Back to Journals » Cancer Management and Research » Volume 13
Application of Contrast-Enhanced Ultrasonography (CEUS) in the Assessment of Kidney Wound Recovery After Nephron-Sparing Surgery
Authors Niu D, Li L, Du H, Shi H, Zhou J, Tai S, Xu H, Chen W, Yang C, Liang C
Received 15 December 2020
Accepted for publication 26 April 2021
Published 13 May 2021 Volume 2021:13 Pages 3925—3934
DOI https://doi.org/10.2147/CMAR.S297270
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Eileen O'Reilly
Di Niu,1,2,* Liang Li,3,* Hexi Du,1,2 Haoqiang Shi,1,2 Jun Zhou,1,2 Sheng Tai,1,2 Hanjiang Xu,1,2 Wei Chen,1,2 Cheng Yang,1,2 Chaozhao Liang1,2
1Department of Urology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 2Institute of Urology and Anhui Province Key Laboratory of Genitourinary Diseases, Anhui Medical University, Hefei, Anhui, People’s Republic of China; 3Department of Ultrasound, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cheng Yang; Chaozhao Liang
Department of Urology, The First Affiliated Hospital of Anhui Medical University, No. 218 Jixi Road, Hefei, 230022, China
Tel +86-18010865815
; +86-13505604595
Email [email protected]; [email protected]
Purpose: To investigate feasibility, repeatability and usefulness of contrast-enhanced ultrasonography (CEUS) in the assessment of kidney wound recovery after laparoscopic nephron-sparing surgery (LNSS) or robot-assisted nephron-sparing surgery (RANSS) and preliminarily research the clinical factors associated with the length of extravasation (LOE).
Patients and Methods: From April 2019 to January 2020, 130 patients that underwent LNSS or RANSS in our hospital were included, and 90 patients (90/130) received CEUS examinations each one day from the postoperative day 1. The discovery of the cessation of contrast medium extravasation from the renal wound was the primary endpoint named “ultrasonic healing”, and LOE ranged from the day of surgery to “ultrasonic healing”. Patient, tumor, perioperative factors and LOE were collected. Univariate analysis and multivariate linear regression analysis were applied for the determination of factors associated with LOE.
Results: The average postoperative LOE was 1.76 days (standard deviation, 1.115; 95% confidence interval: 1.52– 1.99). Ultrasonic healing within three days was observed in 95.6% patients (86/90). Univariable and multivariable analyses showed that R and A components in R.E.N.A.L. nephrometry score were associated with LOE. Anterior location and R component score of 2 (tumor size> 4cm) were related to longer LOE than posterior location and R score of 1 (tumor size< 4cm). The incidence of complications in patients with LOE over one day was higher than those with LOE of one day.
Conclusion: CEUS was feasible, repeatable and useful in the assessment of kidney wound recovery. Tumor size and location were related to LOE after minimally invasive nephron-sparing surgery (MINSS). Length of stay after MINSS within three days might be relatively safe.
Keywords: nephron-sparing surgery, minimally invasive, CEUS, wound healing
Introduction
During recent decades, the incidence of kidney cancer is increasing steadily, reaching an estimate of 403,262 new cases worldwide in 2018.1 The recommended standard treatment for T1 stage renal tumor has been nephron-sparing surgery (NSS).2,3 The length of stay (LOS) after NSS in the early learning curve usually ranged from five to ten days.4,5
The application of robotic and laparoscopic technology has decreased LOS compared with open nephron-sparing surgery. Enhanced recovery after surgery (ERAS) protocols were associated with a considerable reduction in LOS and hospital costs.6 Furthermore, in several centers, postoperative day 1 (POD1) discharge protocols without an increase in post-discharge complications were successfully utilized for many patients after minimally invasive nephron-sparing surgery (MINSS).7–9 But the critical factor associated with the recovery of NSS such as kidney wound healing was mostly unexplored. Although multidetector CT is applied to diagnose the complications about kidney wound such as hemorrhage and urine leaks,10,11 the continuous observation of kidney wound in the early postoperative stage of NSS has not been reported, so we aimed at seeking for an image examination method to reflect the process of kidney wound healing.
