Back to Journals » International Journal of Women's Health » Volume 14
Application of Transperineal Pelvic Floor Ultrasound in Changes of Pelvic Floor Structure and Function Between Pregnant and Non-Pregnant Women
Authors Xu Z, He H, Yu B, Jin H, Zhao Y, Zhou X, Huang H
Received 9 February 2022
Accepted for publication 3 August 2022
Published 24 August 2022 Volume 2022:14 Pages 1149—1159
DOI https://doi.org/10.2147/IJWH.S361755
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Zhihua Xu, Huiliao He, Beibei Yu, Huipei Jin, Yaping Zhao, Xiuping Zhou, Hu Huang
Department of Ultrasonic Diagnosis, The Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University, Zhejiang, People’s Republic of China
Correspondence: Hu Huang, Department of Ultrasonic Diagnosis, The Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University, No. 109 Xueyuan West Road, Lucheng District, Wenzhou City, Zhejiang Province, 325000, People’s Republic of China, Email [email protected]
Abstract:
Objective: To evaluate the changes of pelvic floor tissue structure and function between pregnant and non-pregnant women from the view of transperineal pelvic floor ultrasound.
Methods: Thirty-eight cases of women with a second singleton pregnancy and thirty-two cases of women with a first singleton pregnancy underwent transperineal pelvic floor ultrasound, and their results were compared with forty-two cases of healthy non-pregnant women.
Results: The differences of bladder neck descent (BND), rectal ampulla distance and levator hiatus area (LHA) among the three groups were statistically significant (P< 0.05), and the differences of BND, rectal ampulla distance, LHA between the women with a second singleton pregnancy group and non-pragnent group were statistically significant (P< 0.05). The BND, retrovesical angle at rest (RVA-R) and retrovesical angle underwent Valsalva maneuver (RVA-V) in the group of stress urinary incontinence (SUI) during pregnancy were larger than those in non-SUI group, with significant difference (P< 0.05), especially BND and RVA-V (P = 0.00).
Conclusion: Transperineal pelvic floor ultrasound has a high resolution of pelvic floor structure and function changes during pregnancy, and can dynamically evaluate pelvic floor function, providing a theoretical basis for early diagnosis and prevention of female pelvic floor dysfunction (FPFD) in subsequent pregnancies.
Keywords: pregnancy, pelvic floor dysfunction, ultrasound, transperineal
Introduction
Female pelvic floor dysfunction (FPFD) is a common gynecological disorder, and is the general term for a series of disorders caused by abnormal pelvic floor structure and function, mostly in middle-aged and elderly women. Its clinical manifestations include pelvic organ prolapse (POP), stress urinary incontinence (SUI), overactive bladder syndrome, and fecal incontinence. At present, it is believed that age, pregnancy, childbirth, perineal cleft injury, low estrogen level after menopause, long-term constipation, pelvic surgery and other causes of pelvic floor support structure relaxation injuries and defects are risk factors for FPFD,1–4 of which pregnancy and delivery are considered as the main factors, which are highly related to POP and SUI.5,6 Studies have estimated that 50% of parous women have different degrees of POP and SUI. These disorders are prevalent in adult women and their incidences increase with age.7,8 In 2010, 28 million women presented with FPFD in the United States, and it is believed that this number may increase to 43 million by 2050.9 In China, with the comprehensive liberalization of the three-child policy, there is an increasing number of women who choose to have a second or third pregnancy, thus we believe that FPFD incidence may also increase in China.
It is known that POP and SUI can severely affect people’s daily life. Handa’s study showed that obstetric levator avulsion is strongly associated with pelvic organ prolapse.10 Gonzalez’s research found that levator ani muscle avulsion acts as an independent risk factor for persistent postpartum voiding dysfunction.11 Most women with symptomatic POP and concomitant urinary incontinence (UI) find that their UI is either cured or improved after POP surgery alone.12 Surgical repair improved anal continence as well as sexual function in all patients.13 Therefore early diagnosis, prevention and intervention of pelvic floor disorders are important.
