baseline (median 1656 ng/mL, IQR [38–21,591]). The AFP level significantly decreased to 553 ng/mL (IQR 22 ~ 5282) 4 weeks after LD-CCRT and 50 (8 ~ 1793) during the planned treatment courses (both p<0.001 by Wilcoxon signed ranks test). Likewise, from the baseline (median 2000 mAU/mL, [IQR 266–2167]), the PIVKA-II level significantly decreased to 257 mAU/mL (IQR 41 ~ 2000) 4 weeks after LD-CCRT and 76 mAU/mL (29 ~ 1019) during the planned treatment courses (both p<0.001 by Wilcoxon signed ranks test). Detailed changes in tumor size and their correlations with tumor markers were described in waterfall plots (Supplementary Figure 2A, 2B, and 2C) and the association between radiological and biological responses were summarized in Supplementary Table 2 and Supplementary Figure 3.).
Notably, during the planned treatment courses, 16 patients (10.5%) underwent curative resection or OLT after successful down-staging. The median time from the initiation of LD-CCRT to the last follow-up among these 16 patients was 34.8 (IQR 21.1–60.9) months. Seven cases of death were observed between 7.2 and 61.6 months (median 22.0 months, IQR 15.0–59.0 months). Overall, there was no post-operative complication of > grade III by the Clavien-Dindo classification,39 except one post-OLT mortality. There was one intra-operative complication of weak pulsation after hepatic artery anastomosis during living donor OLT, for which the anastomosis of right hepatic artery of the graft to the common hepatic artery of the recipient was switched to transposition anastomosis with the left gastric artery of the recipient; this patient had no significant post-OLT complication. Two patients developed biliary strictures most likely 6 months after OLT, both of which were successfully treated with the endoscopic and/or percutaneous approaches. Among patients undergoing surgical resection, there was no significant post-operative complication; 5 patients developed the small amount of pleural effusion, all of which resolved spontaneously without any intervention.
Survival Outcomes and Prognosis Factors
Overall, a total of 107 patients died and the median OS was 13.5 months (95% confidence interval [CI] 11.003–16.077) (Figure 1). The median PFS was 6.9 months (95% CI 6.326–7.474) (Figure 2). Treatment modalities after progression of disease included continuing intra-arterial chemotherapy (6.1%), LRTs such as TACE or Transarterial chemoinfusion (TACI) (16.0%), and systemic chemotherapy including systemic 5-fluorouracil plus cisplatin chemotherapy (9.9%) and sorafenib (23.7%). Palliative radiotherapy was delivered to patients with bone metastases (1.5%). The majority of patients with disease progression (32.2%) received supportive care.
 |
Figure 1 Kaplan–Meier analysis of OS. |
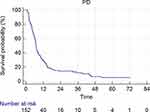 |
Figure 2 Kaplan–Meier analysis of PFS. |
Table 4 shows that only significant prognostic factor for OS was ALBI score (HR 1.523, 95% CI 1.032–2.246; p=0.034).
 |
Table 4 Prognostic Factors for Overall Survival |
Subgroup Analysis for Patients with Elevated AFP or PIVKA-II Levels at Baseline
Among 126 (82.9%) patients with baseline AFP > 20 ng/mL, 54.0% showed the favorable biological response 4 weeks after LD-CCRT. Such AFP responders were more likely to have the higher ORR 4 weeks after LD-CCRT (57.4% vs 34.5%, respectively; p=0.010 by chi-square test), and the longer OS (22.4 vs 10.8 months; p<0.001 by Log rank test) and PFS (9.0 vs 5.1 months; p=0.003 by Log rank test) than non-responders.
Likewise, among 137 (90.1%) patients with baseline PIVKA-II >40 mAU/mL, 58.4% showed the favorable biological response 4 weeks after LD-CCRT. Such PIKVA-II responders were more likely to have the higher ORR 4 weeks after LD-CCRT (65.0 vs 21.1%, respectively; p<0.001 by chi-square test) and the longer OS (20.3 vs 11.5 months, respectively; p=0.001 by Log rank test) and PFS (7.7 vs 5.7 months, respectively; p=0.016 by Log rank test) than non-responders.
