Back to Journals » Journal of Inflammation Research » Volume 15
Application of Baricitinib in Dermatology
Authors Zhang J, Qi F , Dong J , Tan Y, Gao L, Liu F
Received 29 December 2021
Accepted for publication 4 March 2022
Published 18 March 2022 Volume 2022:15 Pages 1935—1941
DOI https://doi.org/10.2147/JIR.S356316
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Jingya Zhang,1 Fei Qi,1 Jie Dong,1 Yaqi Tan,1 Ling Gao,2 Fang Liu1
1Department of Dermatology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China; 2China CDC Key Laboratory of Radiological Protection and Nuclear Emergency, National Institute for Radiological Protection, Chinese Centre for Disease Control and Prevention, Beijing, 100088, People’s Republic of China
Correspondence: Fang Liu, Department of Dermatology, Beijing Chaoyang Hospital, Capital Medical University, 8 Gongti South Road, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +86-10-85231688, Fax +86-10-85231217, Email [email protected]
Abstract: There are four JAK subtypes: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). Small molecule Janus tyrosine kinase (JAK) inhibitors can inhibit a variety of pro-inflammatory cytokines. Baricitinib is the first generation of JAK1/2 inhibitor targeting the ATPase of JAK, which blocks the intracellular transmission of cytokines through JAK-STATs. Thus far, it has been approved for the treatment of rheumatoid arthritis (RA); however, an increasing number of studies have suggested that baricitinib can be used to treat dermatological diseases, such as atopic dermatitis (AD), psoriasis, vitiligo, and alopecia areata. Baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with conventional drugs. We reviewed the application, efficacy, side effects, precautions, limitations and prospect of baricitinib in atopic dermatitis, psoriasis, vitiligo and alopecia areata (AA) in recent 5 years including clinical trials and case reports. Among them, the application in the field of alopecia areata is the most encouraging, and we reviewed the mechanism in detail.
Keywords: JAK inhibitors, JAK/STAT pathway, atopic dermatitis, psoriasis, vitiligo, alopecia areata
Introduction
Baricitinib (OlumiantTM) is a small-molecule, reversible competitive inhibitor of the Janus kinase (JAK) family.1 JAKs are intracellular tyrosine kinases linked to the intracellular domains of many cytokine receptors.2 Type 1 and type 2 cytokine families interact with specific JAK subtypes for signal transduction. When cytokines bind to their corresponding receptors, signal transduction occurs through the JAK/signal transducer and the activator of the transcription (STAT) pathway. JAKs mediate phosphorylation of specific receptor tyrosine residues. As docking sites for STAT proteins and other signalling molecules, they phosphorylate STAT proteins recruited to receptors via a single tyrosine residue. Activated STAT proteins separate from the receptor, dimerise, translocate to the nucleus, and combine with gamma-activated site members to regulate gene transcription.3
There are four JAK subtypes: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). Baricitinib mainly acts on JAK1 and JAK2 subtypes and has a weak potency against other subtypes.4 The adenosine triphosphate enzyme of JAK,5 which blocks the intracellular transduction of STAT proteins, is the target of baricitinib. The molecular structure of baricitinib is C16H17N7O2S (Figure 1), with a molecular weight of 371.42 g/mol. On 13 February 2017 the European Union approved baricitinib for sale in the local market for treatment of rheumatoid arthritis (RA),1 and in 2018, it entered the United States market for treatment of RA.6 Subsequently, it was approved by China for patients with moderate to severe rheumatoid arthritis unresponsive to treatment with other traditional DMARDs (disease-modifying anti-rheumatic drugs). Notably, baricitinib can also be used to treat atopic dermatitis (AD), systemic lupus erythematosus, and other diseases.