With the rapid development and expanding application of ultrasonic technology, transperineal pelvic floor ultrasound and three-dimensional (3D) transperineal pelvic floor ultrasound have become the main means of diagnosis of FPFD. Transperineal pelvic floor ultrasound can clearly and closely observe the bladder, urethra, vagina, uterus, rectum and anal canal. Furthermore, 3D transperineal pelvic floor ultrasound is a simple and accessible method, radiation-free, cost-effective, minimally invasive, and has the benefit of providing a real-time and dynamic appraisal of the pelvic floor from resting state to Valsalva maneuver and constrictive anal state.14–16
Most previous studies of the relationship between pregnancy and pelvic structure only took nulliparous women into consideration.17–19 Despite numerous existing studies on pelvic floor ultrasound imaging in women, there are relatively few studies on the use of ultrasound for assessing female pelvic floor function between a first pregnancy and a second pregnancy. Our study refined pregnant women into women with a first singleton pregnancy and women with a second singleton pregnancy, so as to reflect the effect of an increased number of pregnancies on pelvic floor function. It will help provide an actual assessment of Chinese female pelvic floor function during a second pregnancy. We hypothesized that there are differences in pelvic floor parameters between women with a first singleton pregnancy and women with a second singleton pregnancy.
Through dynamic observation of the pelvic floor structure and function state, the assessment of the three pelvic floor chambers can detect pelvic floor structural abnormalities early. This study aims to observe pelvic floor structure and function changes in the women with a first singleton pregnancy and women with a second singleton pregnancy through transperineal pelvic floor ultrasound, and to explore the correlation between pregnancy and FPFD, so as to provide a theoretical basis for early screening and prevention of FPFD.
Objects and Methods
Research Design
In this prospective study, we evaluated the changes of pelvic floor tissue structure and function in pregnant and non-pregnant women from the view of transperineal pelvic floor ultrasound. All the women were selected from our hospital from October 2018 to January 2020 in China. First, women with a first singleton pregnancy and women with a second singleton pregnancy (at second trimester and third trimester of pregnancy) who visited the outpatient clinic for maternity checkup were asked to participate in the pregnant group. At the same time, we recruited non-pregnant women who came to the gynecological clinic for routine check-ups for the control group at the same clinic (Figure 1). This study was approved by the local Research Ethics Committee (Ethics Reference No. LCKY2019-287), and all the pregnant women signed an informed consent.
 |
Figure 1 Flowchart of enrollment and grouping of the participants. |
Participants
The inclusion criteria were non-pregnant women, women with a first singleton pregnancy and women with a second singleton pregnancy (second or third trimester of pregnancy), age ≥ 20 years, ability to understand Chinese, and provision of written informed consent. Women who met the following criteria were excluded: (a) history of estrogen administration; (b) history of chronic cough and constipation; (c) acute or chronic inflammation of the urogenital tract; (d) history of nervous system disease and pelvic floor injury; (e) history of POP and SUI; (f) pelvic mass. Anthropometrics and sociodemographic data including age, height, body mass index (BMI) and parity were collected.
Power calculation was done for the primary analyses on UI and was based on the results of a former study,20 prevalence rates (48%) of UI in multiparous women, prevalence rates (32%) of UI in nulliparous women. A sample of 30 women in each group was calculated for type I error <0.05 and type II error <0.10, power of 87%.
A total of 70 singleton pregnant women without symptoms of preterm delivery were selected from our hospital. The average age was (30.0±4.5) years old, and the average body mass index (BMI) was (23.5±2.8) kg/m2, including 32 women with a first singleton pregnancy and 38 women with a second singleton pregnancy. Forty-two healthy non-pregnant women, aged (27.0±4.3) and BMI (20.8±3.2) kg/m2, were selected as the control group.
Instruments and Methods
Instruments
Mindray Resona 8T and GE Voluson E10 color Doppler ultrasound diagnostic instruments were used, respectively equipped with D8-4U and RM6C convex array product probe, which frequency was 4~8 MHz, both with 2D and dynamic 3D scanning function and pelvic floor mode.
Preparation Before Examination
Each subject emptied her bladder 15 minutes before the examination, and the residual urine volume in the bladder was less than 50 ml. The sonographer instructed the subjects to conduct Valsalva maneuver and pelvic floor muscle contraction training. Effective Valsalva maneuver refers to the subject holding breath and doing a strenuous attempt to evacuate the bowel for at least 6 seconds. From the view of ultrasound, you can see the organs in the pelvis move to caudal side, and the area of the minimal levator ani hiatus is enlarged. Effective pelvic floor muscle contraction refers to the subject forcibly upward and forward contraction of the anus and vagina. Pelvic organs can be seen moving forward and upward, and the area of the minimum levator ani hiatus becomes smaller from the view of the ultrasound.