In theory, kidney wound after PN gets similar physiopathologic and morphologic changes to trauma; meanwhile, the difference is the wound after PN should be much more stable due to the suture. Hemodynamic stability was a vital factor for severity assessment and management in trauma,12,13 and CEUS was proved feasible to evaluate active hemorrhage around the injuries of solid organ with significant specificity and sensitivity.14–16 Moreover, Xu et al17 have successfully applied real-time 3-dimensional CEUS to detect hemorrhage in blunt renal trauma of New Zealand white rabbits, and Lin et al18 have found CEUS sensitive in evaluating hemorrhagic renal lesions in shock or different status of hemodynamic instability in a canine model, which pushed us to consider CEUS as a new approach to display the possible bleeding or exudation from the wound which might be a probable indicator to wound healing.
The ultrasonic contrast median comprises gas microbubbles similar in size to red blood cells. Each of microbubbles is covered outwardly by a protein, lipid, or polymer coating shell. The gas contained in the microbubbles is excreted by the lungs, and the coating shell is metabolized by the liver. The kidneys have no role in the excretion of the microbubble,19 so it is a pure intravascular medium and not contraindicated in patients with renal insufficiency.
As far as we know, CEUS has not been utilized to assess the recovery of the renal wound. So, the present research aimed at investigating the feasibility, repeatability and usefulness of CEUS in the assessment of kidney wound recovery after LNSS or RANSS and preliminarily analyzing the clinical factors associated with the length of extravasation (LOE).
Patients and Methods
Ethics Approval
This study was conducted according to the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The First Affiliated Hospital of Anhui Medical University (PJ2019-17-19). All participants provided written informed consent.
Patient Population
From April 2019 to January 2020, all patients who were diagnosed with T1 stage renal masses by contrast-enhanced CT or MRI and underwent LNSS or RANSS in the First Affiliated Hospital of Anhui Medical University were included. They were assessed according to inclusion and exclusion criteria. R.E.N.A.L. nephrometry score system was used to assess the anatomy characteristics of each tumor20 (Supplementary Table 1).
Inclusion and Exclusion Criteria
Inclusion criteria included patients who could cooperate with CEUS procedure and sign an informed consent form. Exclusion criteria included age <18 or >80; a known history of allergies to food, medications, albumin, and other blood products; congenital heart disease with intracardiac shunt; severe pulmonary hypertension; asthma; respiratory failure; a lack of cooperation and patients who declined to participate in the study.
Surgical Technique
All RANSSs were conducted using the Da Vinci Si Surgical System (Intuitive, Sunnyvale, CA) through retroperitoneal or transperitoneal approach. Vascular bulldog clamps were used to clamp the main or branch renal artery. Excision of the tumor along with a rim of normal healthy parenchyma (5mm) was performed using cold cut scissors. 3-0 V-Loc 180 sutures on V-20 needles (Covidien, Mansfield, MA) with anchoring Hem-o-Lok clips in the loop of the sutures were utilized for renorrhaphy.21 One or two layers of suture depended on the depth of tumor excision bed. Drainage tube was routinely placed for every patients. The surgical technique of LNSSs was the same as that of RANSSs.
CEUS Examination
US examinations were performed using a Mindray Resona 7 ultrasound system (Mindray Medical, Shenzhen, China) and an SC-5-1U probe with a frequency of 3–5MHz. The mechanical index was 0.082 for CEUS, and perfluoropropane-albumin microsphere injection (Kangrun Pharmaceutical Co, Yueyang, China) was used as a contrast agent. Preoperative US examinations including B-mode and color Doppler ultrasound were performed for every patient to obtain the masses’ size and location. Preoperative ultrasonography and postoperative CEUS were performed by a US specialist with five years of experience in CEUS and a urology specialist experienced in RPN.
During the examination of CEUS, all patients were required to maintain slow shallow breathing in lateral or spine position. The ultrasonic probe was placed near the posterior axillary line to acquire the coronal US images of kidneys. Firstly, conventional US was performed to evaluate the surgical lesion on the surface of the kidney. Then, an intravenous bolus of 2.5–3 mL followed by a 5 mL saline flush was injected through a 20G peripheral intravenous cannula. The video recorder and timer were immediately started after the injection. The CEUS examination lasted about 3 minutes. All images and video clips were stored to confirm the existence of contrast agent extravasation from the lesion. After CEUS, patients’ clinical symptoms and vital signs were monitored to guarantee the safety of patients.
CEUS was performed on the first day after surgery and repeated every 24 hours until the primary endpoint named “ultrasonic healing” of the kidney wound. The “ultrasonic healing” was defined as the cessation of contrast medium extravasation from the renal wound, and LOE ranging from the day of surgery to “ultrasonic healing” of every patient was recorded.