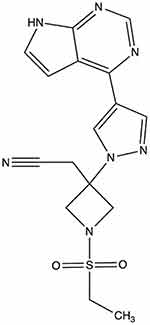 |
Figure 1 Molecular structure of baricitinib. |
Clinical Application
Baricitinib is effective for the treatment of chronic inflammatory diseases, especially rheumatoid arthritis and systemic lupus erythematosus. Recently, it has been reported to have a positive effect on the inflammatory storm associated with the development of coronavirus disease 2019.7 In addition, a few reports have shown that baricitinib has a beneficial effect in the treatment of interferon (IFN)-related diseases8 diabetic nephropathy,9 and refractory juvenile dermatomyositis.10
Application of Baricitinib in Dermatology
Baricitinib has been widely used in dermatology as a new molecular-targeted therapy. Increasing evidence suggests that baricitinib is effective against AD, alopecia areata (AA), psoriasis, and vitiligo. (Table 1) Many inflammatory dermatoses are driven by inflammatory mediators that rely on JAK/STAT signals, and the use of JAK inhibitors has become a new strategy for the treatment of diseases for which conventional drugs have not been effective.11
 |
Table 1 The Application of Baricitinib in Dermatology. vIGA-AD (0, 1): Validated Investigator’s Global Assessment of AD (Complete or Almost Complete Removal of Lesions) |
Atopic Dermatitis
AD is one of the most common chronic inflammatory skin diseases. Elevated inflammatory cytokines in AD affected skin include Th2 (interleukin [IL]-4, IL-13, IL-31, IL-5), Th22 (IL-22), Th1, and thymic stromal lymphopoietin (TSLP).12 These cytokines are associated with increased signalling through all four JAKs. IL-4 and IL-13 bind to IL-4 (either the α or γ chain) and IL-13 (α1) receptors to induce JAK1 and JAK3, respectively, resulting in the activation of STAT6. IL-5 binds to the IL-5 receptor (β chain), thus inducing the expression of JAK1 and JAK2, resulting in the activation of STAT1, STAT2, and STAT5. In addition, TSLP binds to the α unit of the IL-7 heterodimer and TSLP receptor and induces the activation of JAK1 and JAK2, thus activating STAT5.13 Studies have shown that 4 mg of baricitinib combined with glucocorticoids significantly improves the signs and symptoms of moderate to severe AD in adults, with rapid effects and good safety.14
Psoriasis
Psoriasis is a chronic inflammatory skin disease characterised by a high division of keratinocytes. The increased proliferation of keratinocytes is caused by high levels of inflammatory cytokines. The IL-23/Th17 axis plays a key role in psoriasis pathogenesis.15 The IL-23 receptor relies on the heterodimer of JAK2 and TYK2 for signal transduction, which highlights the role of JAKs in the pathogenesis of psoriasis and the therapeutic potential of JAK inhibitors.16 An animal experiment showed that local treatment with baricitinib inhibited the expression of inflammatory markers upregulated by 12-O-tetradecyl phorbol-13-acetate. The injection of baricitinib into mouse ears significantly reduced ear swelling, leukocyte infiltration, epidermal cell proliferation, and dermal angiogenesis. Moreover, baricitinib significantly decreased the phosphorylation of STAT3 and STAT1 and the expression of inflammatory cytokines.17 A double-blind controlled study showed a significant improvement in the incidence and prevention of moderate to severe psoriasis in patients with a 75% reduction in the psoriasis area and severity index (PASI)-75 after 12 weeks of treatment with 8 mg or 10 mg baricitinib compared to patients taking a placebo. The majority of patients reached PASI-75 after the first 12 weeks of baricitinib treatment and maintained this response for the following 12 weeks.18 Of note, there is a case report of a patient with rheumatoid arthritis who developed reverse psoriasis while being treated with baricitinib. This may be due to the baricitinib-induced enhancement of IL6, IL8 (C-X-C motif ligand [CXCL]-8), and IL36 gamma gene expression.19
Vitiligo
The IFN-γ-related chemokine CXCL-10 is involved in the pathogenesis of vitiligo, and IFN-γ signalling is mediated by the JAK/STAT pathway, especially through JAK1 and JAK2. JAK inhibitors can block this pathway, thereby blocking the effects of IFN and CXCL-10. Interestingly, a patient with rheumatoid arthritis and vitiligo showed a reduction in the area of vitiligo lesions after treatment with baricitinib.20,21
Alopecia Areata
Etiology, Pathogenesis, and Advanced Treatments
AA is a polygenic autoimmune disease characterised by temporary scarless alopecia and follicular preservation, affecting nearly 2% of the general population at some point in their lives. AA is divided into four subtypes: ophiasis, sisaipho, sudden greying, and diffuse AA. Many inflammatory cells such as CD8+ T cells, mast cells, and natural killer (NK) cells have been observed in AA tissues. These inflammatory cells attack the growing hair and cause hair loss.22 The primary treatment for patients with small lesions includes the use of topical glucocorticoids, topical injection of glucocorticoids, contact immunotherapy, and topical use of minoxidil. Systemic use of glucocorticoids and immunosuppressants can be recommended for patients in advanced AA or those displaying large lesions.23 However, traditional treatments have limited effects in patients with AA totalis or universalis. Therefore, molecular-targeted drugs have emerged.