Examination Method
A sonographer with more than 10 years of experience in obstetrics and gynecology ultrasonography did the ultrasound examination and completed the measurement, and there was another sonographer with more than 15 years of experience in obstetrics and gynecology ultrasonography present at the same time during the ultrasound examination. These two sonographers were blinded to all other medical information of the women, such as the mode of delivery, parity. The subject took the lithotomy position, heels close to buttocks, and the probe was covered with disposable gloves to avoid infection. The probe surface was coated with a couplant and placed between the labia and adhered tightly, with gentle movements and moderate pressure, clearly showing the midsagittal plane of the pelvic floor, including the pubic symphysis, urethra, bladder neck, vagina, cervix, anal canal and rectal ampulla, respectively (Figure 2). 2D static and dynamic images of the subjects at the Valsalva maneuver and the resting state, as well as static and dynamic 3D images at the constrictive anal state and the resting state, were collected respectively. After the images were stored, the images measurement and analysis were carried out. All the examinations were in strict accordance with the International Urogynecological Association (IUGA) guidelines for pelvic floor ultrasound examination.21
 |
Figure 2 The frame shows the plane of minimal levator hiatal. Anatomical landmarks. Abbreviations: SP, symphysis pubis; U, urethra; V, vagina; R, rectum; LA, levator ani. |
Image Analysis and Detection Indicators
The horizontal line was the line that passes through the inferior symphyseal margin at the plane of the mid sagittal section. It is set as the reference line, which is positive when it is above the reference line and negative when it is below the reference line. Bladder neck-symphysis distance (BSD), retrovesical angle (RVA), the distance of external orifice of cervix which refers to the vertical distance between the external orifice of cervix and the line of the symphysis pubis, rectal ampulla distance were measured at rest state and Valsalva maneuver. Calculation was then done on bladder neck descent (BND), the maximum distance of cervical drop and the maximum distance of rectum ampulla drop. At the same time, it was observed whether the bladder bulges during the Valsalva maneuver, that is, whether the lowest point of the bladder during the Valsalva maneuver is below the horizontal reference line.22 In volumetric imaging, the median sagittal section was shown on plane A by rotating the X, Y and Z axes, and the minimum levator ani hiatus plane was taken as the reference plane (Figure 2). The width of the sampling box was about 1.0~2.0 cm. The volumetric imaging clearly showed the diamond-shaped minimal levator ani hiatus between the posterior aspect of the symphysis pubis and the anterior aspect of the anorectal angle, as well as the urethra, vagina, rectum through the levator ani hiatus. Levator hiatus area (LHA) was measured at resting state (LHA-R) and at the constrictive anal state (LHA-S).
Outcome
Primary outcome was pelvic floor parameters among the women with a first singleton pregnancy group, women with a second singleton pregnancy group and the non-pregnant women group. Secondary outcomes were parameters between SUI group and SUI-free group during pregnancy.
Statistical Analysis
Statistical analysis was performed using SPSS23.0 statistical software. The measurement data conforming to the normal distribution was represented by 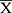 ±s, and the independent sample t-test was used for comparison between the two groups. For measurement data of skewness distribution, Kruskal-Wallis test was used for comparison between multiple groups, and post-hoc test was used for pairwise comparison between groups, statistically significant after Bonferroni correction (P = 0.05).
±s, and the independent sample t-test was used for comparison between the two groups. For measurement data of skewness distribution, Kruskal-Wallis test was used for comparison between multiple groups, and post-hoc test was used for pairwise comparison between groups, statistically significant after Bonferroni correction (P = 0.05).
Results
Demographics
The heights between the non-pregnant women group and the pregnant groups are not statistically significant (P>0.05). The age and BMI of the pregnant groups are higher than those of the non-pregnant women group (P<0.05). However there was no statistically significant difference in BMI before pregnancy between pregnant groups and the non-pregnant women group (P = 0.428) (Table 1). Comparing the basic data of the women with a first singleton pregnancy group and the women with a second singleton pregnancy group, differences were not statistically significant in height, gestational week, BMI (P>0.05). Women in the second singleton pregnancy group were older than women in the first singleton pregnancy group (P = 0.001) (Table 2).
 |
Table 1 Demographic Characteristics Between the Non-Pregnant Women Group and the Pregnant Group (Mean ± SD) |
 |
Table 2 Demographic and Obstetric Characteristics Between the Women with a First Singleton Pregnancy Group and Women with a Second Singleton Pregnancy Group (Mean ± SD) |
Primary Outcome: Parameters of Pelvic Floor Ultrasound
Among the three groups, there were no significant differences in BSD, the distance of external orifice of cervix, the maximum distance of cervical drop, the maximum distance of rectum ampulla drop (P>0.05), and there was a statistically significant difference in BND, rectum ampulla distance (P<0.05). Pairwise comparison among different groups showed that BND in the women with a second singleton pregnancy group was significantly higher than that in the non-pregnant women group (P = 0.006). Transperineal dynamic 3D image of the pelvic floor showed that there were statistically significant differences in LHA among the three groups both at the resting state and the pelvic floor muscle contraction state (P = 0.00). Pairwise comparison among different groups showed that there was a statistically significant difference in LHA between the women with a second singleton pregnancy group and the non-pregnant women group at resting state (P = 0.00). At pelvic floor muscle contraction state, the difference in LHA of the women with a second singleton pregnancy group was statistically significant compared with that of the non-pregnant women group, and that of the women with a first singleton pregnancy group was statistically significantly different compared with that of the non-pregnant women group (P = 0.00, P = 0.038) (Figure 3) (Table 3).
