Back to Journals » Drug Design, Development and Therapy » Volume 9
Apparent Km of mitochondria for oxygen computed from Vmax measured in permeabilized muscle fibers is lower in water enriched in oxygen by electrolysis than injection
Authors Zoll J, Bouitbir J, Sirvent P, Klein A, Charton A, Jimenez L, Péronnet F, Geny B, Richard R
Received 30 January 2015
Accepted for publication 14 April 2015
Published 13 July 2015 Volume 2015:9 Pages 3589—3597
DOI https://doi.org/10.2147/DDDT.S81891
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Joffrey Zoll,1 Jamal Bouitbir,1 Pascal Sirvent,2 Alexis Klein,3 Antoine Charton,1,4 Liliana Jimenez,3 François R Péronnet,5 Bernard Geny,1 Ruddy Richard6
1Physiology Department, Faculty of Medicine and EA3072, Université de Strasbourg, Strasbourg, 2Clermont Université, Université Blaise Pascal, EA 3533, Laboratoire des Adaptations Métaboliques à l’Exercice en Conditions Physiologiques et Pathologiques, Clermont-Ferrand, 3Danone Research, Centre Daniel Carasso, Palaiseau, 4Department of Anesthesia and Critical Care and EA3072, Hôpital de Hautepierre, Université de Strasbourg, France; 5Kinesiology Department, Université de Montréal, Montréal, QC, Canada; 6Department of Sport Medicine and Functional Explorations and INRA UMR 1019, Faculty of Medicine, Université d’Auvergne, Clermont-Ferrand, France
Background: It has been suggested that oxygen (O2) diffusion could be favored in water enriched in O2 by a new electrolytic process because of O2 trapping in water superstructures (clathrates), which could reduce the local pressure/content relationships for O2 and facilitate O2 diffusion along PO2 gradients.
Materials and methods: Mitochondrial respiration was compared in situ in saponin-skinned fibers isolated from the soleus muscles of Wistar rats, in solution enriched in O2 by injection or the electrolytic process 1) at an O2 concentration decreasing from 240 µmol/L to 10 µmol/L (132 mmHg to 5 mmHg), with glutamate–malate or N, N, N', N'-tetramethyl-p-phenylenediamine dihydrochloride (TMPD)–ascorbate (with antimycin A) as substrates; and 2) at increasing adenosine diphosphate (ADP) concentration with glutamate–malate as substrate.
Results: As expected, maximal respiration decreased with O2 concentration and, when compared to glutamate–malate, the apparent Km O2 of mitochondria for O2 was significantly lower with TMPD–ascorbate with both waters. However, when compared to the water enriched in O2 by injection, the Km O2 was significantly lower with both electron donors in water enriched in O2 by electrolysis. This was not associated with any increase in the sensitivity of mitochondria to ADP; no significant difference was observed for the Km ADP between the two waters.
Conclusion: In this experiment, a higher affinity of the mitochondria for O2 was observed in water enriched in O2 by electrolysis than by injection. This observation is consistent with the hypothesis that O2 diffusion can be facilitated in water enriched in O2 by the electrolytic process.
Keywords: saponin-skinned fibers, mitochondrial respiration, glutamate–malate, TMPD–ascorbate, Km O2
Introduction
In the course of studying the possible biological effects of a water enriched in oxygen (O2) by a new electrolytic process,1 we have recently shown that, when compared to water enriched in O2 by injection, intragastric administration of water enriched in O2 by electrolysis lessened the slow decline in peripheral tissue oxygenation observed in anesthetized pigs.2 This observation is consistent with the hypothesis that O2 diffusion can be facilitated in waters enriched in O2 by electrolysis. It has been hypothesized that this phenomenon could be due to the fact that the electrolytic process could generate water susperstructures similar to clathrates,3–5 which could trap O2 molecules and reduce local pressure/content relationships for O2, thus facilitating O2 diffusion along PO2 gradients. Interestingly, a series of studies conducted in vitro and in animal models suggests that isotonic saline submitted to a process involving Taylor–Couette–Poiseuille flow in the presence of O2 (electrokinetically modified water [EMW]) can interfere with cell signaling pathways involved in inflammation, cell death, and survival, and could have beneficial effects in various situations where inflammation is present.6–11 In human, EMW tends to increase VO2max and reduces the rate of perceived exertion during aerobic exercise,7 significantly reduces muscle fatigue during resistance exercise,12 and prevents or attenuates muscle damage and inflammation in both types of exercise.12–14 The authors hypothesized that these biological effects of EMW can also be due to the fact that this water could contain “charge-stabilized nanostructures”.