The Mechanism of Action of Baricitinib
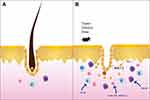
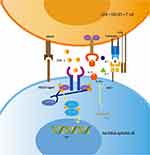
AA is an autoimmune disease caused by disturbances in follicular immune privilege, a state of protection of certain sites of the body which prevents an inflammatory immune response when exposed to antigens.24 Low expression of major histocompatibility (MHC) class I and II molecules in the normal population exempts hair follicles from autoimmune attack.25 In some conditions, such as cancer, infection, or stress, immune functions are inhibited through a decline in transforming growth factor β1 (TGFβ1), insulin-like growth factor 1(IGF-1), and α melanocyte-stimulating hormone (α-MSH). T lymphocytes, NK cells, and other inflammatory cells;26 in particular, CD8+ NKG2D+ T lymphocytes congregate around hair follicles.22 These cells can produce large amounts of IFN-γ, resulting in upregulation of MHC class I expression in the human follicle epithelium (Figure 2). Consequently, the immune privilege of follicles is compromised.27 CD8+ NKG2D+ T cells play an important role in the genetic development of AA. In 2010, a genome-wide association study (GWAS) found that the cytomegalovirus UL16-binding protein (ULBP) gene cluster on chromosome 6q25.1, which encodes the activating ligands of the natural killer cell receptor NKG2D, is strongly associated with AA. During the active phase of this disease, expression of the ULBP gene cluster in the diseased scalp, particularly in the hair follicle dermal sheath, of AA patients was significantly upregulated.28 IFN-γ acts on the IFN-γ receptor in hair follicle epithelial cells, which relies on the JAK/STAT signalling pathway. Simultaneously, CD8+ T cells produce IFN-γ, which signals JAK1 and JAK2 to enhance IL-15 production. After binding to the IL-15a receptor (a chaperone protein), IL-15 binds to the surface of CD8+ T cells and activates IFN-γ production through JAK1 and JAK3 signalling.22 This causes the hair follicles to break down and massive numbers of lymphocytes to attack the epithelial cells of the hair follicles, causing hair loss. IFN-γ is considered the main immune factor that causes AA. Each cytokine receptor is linked to two parallel JAK isomers that exist as homodimers or heterodimers.4 When the IFN-γ receptor receives the signal, the JAK enzyme located in the intracellular part cannot be phosphorylated, so it cannot conduct intracellular signal transduction (Figure 3). Therefore, the IFN-γ signalling pathway, which controls the immune response, is blocked.