 |
Table 3 Comparison of Pelvic Floor Parameters Between the Women with a Second Singleton Pregnancy Group, the Women with a First Singleton Pregnancy Group and the Non-Pregnant Women Group |
Secondary Outcomes: Parameters of the SUI and Non-SUI Group
In this study, SUI were 20 cases, all of which were Grade I. SUI severity was classified using the Ingelman-Sundberg scale: Grade I urinary incontinence was characterized as being induced by coughing or sneezing, grade II urinary incontinence was induced by running or picking up an object from the floor and grade III urinary incontinence was induced by walking or climbing stairs.23 The incidence of SUI during pregnancy was 28.6%, including 7 cases of women with a first singleton pregnancy, and 13 cases of women with a second singleton pregnancy. Among the multiparous women group with SUI, there were 10 cases of the first child delivered by vagina, 3 cases of cesarean section. No cases of SUI occurred among the non-pregnant women group. BND, RVA-R, RVA-V in the SUI group during pregnancy was higher than that in the non-SUI group (t = 4.424, 2.626, 3.918), and the difference was statistically significant (P<0.05) (Figure 4). Meanwhile BSD showed no statistically significant difference (P>0.05) (Table 4). Mild cystocele occurred at maximum Valsalva movement in 3 patients in the SUI group. Cystocele classification was according to the Green standard.24
 |
Table 4 Comparison of Parameters Between SUI Group and SUI-Free Group During Pregnancy (Mean ± SD) |
Discussion
In this prospective study, we compared ultrasound measures of functional pelvic floor anatomy between three different groups to determine whether parity may have a cumulative impact on pelvic organ mobility and pelvic floor function. We found no significant differences between women with a first singleton pregnancy and women with a second singleton pregnancy in sonographic parameters. Hans Peter Dietz’s25 study also found no significant differences between women with one, two, or three and more cesarean births in any clinical or sonographic parameters of pelvic organ support and pelvic floor muscle function. However Dan Luo’s20 study showed that multiparous women experienced more obvious UI symptoms and pelvic floor structure changes during pregnancy than did nulliparous women. Our study also showed that women with a second singleton pregnancy more likely to have SUI (13/38 = 34.3%) than women with a first singleton pregnancy (7/32 = 21.9%). There were also statistically significant differences between women with a second singleton pregnancy and non-pregnant women in BND, distance of rectum ampullary LHA-R, LHA-S. These indicated that the increased number of pregnancies may cause some changes of pelvic organ support and pelvic floor muscle function, which need more research to support it.
According to statistics, the incidence of FPFD ranges from 10–58%,26 and its incidence is closely related to changes in the structure of the female pelvic floor. Pregnancy and childbirth are considered to be independent high-risk factors.5,6 During pregnancy, as the weight of pregnant women and fetuses gradually increases, the levator ani hiatus and the surrounding supporting tissues are under increasing pressure. At the same time, the changes in hormone levels during pregnancy and the process of delivery all cause pelvic floor structure changes.27 With further damage, the pelvic floor tissue adapts to this change through a series of remodeling processes.28 Once obvious morphological changes occur, POP and SUI will be exhibited, seriously affecting the physical and mental health of female patients.29,30
The levator ani muscle (LAM) differs from most other skeletal muscles, in that it maintains a constant tone, except during voiding, defecation and the Valsalva maneuver.31 It has the ability to contract quickly with a sudden increase in abdominal pressure during the physical action of a sneeze or a cough,31–33 thereby minimizing the risk of SUI and POP.34,35 Paradoxically, it has to stretch during parturition even beyond its limits36,37 in order to allow the passage of the term infant. Vaginal delivery can cause an injury to the LAM, resulting in reduced pelvic floor muscle strength, enlargement of the vaginal hiatus and pelvic organ prolapse. Pregnancy and childbirth can change the morphology, structure and function of the LAM. LHA reflects the compliance and elasticity of pelvic floor and levator ani muscle; a larger LHA indicates a weaker pelvic floor support structure that will be more prone to POP.38,39
In this study, through the comparative analysis of the pelvic floor parameters of groups of women with a second singleton pregnancy, women with a first singleton pregnancy, and non-pregnant women, the results showed that the BND, LHA-R and LHA-S of the three groups gradually decreased, and the differences were statistically significant (P<0.05). Pairwise comparison showed statistically significant differences in the BND, LHA-R and LHA-S between the women with a second singleton pregnancy group and the non-pregnant women group (P<0.05), which was due to the weakened pelvic floor support structure, damage to the LAM and increased mobility of the bladder neck caused by pregnancy and delivery. However there are no significant difference between the women with a second singleton pregnancy group and the women with a first singleton pregnancy group in LHA, which was consistent with a previous study.20
SUI is a common gynecological urological disorder, which is defined by the International Continence Society (ICS) as the complaint of involuntary loss of urine on effort or physical exertion or sneezing or coughing.40 It is the most common type of urinary incontinence (UI) in pregnant women. However, the true prevalence of SUI is still unknown.