The objective of the present study was to further investigate the biological effects of water enriched in O2 by electrolysis by comparing mitochondrial respiration (Vmax) studied in situ in permeabilized muscle fibers15 in waters enriched by injection and by the electrolytic process. The observations were made over a wide range of PO2 and O2 concentrations in order to compute the apparent Km of mitochondria for O2. Since the rate of O2 consumption by mitochondria depends on the flux of both O2 and electrons,16 the experiments were performed with glutamate–malate, as well as with tetramethyl-p-phenylenediamine (TMPD)-ascorbate, an artificial substrate that provides electrons to complex IV of the respiratory chain at a much larger rate than glutamate–malate.17 Under the hypothesis that O2 diffusion is facilitated in water enriched in O2 by the electrolytic process, when compared to the water enriched in O2 by injection, Km will be lower with both electron donors and the difference will be larger with TMPD–ascorbate than with glutamate–malate because of the higher flux of electrons. We also verified that the possible differences in mitochondrial respiration between the two waters and the two electron donors were not due to differences in the affinity of mitochondria to adenosine diphosphate (ADP) by measuring the apparent Km of mitochondria for ADP.
Materials and methods
Animal procedures were conducted in accordance with the Declaration of Helsinki and were approved by our local ethics committee Comité Régional d’Éthique en Matière d’Expérimentation Animale, (CREMEAS).
Mitochondrial respiration was studied in situ in muscle fibers permeabilized with saponin.15,18,19 As reviewed in detail by Kuznetsov et al,15 saponin is a detergent with a large affinity for cholesterol, which specifically destroys the cholesterol-rich sarcolemma (0.5 mmol cholesterol/mmol phospholipids) without altering the cholesterol-poor mitochondrial membrane (0.07 and 0.01 cholesterol/mmol phospholipids for the outer and inner mitochondrial membranes, respectively). The end result is a preparation with intracellular structures that are intact (mitochondrion, endoplasmic reticulum, myofilaments, and cytoskelton) within an intracellular space devoid of all solutes, which have been washed out, and in equilibrium with the incubation milieu.
Male Wistar rats (body mass ~300 g) were anesthetized (sodium pentobarbitate: 0.2 g/100 g body mass, intraperitoneally) and the soleus muscles were removed and placed in solution S (as will be discussed). Muscle fibers were immediately separated under binocular microscope and permeabilized with 50 μg/mL of saponin for 30 minutes at 4°C. After being placed for 10 minutes in solution R (as will be discussed) to wash out adenine nucleotides and creatine, skinned separated fibers were transferred in a 3 mL water-jacketed oxygraphic cell (Strathkelvin Instruments Limited, North Lanarkshire, Scotland) equipped with a Clark electrode, as previously described.20
Solutions R and S (prepared from tap water demineralized by reverse osmosis and then remineralized with Na2SO4 + Na3PO4, 12H2O) both contained 2.77 mM of CaK2EGTA, 7.23 mM of K2EGTA (100 nM of free Ca2+), 6.56 mM of MgCl2 (1 mM of free Mg2+), 20 mM of taurine, 0.5 mM of dithiothreitol, 50 mM of potassium methanesulfonate (160 mM ionic strength), and 20 mM of imidazole (pH 7.1). Solution S also contained 5.7 mM of Na2 ATP and 15 mM of creatine phosphate, while solution R contained 5 mM of glutamate, 2 mM of malate, 3 mM of phosphate, and 2 mg/mL of fatty acid-free bovine serum.