Subgroup Analysis for Patients with HBV-Related HCC
Among 129 patients with HBV-related HCC, the ORRs 4 weeks after LD-CCRT and during the planned treatment courses were 49.6% and 55.8%, respectively. Likewise, 43.4% and 53.5% had favorable biological response by AFP and PIVKA-II, respectively, 4 weeks after LD-CCRT. They were 63.6% and 65.9%, respectively, during the planned treatment courses. The median OS and PFS were 14.1 (95% CI 11.358–16.902) and 7.2 (95% CI 6.553–7.847) months, respectively.
Treatment-Related Adverse Events
Treatment-related adverse events during the planned treatment schedules are summarized in Table 5. Three key events associated with RT for liver, ie ascites, hepatic encephalopathy, and melena were evaluated using the SOMA-LENT grading classification.40 Overall, deterioration in liver function (defined as a shift in Child-Pugh score by 2 or more points from the baseline) after LD-CCRT was observed in 46 patients (30.3%), while 38 patients (25.0%) demonstrated newly developed ascites. Among 129 patients with chronic HBV infection, three patients (2.3%) developed virologic breakthrough, all of whom were treated with appropriate antiviral therapy.41 After LD-CCRT, 32 patients (21.1%) developed radiation gastritis, while 16 patients (10.5%) experienced upper gastrointestinal bleeding, which was successfully managed with endoscopic hemostasis and medical management. Among patients receiving maintenance HAIC after LD-CCRT, HAIC was discontinued in one patient due to HCC rupture, in one patient due to hepatic encephalopathy, and in two patients due to sepsis.
 |
Table 5 Treatment-Related Adverse Events |
Discussion
HCC with major portal vein invasion has poor prognosis and the median OS among such patients is expected to range from 7 to 8.5 months according to the real-life experience in the Republic of Korea.2,8 An effective systemic therapy is an important option for the treatment of HCC in the subset of patients with extrahepatic metastasis. However, considering that HCC is a rapidly growing locally aggressive disease frequently leading to the patients’ death before extrahepatic metastasis have developed, there has been various attempts to locally control the disease with LRTs, so far. For instance, the previous study showed that locally advanced HCC patients treated with LD-CCRT showed the higher ORRs at post-treatment 1 (46.8% VS 16.1%, respectively, P<0.001) and 3 (39.3% vs 21.4%, P=0.04) months than those treated with selective internal radiation therapy (SIRT), however, long-term response rates and survival rates were comparable.42 Two recent comparative trials comparing SIRT vs sorafenib in locally advanced HCC patients, ie SARAH and SIRveNIB, showed comparable median OS between the two treatment groups, but the response rate was higher in the SIRT as compared to sorafenib group, suggesting the potential of SIRT as more likelihood to control the tumor within the liver.18,43
In this study, we present locally advanced-stage HCC patients with major PV invasion receiving LD-CCRT with HAIC maintenance. Out of the total study population, 143 patients (94.0%) had poor oncological prognostic factors, including massive tumor (≥10 cm), infiltrative tumor morphology, tumor invasion into the main portal trunk (Vp4), tumor invasion into the IVC and/or right atrium, AFP level ≥400 ng/mL, or PIVKA-II level ≥1000 mAU/mL. In this population, LD-CCRT followed by maintenance HAIC demonstrated encouraging results, not only producing an objective response in 55.3% of subjects but also improving the median OS to 13.5 months. The median OS of 13.5 months in our study population with Vp3 or Vp4 invasion is notable given that patients with major PV invasion at baseline were excluded in that phase III trial. Even though our study is only a one-arm study with a retrospective design, our findings could at least reappraise the necessity of future prospective randomized clinical trials comparing the efficacy between active LRT and systemic chemotherapy in patients with advanced HCC with major PV invasion. Despite major portal vein invasion, LD-CCRT showed potential to convert unresectable HCC to resectable HCC. During the planned treatment courses, 10.5% of patients achieved successful down-staging from LD-CCRT and underwent curative resection or transplantation. The optimal treatment strategy for successful down-staging remains to be determined. Despite several positive results, repeated TACE alone is considered to be contraindicated in this population due to the potential of hepatic insufficiency resulting from ischemic insult.44–47 HAIC may be an eligible LRT option; however, the evidence for its efficacy remains weak.25 EBRT is also complicated by issues of systemic or local failure outside the RT field, notwithstanding the excellent intra-RT field disease control rate. Our findings indicate that EBRT-based LRT may be a useful strategy for down-staging of advanced-stage HCC.48
Our treatment protocol for LD-CCRT has several advantages for the curative treatment for advanced-stage HCC. Most importantly, the FRL increased substantially after LD-CCRT,15 which is induced by a marked atrophy of the irradiated region and a compensatory enlargement of the non-irradiated region.49 Another advantage is that, optimal candidates for curative treatments could be selected through better assessment of the biological behaviors of advanced tumors during the period of so-called “neo-adjuvant” treatment. Finally, 5-fluorouracil could be useful as a radio-sensitizer for treating HCC as well as its anti-cancer effect;50–55 therefore, a synergistic effect against HCC might be expected. Although not all patients with advanced-stage HCC with major PV invasion would be eligible for EBRT-based active LRTs in real-world practice, the feasibility of EBRT-based active LRTs should be carefully assessed through a multi-disciplinary approach.