A large population survey conducted in China found that 26.7% of pregnant women presented with urinary incontinence (UI), including 18.6% with SUI, 4.3% with mixed urinary incontinence MUI, and 2.0% with urgency urinary incontinence (UUI).41 Wesnes et al42 found that the most common type of UI was SUI in high prevalence in both nulliparous and multiparous women, 31% and 42%, respectively. Whitford et al43 in the UK found the prevalence of SUI during pregnancy was 54.3%. In this study, pregnant women were more likely to have SUI (20/70 = 28.6%) than non-pregnant women (0/40 = 0%). Women with a second singleton pregnancy were more likely to have SUI (13/38 = 34.3%) than women with a first singleton pregnancy (7/32 = 21.9%). Ayten Dinç’s study44 showed that 38% of primiparous women, and 20.3% of multiparous women experienced urinary incontinence, which was not consistent with our study. Our study showed that multiparous women were more likely to develop SUI during pregnancy than the primiparous, consistent with a previous study.45
With the comprehensive liberalization of the three-child policy in China, the growing number of multiparous women has drawn attention to the screening and prevention of SUI during pregnancy.
Pregnancy has a significant effect on lower urinary tract function,46 this increases the risk of SUI in pregnant women. In this study, by comparing the pelvic floor ultrasound parameters of the SUI group and the Non-SUI group during pregnancy, it was found that the BND (15.6±6.9 mm), RVA-V (152.1°±16.8°) of the SUI group were significantly higher than the BND (8.1± 4.9 mm), RVA-V (135.3°±16.0°) of the Non-SUI group during pregnancy. The difference was statistically significant (P = 0.00), indicating that SUI during pregnancy was associated with increased activity of the bladder neck. Another study also reported that BND had a strong association between BND measurement and severity of SUI.47 In our study, 3 patients in the SUI group had SUI accompanied with mild cystocele, while none in the non-pregnant group had SUI. Thus, pregnancy and delivery may cause damage to the pelvic floor muscle and fascia tissue, increasing the activity of the bladder neck, and resulting in a downward shift of bladder position, which may lead to SUI and even POP in pregnant women.
However, the etiology of SUI and POP is multifactorial and complex, and recent studies suggested ethnicity may be an important factor.48,49 Abdool, Z.’ study50 found significant ethnic differences in clinical prolapse stage, levator distensibility and pelvic organ descent in a multiethnic South African population. Nepali women appear to have a lower rate of obstetric anal sphincter tears and levator avulsion and a higher rate of uterine retroversion compared with other populations.51,52 However, another study showed that ethnicity had not been demonstrated to have an important impact on pelvic floor symptoms.53 Thus, our population of mainly Chinese women may not be representative of other populations.
Our study will provide basic research of Chinese female pelvic floor function during a second pregnancy, which will be of clinical significance around the world, as well as in China. China will keep promoting further the comprehensive liberalization of the three-child policy, as the aging population is increasing. If the developing countries want to promote second pregnancies, they have basic research and data to instruct the affected women.
Limitations
First, we collected women with a second singleton pregnancy, and the way they gave birth the first time was overlooked. Second, all the women were at the second and third trimester of pregnancy, we should have subdivided gestation into second trimester or third trimester, and analysed them respectively. Third, it is more meaningful to compare the changes of pelvic floor function parameters in the same pregnant woman during the first and second pregnancy, however there are some limitations of clinical application. Fourth, all the study group were Chinese, which implies that our result may not apply to other ethnic groups or those with substantially different obstetric management.