Statistical Analysis
IBM SPSS Statistics, Version 20.0 (IBM, Armonk, NY, USA) was used for data analysis. Categorical variables were presented as number and percentage, and continuous variables were presented as mean±standard deviation. The t test, one-way analysis of variance (ANOVA) followed by Fisher LSD (Least Significant Difference) Post hoc test and Pearson correlation analysis were used for binary, polytomous categorical and continuous variables, respectively. Kruskal–Wallis test and Mann–Whitney U-test were applied for continuous variables with non-normal distribution. Predictors of LOE were determined by multivariable linear regression analysis. Univariate analysis was applied to determine the relationship between LOE and Clavien-Dindo Classification. P<0.05 was considered statistically significant.
Results
Baseline Patient Characteristics
Before surgery, 130 patients received US scanning and 25 patients were excluded because of a known history of allergy (n=15), over 80 years old (n=2) and a lack of cooperation (n=8). After surgery, 105 patients received US scanning, and ten patients with subcutaneous emphysema over the kidney wound and five patients allergic to antibiotics after surgery were excluded, so 90 of 130 patients received CEUS examinations. No adverse reactions nor complications related to CEUS were found.
These 90 patients underwent surgeries by three surgeons with different seniority. Baseline patient and tumor characteristics, surgical and postoperative factors are presented in Tables 1 and 2. Categorical variables are listed in Table 1 and continuous variables are summarized in Table 2.
 |
Table 1 Univariate Analysis of Categorical Variables with LOE |
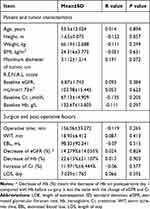 |
Table 2 Univariate Analysis of Continuous Variables with LOE |
Features of US and CEUS
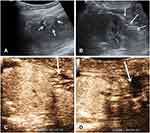
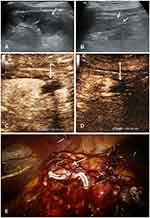
In conventional US, with reference to preoperative localization, the renal wound was presented as a region of local heterogeneous echo with unclear irregular border (Figures 1A and B, 2A and B). The renal capsule of the wound was rugged; meanwhile, the severity depended on the method of suture ligation. Free fluid as hypoecho or anecho around the capsule is displayed in all cases; however, the volume could not be exactly measured because of the irregular distribution. Major resection range may result in more renal units lost which displayed a hollow on the surface, even worse conspicuous parenchyma lost in the images. During the medullary phase, CEUS represented the continuous extravasation of the contrast agent as a drip or a band-like spot with a low velocity out of the sutured capsule (Figures 1C and 2C). With the healing of kidney wound, contrast agent extravasation gradually stopped (Figures 1D and 2D).
Constituent Ratio of LOE
The average postoperative LOE was 1.76 days (standard deviation, 1.115; 95% confidence interval: 1.52–1.99). The median (interquartile range) LOE was 1 (1–2) days. 49 patients (54.4%), 24 patients (26.7%) and 13 patients (14.4%) achieved ultrasonic healing on the first, second and third day after surgery, respectively. There were 73 patients (81.1%) with LOE within two days and 86 patients (95.6%) within three days. LOE longer than three days was observed in only four patients (4.4%).
Univariate Analysis of Factors with LOE
In univariate analysis. Patient, surgical and postoperative factors were not associated with LOE. Only R and A components in R.E.N.A.L. score were found associated with LOE (Table 1, P<0.05). We noted a significant increase in mean LOE in patients (19/90, 21.1%) with R component score of 2 compared with patients (71/90, 78.9%) with R score of 1 (2.25 days vs 1.62 days, P=0.025). Fisher LSD Post hoc test showed the mean LOE in patients (29/90, 32.2%) with anterior masses was 2.14 days, which was significantly longer than that of 1.42 days in patients (33/90, 36.7%) with posterior masses (P=0.012). No difference was found between “A” and “X” and between “P” and “X”.
Multivariate Analysis of Factors with LOE
In multivariate linear regression analysis, the only factors that remained significant were R and A components in R.E.N.A.L. score. Patients with R component score of 2 were related to significantly longer LOE than those with R score of 1 (β=0.647, P=0.02). Anterior location was associated with a 0.717 day increase of LOE (β=0.717, P=0.009) compared with posterior location. The prediction formula for LOE was as follows: LOE=0.640+0.647×R scores+ 0.717×”A”+0.324×”X” (reference= posterior, A=0, X=0; anterior, A=1, X=0; unknown A or P, A=0, X=1).