This study has several limitations. In the first place, it was a single-arm study with a retrospective design from the single institution and the higher proportion of male patients might be another problem, both of which can lead to selection bias. Therefore, additional multi-center, randomized controlled trials are required to prove the utility of LD-CCRT. However, our findings provided a rationale for further prospective clinical trials to assess the efficacy of alternative LRTs other than molecular-targeted agents alone as a recommended first-line modality in treating advanced-stage HCC with major PV invasion. Similarly, given that novel systemic agents such as atezolizumab plus bevacizumab will presently become available in the clinical setting,56 the potential benefits of induction LD-CCRT as a neo-adjuvant treatment should be studied further. Another limitation is that our results might not be generalizable to all HCC patients, since chronic HBV infection was the most predominant etiology among our study population.41,57,58 Furthermore, there was limited availability of second-line systemic agents in South Korea during the study period. Additional long-term follow-up to evaluate the entire clinical course of advanced-stage HCC is advisable.
Conclusion
In conclusion, LD-CCRT followed by maintenance HAIC demonstrated not only favorable outcomes through initial tumor size reduction but also acceptable tolerability in patients with advanced-stage HCC with major PV invasion. To confirm these results, further prospective clinical trials are required.
Disclosure
The authors declare no conflicts of interest.
References
1. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
2. Kim BK, Kim DY, Han KH, Seong J. Changes in real-life practice for hepatocellular carcinoma patients in the Republic of Korea over a 12-year period: a nationwide random sample study. PLoS One. 2019;14(10):e0223678. doi:10.1371/journal.pone.0223678
3. Galle PR, Forner A, Llovet JM, EASL Clinical Practice. Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
4. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
5. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
6. Lee JM, Han KH. Positioning and indication of sorafenib in the treatment algorithm and real practice setting: western and eastern approach–Asian perspective. Oncology. 2010;78(Suppl 1):167–171. doi:10.1159/000315246
7. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67(5):999–1008. doi:10.1016/j.jhep.2017.06.026
8. Kim DY, Kim HJ, Han KH, et al. Real-life experience of sorafenib treatment for hepatocellular carcinoma in Korea: from gideon data. Cancer Res Treat. 2016;48(4):1243–1252.