Conclusions
Our results indicate that women with a second singleton pregnancy had a higher prevalence of SUI and more changes in pelvic floor parameters than did women with a first singleton pregnancy. Since Professor Dietz first applied 3D ultrasound to the observation of pelvic floor tissue, it has now become the preferred imaging examination method for the diagnosis of FPFD. Transperineal pelvic floor ultrasound is a non-invasive examination, it can objectively and dynamically evaluate the pelvic floor structure and function during pregnancy and allow the conduct of quantitative analysis. It can provide abundant dynamic diagnostic information of pelvic floor morphology and function for clinical application. Whether subsequent pregnancies have an effect on pelvic floor structures or not needs more research.
Key points
- FPFD is a common gynecological disorder.
- Transperineal pelvic floor ultrasound is the main means of diagnosis of FPFD.
- Transperineal pelvic floor ultrasound can evaluate the pelvic floor structure and function.
- Women with a second singleton pregnancy had a higher prevalence of SUI and more changes in pelvic floor parameters than did women with a first singleton pregnancy.
Abbreviations
BND, Bladder neck descent; LHA, Levator hiatus area; RVA-R, Retrovesical angle at rest; RVA-V, Retrovesical angle underwent Valsalva maneuver; SUI, Stress urinary incontinence; FPFD, Female pelvic floor dysfunction; POP, Pelvic organ prolapse; 3D, Three-dimensional; BMI, Body mass index; IUGA, International Urogynecological Association; BSD, Bladder neck-symphysis distance; RVA, Retrovesical angle; LAM, Levator ani muscle; ICS, International Continence Society; UI, Urinary incontinence; UUI, Urgency urinary incontinence; ACR, American College of Radiology.
Data Availability Statements
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
The present retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and the study was approved by The Second Affiliated Hospital of Wenzhou Medical University Research Ethics Committee (No. LCKY2019-287). The authors thank Ying Hua, Rongyue Wang, Ke Dong from the Department of Obstetrics and Guifeng Lin, Yinhong Zhang from the Department of Ultrasonic Diagnosis who contributed to data collection and conduct of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Projects supported by the Scientific Research Fund of the Wenzhou Science and Technology Division. Grant Numbers:Y20180755.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dwyer PL. Female pelvic floor dysfunction and estrogen therapy. Climacteric. 2001;4:179–180. doi:10.1080/cmt.4.3.179.180
2. Tinelli A, Malvasi A, Rahimi S, et al. Age-related pelvic floor modifications and prolapse risk factors in postmenopausal women. Menopause. 2010;17:204–212. doi:10.1097/gme.0b013e3181b0c2ae
3. Kenton K, Sadowski D, Shott S, Brubaker L. A comparison of women with primary and recurrent pelvic prolapse. Am J Obstet Gynecol. 1999;180:1415–1418. doi:10.1016/S0002-9378(99)70027-X
4. Uustal Fornell E, Wingren G, Kjølhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand. 2004;83:383–389. doi:10.1111/j.0001-6349.2004.00367.x
5. Boyle R, Hay-Smith EJ, Cody JD, Morkved S. Pelvic floor muscle training for prevention and treatment of urinary and fecal incontinence in antenatal and postnatal women: a short version Cochrane review. Neurourol Urodyn. 2014;33:269–276. doi:10.1002/nau.22402
6. Van Geelen H, Ostergard D, Sand P. A review of the impact of pregnancy and childbirth on pelvic floor function as assessed by objective measurement techniques. Int Urogynecol J. 2018;29:327–338. doi:10.1007/s00192-017-3540-z
7. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi:10.1016/S0029-7844(97)00058-6
8. Fitz FF, Resende AP, Stupp L, Sartori MG, Girao MJ, Castro RA. Biofeedback for the treatment of female pelvic floor muscle dysfunction: a systematic review and meta-analysis. Int Urogynecol J. 2012;23:1495–1516. doi:10.1007/s00192-012-1707-1
9. Memon H, Handa VL. Pelvic floor disorders following vaginal or cesarean delivery. Curr Opin Obstet Gynecol. 2012;24:349–354. doi:10.1097/GCO.0b013e328357628b
10. Handa VL, Blomquist JL, Roem J, Muñoz A, Dietz HP. Pelvic floor disorders after obstetric avulsion of the levator ani muscle. Female Pelvic Med Reconstr Surg. 2019;25:3–7. doi:10.1097/SPV.0000000000000644