LOE with Surgical Complications
No complications were found in 35 patients (35/90, 38.9%). In the Clavien-Dindo classification, there were 45 patients, 9 patients and one patient with grade I, grade II and grade III, respectively. No statistical significance was found between LOE and grades of the Clavien-Dindo classification (Spearman correlation rho=0.185, P=0.081). Given the limited numbers of our study, these 90 patients were splitted into two groups: 49 patients (54.4%) with LOE of one day and 41 patients (45.6%) with LOE over one day. The incidence of complications in patients with LOE over one day (31/41, 75.6%) was higher than those with LOE of one day (24/49, 49%, P=0.016). Surgical complications are summarized in Supplemental Table 2. Although intraoperative collecting system entry was recognized in 25 patients, no complications of urinary leaks were found.
Discussion
With the application of laparoscopic and robotic techniques and ERAS protocols, LOS after NSSs has been significantly decreased. Discharge on POD1 after MINSS without an increase in post-discharge complications was reported in several centers, and it was more likely that MINSSs with next-day discharge were performed in patients with small (tumor size <4cm) and non-complex masses (R.E.N.A.L. score ≤9) in these studies.7–9 However, it was unknown that whether ERAS protocol was suitable for patients with large or complex masses, and to date, there have been no prospective studies that have determined which perioperational factors allowed early discharge.8
Kidney wound healing which was one of the critical factors associated with the recovery of NSS may be crucial for early discharge, and the morphological evidence of kidney wound healing was lacking. Gregory et al22 checked the leakage from partial nephrectomy defects by injecting indigo carmine into the renal arteries of porcine kidneys to compare the suture effect, which showed leakage or not of vascular contents could reflect the state of wound recovery. As far as we know, CEUS has not been utilized to assess the recovery of the renal wound, so we first applied CEUS with pure intravascular microbubbles to evaluate the kidney wound healing after MINSS and found some significant appearance and changes of kidney wound in CEUS.
CEUS is widely applied to evaluate the solid organ injury in blunt abdominal trauma,23 but few researches reported the application of CEUS in the follow-up of abdominal trauma without a standardized protocol.24–26 Miele et al27 found CEUS at 24 h and 72 h from trauma a very good correlation with onset contrast-enhanced CT. In another study, CEUS was performed at 12, 24, and 48 h after mild liver and spleen trauma to ensure a safe discharge from ICU to the general ward.28 What’s more, Tagliati et al published three researches with the same patients with splenic trauma in 2019,29–31 patients underwent serial CEUS at short time intervals (1, 3, 8, 15, 30, 60, 90 and 180 days post-trauma) until splenic trauma became no more visible. But no change in injury was observed within three days post-trauma, and CEUS was applied to diagnose delay active hemorrhage or assess healing time of solid organ injuries in these studies. Our study first reported the process of gradual cessation of ultrasonic contrast agent extravasation from kidneys in the early postoperative stage.
In the arterial phases of CUES, active bleeding is presented as a region of hyperechoic perfusion associated with contrast agent extravasation. Active bleeding from a solid organ trauma involving the organ capsule appeared as a fountain or spring with a high velocity.14 In detecting active bleeding of blunt renal trauma using real-time three-dimensional CEUS, Xu et al15 found that consistent enhanced signal gushed from the injured site. In contrast, continuous extravasation of contrast agent as a drip or a band-like spot with a low velocity out of the sutured capsule was found in our study. Because CEUS could evaluate capillary perfusion in real time,17 we assumed incomplete closure of the microvasculature in kidney wound resulted in this appearance. The area of wound exstrophy which showed the unsatisfactory closure after suture may be the site of extravasation (Figure 2E). Contrast agent leaked from the broken end of capillary and gradually stopped extravasating from the kidney wound with its healing process. We represented “ultrasonic healing” as the judgment standard of primary healing of the kidney wound, but further animal models and histological verification are needed.
The extent and stability of wound were influenced by tumor and surgical factors such as suture methods. CEUS as an imaging method could objectively reflect and predict it, lest the influence of subjective surgical factors to an extent. Moreover, location (anterior/posterior) and R score were found related to LOE in our study, which also influenced the extent and stability of wound. CEUS is a non-invasive, repeatable and radiation-free method that could be implemented at the bedside of patients and is convenient for the follow-up of patients in bed after surgery. Microbubbles are safer than iodine contrast because of low risk of allergies. However, injury of the kidney collecting system such as urine leakage may be neglected in CEUS due to a lack of microbubble urinary excretion by intravenous injection. Moreover, subcutaneous emphysema could hinder the implementation of CEUS, and the microbubble extravasation from wound is subject to the subjective judgment of the examiner.