9. Liu PH, Huo TI, Miksad RA. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis. 2018;38(3):242–251. doi:10.1055/s-0038-1666805
10. Lee D, Lee HC, An J, et al. Comparison of surgical resection versus transarterial chemoembolization with additional radiation therapy in patients with hepatocellular carcinoma with portal vein invasion. Clin Mol Hepatol. 2018;24(2):144–150. doi:10.3350/cmh.2017.0041
11. Rim CH, Seong J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat Oncol J. 2016;34(3):160–167. doi:10.3857/roj.2016.01970
12. Byun HK, Kim HJ, Im YR, Kim DY, Han KH, Seong J. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol. 2019;133:1–8. doi:10.1016/j.radonc.2018.12.025
13. Feng M, Suresh K, Schipper MJ, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 2018;4(1):40–47. doi:10.1001/jamaoncol.2017.2303
14. Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67(1):92–99. doi:10.1016/j.jhep.2017.02.022
15. Lee HS, Choi GH, Choi JS, et al. Surgical resection after down-staging of locally advanced hepatocellular carcinoma by localized concurrent chemoradiotherapy. Ann Surg Oncol. 2014;21(11):3646–3653. doi:10.1245/s10434-014-3652-3
16. Kim BK, Kim DY, Byun HK, et al. Efficacy and safety of liver-directed concurrent chemoradiotherapy and sequential sorafenib for advanced hepatocellular carcinoma; a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2020.
17. Han KH, Seong J, Kim JK, Ahn SH, Lee DY, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008;113(5):995–1003. doi:10.1002/cncr.23684
18. Shim JH, Park JW, Choi JI, Park BJ, Kim CM. Practical efficacy of sorafenib monotherapy for advanced hepatocellular carcinoma patients in a hepatitis B virus-endemic area. J Cancer Res Clin Oncol. 2009;135(4):617–625. doi:10.1007/s00432-008-0496-x
19. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
20. Ishikawa T, Imai M, Kamimura H, et al. Improved survival for hepatocellular carcinoma with portal vein tumor thrombosis treated by intra-arterial chemotherapy combining etoposide, carboplatin, epirubicin and pharmacokinetic modulating chemotherapy by 5-FU and enteric-coated tegafur/uracil: a pilot study. World J Gastroenterol. 2007;13(41):5465–5470.
21. Choi JH, Chung WJ, Bae SH, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82(3):469–478. doi:10.1007/s00280-018-3638-0
22. Song MJ, Bae SH, Chun HJ, et al. A randomized study of cisplatin and 5-FU hepatic arterial infusion chemotherapy with or without adriamycin for advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2015;75(4):739–746. doi:10.1007/s00280-015-2692-0
23. Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi:10.1016/j.jhep.2018.02.008
24. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
25. Moriya K, Namisaki T, Sato S, et al. Bi-monthly hepatic arterial infusion chemotherapy as a novel strategy for advanced hepatocellular carcinoma in decompensated cirrhotic patients. Clin Mol Hepatol. 2019;25(4):381–389. doi:10.3350/cmh.2019.0037
26. Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019;25(3):245–263. doi:10.3350/cmh.2018.0090
27. Kim YY, Park MS, Aljoqiman KS, Choi JY, Kim MJ. Gadoxetic acid-enhanced magnetic resonance imaging: hepatocellular carcinoma and mimickers. Clin Mol Hepatol. 2019;25(3):223–233. doi:10.3350/cmh.2018.0107
28. Park JY, Ahn SH, Yoon YJ, et al. Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer. 2007;110(1):129–137. doi:10.1002/cncr.22759
29. Bannangkoon K, Hongsakul K, Tubtawee T, Piratvisuth T. Safety margin of embolized area can reduce local recurrence of hepatocellular carcinoma after superselective transarterial chemoembolization. Clin Mol Hepatol. 2019;25(1):74–85. doi:10.3350/cmh.2018.0072
30. Miyayama S. Ultraselective conventional transarterial chemoembolization: when and how? Clin Mol Hepatol. 2019;25(4):344–353. doi:10.3350/cmh.2019.0016
31. Park MS, Kim SU, Park JY, et al. Combination treatment of localized concurrent chemoradiation therapy and transarterial chemoembolization in locally advanced hepatocellular carcinoma with intrahepatic metastasis. Cancer Chemother Pharmacol. 2013;71(1):165–173. doi:10.1007/s00280-012-1993-9
32. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
33. Ahn KS, Kang KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: radiofrequency ablation vs. resection vs. transplantation? Clin Mol Hepatol. 2019;25(4):354–359. doi:10.3350/cmh.2018.0096
34. Chong JU, Choi GH, Han DH, et al. Downstaging with localized concurrent chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2018;25(11):3308–3315. doi:10.1245/s10434-018-6653-9
35. Han DH, Choi GH, Park JY, et al. Lesson from 610 liver resections of hepatocellular carcinoma in a single center over 10 years. World J Surg Oncol. 2014;12.