11. Gonzalez-Díaz E, Biurrun GP. Levator ani muscle avulsion: a risk factor for persistent postpartum voiding dysfunction. Int Urogynecol J. 2020;31:2327–2335. doi:10.1007/s00192-020-04412-3
12. Khayyami Y, Elmelund M, Klarskov N. Urinary incontinence before and after pelvic organ prolapse surgery-A national database study. Int Urogynecol J. 2021;32:2119–2123. doi:10.1007/s00192-021-04738-6
13. Alketbi MSG, Meyer J, Robert-Yap J, et al. Levator ani and puborectalis muscle rupture: diagnosis and repair for perineal instability. Tech Coloproctol. 2021;25:923–933. doi:10.1007/s10151-020-02392-6
14. Iacobellis F, Reginelli A, Berritto D, et al. Pelvic floor dysfunctions: how to image patients? Jpn J Radiol. 2020;38:47–63. doi:10.1007/s11604-019-00903-6
15. Valsky DV, Yagel S. Three-dimensional transperineal ultrasonography of the pelvic floor: improving visualization for new clinical applications and better functional assessment. J Med Ultrasound. 2007;26:1373–1387. doi:10.7863/jum.2007.26.10.1373
16. Tan L, Shek KL, Atan IK, Rojas RG, Dietz HP. The repeatability of sonographic measures of functional pelvic floor anatomy. Int Urogynecol J. 2015;26:1667–1672. doi:10.1007/s00192-015-2759-9
17. Nishibayashi M, Okagaki R. Ultrasonographic evaluation of pelvic floor structure at antepartum and postpartum periods using three-dimensional transperineal ultrasound. J Med Ultrasound. 2021;48:345–351. doi:10.1007/s10396-021-01100-7
18. Mao YJ, Zheng ZJ, Xu JH, Xu J, Zhang XL. Pelvic floor biometry in asymptomatic primiparous women compared with nulliparous women: a single-center study in Southern China. J Int Med Res. 2020;48:300060520920393. doi:10.1177/0300060520920393
19. van Veelen A, Schweitzer K, van der Vaart H. Ultrasound assessment of urethral support in women with stress urinary incontinence during and after first pregnancy. Obstet Gynecol. 2014;124:249–256. doi:10.1097/AOG.0000000000000355
20. Luo D, Chen L, Yu X, et al. Differences in urinary incontinence symptoms and pelvic floor structure changes during pregnancy between nulliparous and multiparous women. Peer J. 2017;5:e3615. doi:10.7717/peerj.3615
21. Hashimoto B, Sheth S, Mueller E, et al. AIUM/IUGA practice parameter for the performance of Urogynecological ultrasound examinations: developed in collaboration with the ACR, the AUGS, the AUA, and the SRU. Int Urogynecol J. 2019;30:1389–1400.
22. Dietz HP, Haylen BT, Broome J. Ultrasound in the quantification of female pelvic organ prolapse. Ultrasound Obstet Gynecol. 2001;18:511–514. doi:10.1046/j.0960-7692.2001.00494.x
23. Ingelman-Sundberg A, Ulmsten U. Surgical treatment of female urinary stress incontinence. Contrib Gynecol Obstet. 1983;10:51–69.
24. Green TH
25. Dietz HP, Scoti F, Subramaniam N, Friedman T, Shek KL. Impact of subsequent pregnancies on pelvic floor functional anatomy. Int Urogynecol J. 2018;29:1517–1522. doi:10.1007/s00192-018-3567-9
26. Mallett VT, Bump RC. The epidemiology of female pelvic floor dysfunction. Curr Opin Obstet Gynecol. 1994;6:308–312. doi:10.1097/00001703-199408000-00002
27. Hilton P, Dolan LM. Pathophysiology of urinary incontinence and pelvic organ prolapse. BJOG. 2004;111(Suppl 1):5–9. doi:10.1111/j.1471-0528.2004.00458.x
28. Eickmeyer SM. Anatomy and physiology of the pelvic floor. Phys Med Rehabil Clin N Am. 2017;28:455–460. doi:10.1016/j.pmr.2017.03.003
29. Irwin DE, Milsom I, Kopp Z, Abrams P, Cardozo L. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int. 2006;97:96–100. doi:10.1111/j.1464-410X.2005.05889.x
30. Verbeek M, Hayward L. Pelvic floor dysfunction and its effect on quality of sexual life. Sex Med Rev. 2019;7:559–564. doi:10.1016/j.sxmr.2019.05.007
31. Barber MD, Bremer RE, Thor KB, Dolber PC, Kuehl TJ, Coates KW. Innervation of the female levator ani muscles. Am J Obstet Gynecol. 2002;187:64–71. doi:10.1067/mob.2002.124844
32. Junginger B, Baessler K, Sapsford R, Hodges PW. Effect of abdominal and pelvic floor tasks on muscle activity, abdominal pressure and bladder neck. Int Urogynecol J. 2010;21:69–77. doi:10.1007/s00192-009-0981-z
33. Sapsford RR, Hodges PW. Contraction of the pelvic floor muscles during abdominal maneuvers. Arch Phys Med Rehabil. 2001;82:1081–1088. doi:10.1053/apmr.2001.24297
34. Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci. 2007;1101:266–296. doi:10.1196/annals.1389.034
35. Herschorn S. Female pelvic floor anatomy: the pelvic floor, supporting structures, and pelvic organs. Rev Urol. 2004;6(Suppl 5):S2–s10.
36. Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103:31–40. doi:10.1097/01.AOG.0000109207.22354.65
37. Hoyte L, Damaser MS, Warfield SK, et al. Quantity and distribution of levator ani stretch during simulated vaginal childbirth. Am J Obstet Gynecol. 2008;199:
38. Handa VL, Roem J, Blomquist JL, Dietz HP, Muñoz A. Pelvic organ prolapse as a function of levator ani avulsion, hiatus size, and strength. Am J Obstet Gynecol. 2019;221:
39. Lu R, Zhang Y, Yu YP. 超声测量肛提肌裂孔面积在子宫脱垂诊断中的应用 [Application of ultrasound in diagnosis of uterine prolapse by measuring area of levator hiatus]. Zhonghua Yi Xue Za Zhi. 2019;99:2315–2318. Chinese. doi:10.3760/cma.j.issn.0376-2491.2019.29.014
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20. doi:10.1002/nau.20798
41. Zhu L, Li L, Lang JH, Xu T. Prevalence and risk factors for peri- and postpartum urinary incontinence in primiparous women in China: a prospective longitudinal study. Int Urogynecol J. 2012;23:563–572. doi:10.1007/s00192-011-1640-8
42. Wesnes SL, Rortveit G, Bo K, Hunskaar S. Urinary incontinence during pregnancy. Obstet Gynecol. 2007;109:922–928. doi:10.1097/01.AOG.0000257120.23260.00
43. Whitford HM, Alder B, Jones M. A cross-sectional study of knowledge and practice of pelvic floor exercises during pregnancy and associated symptoms of stress urinary incontinence in North-East Scotland. Midwifery. 2007;23:204–217. doi:10.1016/j.midw.2006.06.006
44. Dinç A. Prevalence of urinary incontinence during pregnancy and associated risk factors. Low Urin Tract Symptoms. 2018;10:303–307. doi:10.1111/luts.12182
45. Lin KL, Shen CJ, Wu MP, Long CY, Wu CH, Wang CL. Comparison of low urinary tract symptoms during pregnancy between primiparous and multiparous women. Biomed Res Int. 2014;2014:303697. doi:10.1155/2014/303697
46. Wijma J, Weis Potters AE, de Wolf BT, Tinga DJ, Aarnoudse JG. Anatomical and functional changes in the lower urinary tract during pregnancy. BJOG. 2001;108:726–732. doi:10.1111/j.1471-0528.2001.00123.x
47. Dietz HP, Nazemian K, Shek KL, Martin A. Can urodynamic stress incontinence be diagnosed by ultrasound? Int Urogynecol J. 2013;24:1399–1403. doi:10.1007/s00192-012-2032-4
48. Shek KL, Krause HG, Wong V, Goh J, Dietz HP. Is pelvic organ support different between young nulliparous African and Caucasian women? Ultrasound Obstet Gynecol. 2016;47:774–778. doi:10.1002/uog.15811
49. Cheung RY, Shek KL, Chan SS, Chung TK, Dietz HP. Pelvic floor muscle biometry and pelvic organ mobility in East Asian and Caucasian nulliparae. Ultrasound Obstet Gynecol. 2015;45:599–604. doi:10.1002/uog.14656
50. Abdool Z, Dietz HP, Lindeque BG. Interethnic variation in pelvic floor morphology in women with symptomatic pelvic organ prolapse. Int Urogynecol J. 2018;29:745–750. doi:10.1007/s00192-017-3391-7
51. Turel F, Caagbay D, Dietz HP. Functional pelvic floor anatomy in Nepali women attending a general gynaecology clinic. Int Urogynecol J. 2018;29:1435–1440. doi:10.1007/s00192-017-3534-x
52. Turel F, Caagbay D, Dietz HP. Prevalence of maternal birth trauma in Nepali women. J Med Ultrasound. 2018;37:2803–2809. doi:10.1002/jum.14637
53. Dessie SG, Adams SR, Modest AM, Hacker MR, Elkadry EA. Bladder symptoms and attitudes in an ethnically diverse population. Female Pelvic Med Reconstr Surg. 2016;22:37–42. doi:10.1097/SPV.0000000000000213
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