49 of 90 patients (54.4%) achieved ultrasonic healing on POD1, which may support next-day discharge was feasible for patients after MINSS. Because of only four patients (4.4%) with LOE over three days, it may be relatively safe for discharge within three days after MINSS. Several retrospective studies showed larger tumor size was related to LOS>1 day and prolonged LOS after RANSS.32,33 Correspondingly, R score of two points (tumor size>4cm) was associated with longer LOE in our study. The prediction formula could provide a reference for the evaluation of LOE and relatively safe time of discharge before surgery, especially helpful for hospitals without the condition of CEUS examination in an underdeveloped region. What’s more, the evaluation of LOE was useful for surgeons to decide upon the surgical strategy and improve the suture methods such as an increase in suture tightness, leading to the enhancement of kidney wound’s stability. No statistical significance was found between LOE and grades of Clavien-Dindo classification, the limited number of patients with different grades of complications may account for it. Patients with LOE over one day were more likely to have surgical complications than those with LOE of one day (75.6% vs 49%, P=0.016), which demonstrated that LOE was associated with a clinically relevant outcome such as the increased risk of complications that would be a true finding. The postoperative management for the patients with LOE over one day might be more conservative.
There are some limitations in our study. A relatively small sample size may increase the probability of type II errors and reduce the statistical power, which would affect the conclusions of the study. The ultrasonic healing represents which stage of kidney wound healing needs further animal models and histological verification. More researches are needed to determine whether there is an increase in postoperative complications to discharge after ultrasonic healing.
Conclusions
CEUS was feasible, repeatable and useful in the assessment of kidney wound recovery. Tumor size and location were related to LOE after MINSS. LOS after MINSS within three days might be relatively safe.
Acknowledgments
This study has been previously presented and published in Abstract form from a meeting presentation. The link to the abstract is following: https://doi.org/10.1111/iju.14397.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81700662), Natural Science Research Project Funding of Higher Education Institutions of Anhui Province (No. KJ2019A0279), Special Projects of the Central Government Guiding Local Science and Technology Development (No.2017070802D148), Clinical Medical Research Center of Urinary System Diseases of Anhui Province and Clinical Key Subjects Program of the Ministry of Public Health (Urology), and Scientific Research Fund Project of Anhui Medical university (2020xkj178).
Disclosure
The authors declare no conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198(3):520–529.
3. Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810.
4. Leach GE, Lieber MM. Partial nephrectomy: Mayo Clinic experience 1957–1977. Urology. 1980;15(3):219–228.
5. Chughtai B, Abraham C, Finn D, et al. Fast track open partial nephrectomy: reduced postoperative length of stay with a goal-directed pathway does not compromise outcome. Adv Urol. 2008;2008:507–543.
6. Azhar RA, Bochner B, Catto J, et al. Enhanced recovery after urological surgery: a contemporary systematic review of outcomes, key elements, and research needs. Eur Urol. 2016;70(1):176–187.
7. Bernhard JC, Robert G, Ricard S, et al. Day-case robotic-assisted partial nephrectomy: feasibility and preliminary results of a prospective evaluation (UroCCR-25 AMBU-REIN study) [published online ahead of print, 2020 Jun 8]. World J Urol. 2020;2020(Jun):8.
8. Berger I, Xia L, Sperling C, et al. Next-day discharge after minimally invasive partial nephrectomy: an analysis of the US National Surgical Quality Improvement Program. World J Urol. 2019;37(5):831–836.
9. Sentell KT, Badani KK, Paulucci DJ, et al. A single overnight stay after robotic partial nephrectomy does not increase complications. J Endourol. 2019;33(12):1003–1008.
10. Tonolini M, Ierardi AM, Varca V, et al. Multidetector CT imaging of complications after laparoscopic nephron-sparing surgery. Insights Imaging. 2015;6(4):465–478.
11. Saddala P, Ramanathan S, Tirumani SH, et al. Complications of minimally invasive procedures of the abdomen and pelvis: a comprehensive update on the clinical and imaging features. Emerg Radiol. 2015;22(3):283–294.
12. Bjurlin MA, Fantus RJ, Fantus RJ, et al. Comparison of nonoperative and surgical management of renal trauma: can we predict when nonoperative management fails? J Trauma Acute Care Surg. 2017;82(2):356–361.