36. Burt AD, Alves V, Bedossa P, et al. Data set for the reporting of intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma and hepatocellular carcinoma: recommendations from the international collaboration on cancer reporting (ICCR). Histopathology. 2018;73(3):369–385. doi:10.1111/his.13520
37. Reynolds AR, Furlan A, Fetzer DT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics. 2015;35(2):371–386. doi:10.1148/rg.352140114
38. Demirjian A, Peng P, Geschwind JF, et al. Infiltrating hepatocellular carcinoma: seeing the tree through the forest. J Gastrointest Surg. 2011;15(11):2089–2097. doi:10.1007/s11605-011-1614-7
39. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
40. Routledge JA, Burns MP, Swindell R, Khoo VS, West CM, Davidson SE. Evaluation of the LENT-SOMA scales for the prospective assessment of treatment morbidity in cervical carcinoma. Int J Radiat Oncol Biol Phys. 2003;56(2):502–510. doi:10.1016/S0360-3016(02)04578-9
41. KASL. KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25(2):93–159. doi:10.3350/cmh.2019.1002
42. Song JE, Jung KS, Kim DY, et al. Transarterial radioembolization versus concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma: a propensity score matching analysis. Int J Radiat Oncol Biol Phys. 2017;99(2):396–406.
43. Gebski V, Gibbs E, Gandhi M, et al. VESPRO: an individual patient data prospective meta-analysis of selective internal radiation therapy versus sorafenib for advanced, locally advanced, or recurrent hepatocellular carcinoma of the SARAH and SIRveNIB trials. JMIR Res Protoc. 2017;6(2):e17. doi:10.2196/resprot.7016
44. Kim JH, Yoon HK, Kim SY, et al. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29(12):1291–1298. doi:10.1111/j.1365-2036.2009.04016.x
45. Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148(2):397–401. doi:10.1148/radiology.148.2.6306721
46. Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79(11):2087–2094. doi:10.1002/(SICI)1097-0142(19970601)79:11<2087::AID-CNCR5>3.0.CO;2-M
47. Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX. Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy. Liver Cancer. 2017;6(4):360–374. doi:10.1159/000481315
48. Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4(5):661–669. doi:10.1001/jamaoncol.2017.5847
49. Imada H, Kato H, Yasuda S, et al. Compensatory enlargement of the liver after treatment of hepatocellular carcinoma with carbon ion radiotherapy - relation to prognosis and liver function. Radiother Oncol. 2010;96(2):236–242. doi:10.1016/j.radonc.2010.03.025
50. Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23(34):8739–8747. doi:10.1200/JCO.2005.01.5354
51. Lee IJ, Seong J. Radiosensitizers in hepatocellular carcinoma. Semin Radiat Oncol. 2011;21(4):303–311.
52. McIntosh A, Hagspiel KD, Al-Osaimi AM, et al. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer. 2009;115(21):5117–5125. doi:10.1002/cncr.24552
53. Jang JW, Kay CS, You CR, et al. Simultaneous multitarget irradiation using helical tomotherapy for advanced hepatocellular carcinoma with multiple extrahepatic metastases. Int J Radiat Oncol Biol Phys. 2009;74(2):412–418. doi:10.1016/j.ijrobp.2008.08.034
54. Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a radiation therapy oncology group study. Int J Radiat Oncol Biol Phys. 1989;17(6):1223–1229. doi:10.1016/0360-3016(89)90530-0
55. Kim JS, Han KH, Lee DY, et al. Concurrent chemo-radiation therapy for advanced hepatocellular carcinoma with portal vein thrombosis. Taehan Kan Hakhoe Chi. 2002;8(1):71–79.
56. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
57. Kim BH, Park JW. Epidemiology of liver cancer in South Korea. Clin Mol Hepatol. 2018;24(1):1–9. doi:10.3350/cmh.2017.0112
58. Liang LY, Wong GL. Unmet need in chronic hepatitis B management. Clin Mol Hepatol. 2019;25(2):172–180. doi:10.3350/cmh.2018.0106
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