Clinical Research
A Phase II randomized controlled study where baricitinib was used to treat adult AA showed that 33.3% and 51.9% of patients with AA had alopecia tool scores of <20 at 36 weeks after oral administration of 2 mg and 4 mg dose, respectively, and baricitinib was well-tolerated.29 One case report of a patient with AA, who lost all of her hair after a local injection of triamcinolone acetonide, showed that 97% of the scalp hair, eyebrows, and eyelashes regrew after receiving 2 mg baricitinib treatment. This patient received treatment for 13 months without any adverse effects.30 In another study, the initial dose of baricitinib was 7 mg/day. After six months, the dose was changed to 7 mg in the morning and 4 mg in the evening, and dose of oral corticosteroids was gradually reduced to 3 mg/day for patients with AA. After nine months, the hair of the patients recovered completely. In follow-up animal experiments, mice treated with baricitinib showed a significant reduction in inflammation, decreased CD8+ T cell infiltration, and decreased expression of MHC class I and II. The results of an IFN gene expression assay showed that IFN gene expression returned to normal after using baricitinib,31 which again demonstrates the role of baricitinib in modulating MHC class I and II expression through the IFN pathway.
Perspective
Baricitinib is a first generation JAK1/2 inhibitor. JAK is at the end of the cytokine receptors located in the cell membrane and controls the signal transduction of many cytokines, such as the IL-6, IL-10, IL-3, and IL-5 families.2 Each cytokine receptor is linked to two parallel isomers of JAK that exist as homodimers or heterodimers. When cytokines bind to their receptors, JAK phosphorylation occurs, which leads to the phosphorylation of STAT proteins in cells. Subsequently, these proteins are transported to the nucleus to act directly on the cell’s DNA and ultimately regulate gene expression.2 Signal transmission is directed from the outside to the inside of the cell. Chronic inflammatory diseases associated with upstream cytokine disorders can be treated with baricitinib. In addition, long-term treatment with low dose baricitinib was well tolerated, and only few patients reported serious adverse reactions and complications after baricitinib treatment. Baricitinib is currently approved for treatment of rheumatoid arthritis; however, numerous animal and clinical trials have confirmed its role in other chronic inflammatory diseases. AA is an immune disorder, and traditional treatments are inadequate for some AA patients with a large shedding area. Baricitinib has shown good potential for the treatment of intractable AA.
The first generation of JAK inhibitors have the side effects of immunosuppression. Studies on JAK-deficient mice suggest that offspring without JAK are not viable and that JAK3 knockout (KO) mice present a strong reduction in T and B cell numbers. Mutations in the JAK3 gene manifest as severe combined immunodeficiency syndrome (SCID) in humans.32 Some clinical studies have confirmed that the application of JAK inhibitors can have side effects, such as infection, malignancy, and major adverse cardiovascular events (MACEs). A long-term study of 3770 patients with rheumatoid arthritis receiving baricitinib treatment showed that the standardized incidence ratios (IRs) of severe infection, herpes zoster, and MACEs were 2.6, 3.0, and 0.5, respectively. The IR of malignant tumours was 0.6 in the first 48 weeks, and remained stable thereafter (IR 1.0).33 Clinical disease screening of patients before application of JAK inhibitors and continuous monitoring during application are essential.34 Some experts believe that more targeted second-generation JAK inhibitors for only one subtype or topical application show better effects and lower adverse reactions.35,36
Statement
This study was exempted from ethics requirements which was approved by Beijing Chaoyang Hospital.