13. Loggers SAI, Koedam TWA, Giannakopoulos GF, et al. Definition of hemodynamic stability in blunt trauma patients: a systematic review and assessment amongst Dutch trauma team members. Eur J Trauma Emerg Surg. 2017;43(6):823–833.
14. Feng C, Wang L, Huang S, et al. Application of contrast-enhanced real-time 3-dimensional ultrasound in solid abdominal organ trauma. J Ultrasound Med. 2020;39(5):869–874.
15. Kummer T, Oh L, Phelan MB, et al. Emergency and critical care applications for contrast-enhanced ultrasound. Am J Emerg Med. 2018;36(7):1287–1294.
16. Lv F, Tang J, Luo Y, et al. Contrast-enhanced ultrasound imaging of active bleeding associated with hepatic and splenic trauma. Radiol Med. 2011;116(7):1076–1082.
17. Xu R-X, Li Y-K, Li T, et al. Real-time 3-dimensional contrast-enhanced ultrasound in detecting hemorrhage of blunt renal trauma. Am J Emerg Med. 2013;31(10):1427–1431.
18. Lin Q, Lv F, Luo Y, et al. Contrast-enhanced ultrasound for evaluation of renal trauma during acute hemorrhagic shock: a canine model. J Med Ultrasonics. 2015;42(2):199–205.
19. Alenezi AN, Karim O. Role of intra-operative contrast-enhanced ultrasound (CEUS) in robotic-assisted nephron-sparing surgery. J Robot Surg. 2015;9(1):1–10.
20. Kutikov A, Uzzo RG, The RENAL. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844–853.
21. Hennessey DB, Wei G, Moon D, et al. Strategies for success: a multi-institutional study on robot-assisted partial nephrectomy for complex renal lesions. BJU Int. 2018;121(Suppl 3):40–47.
22. Rosenblatt GS, Fuchs GJ. A comparison of running suture versus figure-8 sutures as the initial step in achieving hemostasis during laparoscopic partial nephrectomy. J Endourol. 2010;24(3):421–424.
23. Miele V, Piccolo CL, Galluzzo M, et al. Contrast-enhanced ultrasound (CEUS) in blunt abdominal trauma. Br J Radiol. 2016;89(1061):20150823.
24. Di Renzo D, Persico A, Lisi G, et al. Contrast-enhanced ultrasonography (CEUS) in the follow-up of pediatric abdominal injuries: value and timing. J Ultrasound. 2020;23:151–155.
25. Durkin N, Deganello A, Sellars ME, et al. Post-traumatic liver and splenic pseudoaneurysms in children: diagnosis, management, and follow-up screening using contrast enhanced ultrasound (CEUS). J Pediatr Surg. 2016;51(2):289–292.
26. Brillantino A, Iacobellis F, Robustelli U, et al. Non operative management of blunt splenic trauma: a prospective evaluation of a standardized treatment protocol. Eur J Trauma Emerg Surg. 2016;42(5):593–598.
27. Miele V, Piccolo CL, Sessa B, et al. Comparison between MRI and CEUS in the follow-up of patients with blunt abdominal trauma managed conservatively. Radiol Med. 2016;121(1):27–37.
28. Manetta R, Pistoia ML, Bultrini C, et al. Ultrasound enhanced with sulphur-hexafluoride-filled microbubbles agent (SonoVue) in the follow-up of mild liver and spleen trauma. Radiol Med. 2009;114(5):771–779.
29. Tagliati C, Argalia G, Giuseppetti GM. Contrast-enhanced ultrasound performance in predicting blunt splenic injuries requiring only observation and monitoring. Med Ultrason. 2019;21(1):16–21.
30. Tagliati C, Argalia G, Graziani B, et al. Contrast-enhanced ultrasound in the evaluation of splenic injury healing time and grade. Radiol Med. 2019;124(3):163–169.
31. Tagliati C, Argalia G, Polonara G, et al. Contrast-enhanced ultrasound in delayed splenic vascular injury and active extravasation diagnosis. Radiol Med. 2019;124(3):170–175.
32. Bazzi WM, Sjoberg DD, Grasso AA, et al. Predicting length of stay after robotic partial nephrectomy. Int Urol Nephrol. 2015;47(8):1321–1325.
33. Shumate AM, Roth G, Ball CT, et al. Factors associated with prolonged length of stay following robotic-assisted partial nephrectomy. Can J Urol. 2019;26(2):9726–9732.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.