Acknowledgments
We would like to thank Editage for English language editing.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Markham A. Baricitinib: first global approval. Drugs. 2017;77(6):697–704. doi:10.1007/s40265-017-0723-3
2. O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi:10.1146/annurev-med-051113-024537
3. Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285(1–2):1–24. doi:10.1016/s0378-1119(02)00398-0
4. Choy EH. Clinical significance of Janus kinase inhibitor selectivity. Rheumatol. 2019;58(6):1122. doi:10.1093/rheumatology/kez002
5. Xin P, Xu X, Deng C, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 2020;80:106210. doi:10.1016/j.intimp.2020.106210
6. Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17(7):460. doi:10.1038/nrd.2018.112
7. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi:10.1056/NEJMoa2031994
8. Sanchez GAM, Reinhardt A, Ramsey S, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. 2018;128(7):3041–3052. doi:10.1172/JCI98814
9. Tuttle KR, Brosius FC, Adler SG, et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant. 2018;33(11):1950–1959. doi:10.1093/ndt/gfx377
10. Kim H, Dill S, O’Brien M, et al. Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann Rheum Dis. 2020. doi:10.1136/annrheumdis-2020-218690
11. Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736–744. doi:10.1016/j.jaad.2016.12.005
12. Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913–921.e9. doi:10.1016/j.jaad.2018.01.018
13. Rodrigues MA, Torres T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J Dermatolog Treat. 2020;31(1):33–40. doi:10.1080/09546634.2019.1577549
14. Reich K, Kabashima K, Peris K, et al. Efficacy and safety of Baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–1343. doi:10.1001/jamadermatol.2020.3260
15. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397(10281):1301-1315. doi: 10.1016/S0140-6736(20)32549-6
16. Kvist-Hansen A, Hansen PR, Skov L. Systemic treatment of psoriasis with JAK inhibitors: a review. DermatolTher. 2020;10(21):29–42. doi:10.1007/s13555-019-00347-w
17. Hiraganahalli Bhaskarmurthy D, Evan Prince S. Effect of Baricitinib on TPA-induced psoriasis like skin inflammation. Life Sci. 2021;279:119655. doi:10.1016/j.lfs.2021.119655
18. Papp KA, Menter MA, Raman M, et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2016;174(6):1266–1276. doi:10.1111/bjd.14403
19. Di Domizio J, Castagna J, Algros MP, et al. Baricitinib-induced paradoxical psoriasis. J Eur Acad Dermatol Venereol. 2020;34(8):e391–e393. doi:10.1111/jdv.16293
20. Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61(4):374–376. doi:10.1111/ajd.13348
21. Qi F, Liu F, Gao L. Janus kinase inhibitors in the treatment of vitiligo: a review. Front Immunol. 2021;12:790125. doi:10.3389/fimmu.2021.790125
22. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1–12. doi:10.1016/j.jaad.2017.04.1141
23. Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British association of dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166(5):916–926. doi:10.1111/j.1365-2133.2012.10955.x
24. Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018;179(5):1033–1048. doi:10.1111/bjd.16808
25. Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi:10.1038/nrdp.2017.11
26. Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366(16):1515–1525. doi:10.1056/NEJMra1103442
27. Gilhar A, Laufer-Britva R, Keren A, Paus R. Frontiers in alopecia areata pathobiology research. J Allergy Clin Immunol. 2019;144(6):1478–1489. doi:10.1016/j.jaci.2019.08.035
28. Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–117. doi:10.1038/nature09114
29. King B, Ko J, Forman S, et al. Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: phase 2 results from a randomized controlled study. J Am Acad Dermatol. 2021;85(4):847–853. doi:10.1016/j.jaad.2021.05.050
30. Olamiju B, Friedmann A, King B. Treatment of severe alopecia areata with baricitinib. JAAD Case Rep. 2019;5(10):892–894. doi:10.1016/j.jdcr.2019.07.005
31. Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor Baricitinib. EBiomedicine. 2015;2(4):351–355. doi:10.1016/j.ebiom.2015.02.015
32. Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847. doi:10.3389/fimmu.2019.02847
33. Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis. 2021;81(3):335–343. doi:10.1136/annrheumdis-2021-221276
34. Atzeni F, Talotta R, Nucera V, et al. Adverse events, clinical considerations and management recommendations in rheumatoid arthritis patients treated with JAK inhibitors. Expert Rev Clin Immunol. 2018;14(11):945–956. doi:10.1080/1744666X.2018.1504678
35. Dai Z, Chen J, Chang Y, Christiano AM. Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI Insight. 2021;6:7. doi:10.1172/jci.insight.142205
36. Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14(6):1235–1242. doi:10.1021/acschembio.9b00188
37. Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: Results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85(1):62–70.
38. Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.