Mitochondrial respiration was measured in situ in solution R at 22°C starting with an initial O2 concentration of 240 μmol/L (PO2=133 mmHg).21 In the solution used as control, this concentration was obtained by injection. In the experimental solution, this concentration was obtained by electrolysis.1 In this process, remineralized water is pumped between two electrodes separated by a membrane permeable to electrical charges but not to gases, and the water enriched in O2, which is recovered on the negative electrode (pH =7.1–7.2; conductivity =750–770 μS/cm; 4,375–5,000 μmol O2/L), is used to prepare the final product. Both solutions, which were supplied by Danone Research, (Palaiseau, France), were prepared from demineralized water, which was remineralized with Na+ (200 mg/L), SO42− (250 mg/L), and PO42− (240 mg/L). It was suspected that the electrolytic process could produce ozone which, in turn, could interfere with mitochondrial function through the generation of reactive oxygen species. However, in line with the observation that ozone is quickly converted into O2 (2 O3 → 3 O2, with a half-life of only about 20 minutes), and because the solution enriched by electrolysis was prepared and conserved in sealed bottles several weeks before the experiment, the level of ozone was found to be very low (6.8±5.5 μg/L).
In the first series of experiments, after the determination of basal O2 consumption (nonstimulated respiration, V0), Vmax was measured under continuous stirring in the presence of saturating amounts of ADP (2 mM) as a phosphate acceptor and glutamate–malate as substrates (5 mM and 2 mM). The Vmax was monitored until the O2 content decreased from the initial value of 240 μmol/L to 10 μmol/L, corresponding to a PO2 of 5 mmHg21 – ie, a value close to the range of PO2 estimated in muscle cells (2–4 mmHg).22 In the second series of experiments, the protocol was similar, but complex III of the respiratory chain was blocked with antimycin A (6.5 μM), and N, N, N′, N′-tetramethyl-p-phenylenediamine dihydrochloride (TMPD; 0.5 mM) and ascorbate (0.5 mM) were added as artificial electron donor to cytochrome c. In the third series of experiments, mitochondrial respiration was measured at increasing concentrations of ADP (from 2.5 μmol/L to 1.5 mmol/L) and decreasing PO2 (from the initial value of 240 μmol/L to final values <10 μmol/L at the end of titration) using glutamate–malate as substrates (5 mM and 2 mM) and without creatine. Each of the first and second series of experiments was conducted on 12 preparations, while the third series of experiments was conducted on eleven preparations. Each preparation was obtained from a different animal.
In all the preparations, at the end of each experiment, cytochrome c was added to the oxygraphic cell. No increase in O2 consumption was observed in any of the preparations, confirming the integrity of the outer mitochondrial membrane.23 In addition, the acceptor control ratio (Vmax/V0) was computed for each preparation in the first series of experiments (glutamate–malate as substrates) and was found to be 3.7±0.3, which is in good agreement with values reported in the literature.24–26
The fibers were then harvested and dried, and respiration rates were expressed as μmol O2/min/g dry weight.
All reagents were purchased from Sigma-Aldrich Co. (St Louis, MO, USA), except ADP, which was obtained from Boehringer Ingelheim (Ingelheim, Germany).
Data are reported as the mean ± standard deviation. The apparent Km of mitochondria for O2 (for the two types of water and with the two electron donors) and for ADP (for the two types of water and glutamate–malate) were calculated using nonlinear regression of the individual relationships between O2 or ADP concentration and Vmax, using a Lineweaver–Burk plot. The apparent Km for O2 (Km O2) was compared using a two-way analysis of variance (ANOVA) (water × electron donor), while the apparent Km for ADP for the two waters was compared using one-way ANOVA. The level of significance was set at 0.05. All data are available in the Supplementary materials.
Results
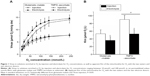
Figure 1A shows the increase in Vmax with O2 concentration measured for the two types of water and the two electron donors. As shown in Figure 1B, the Km O2 computed from these curves was significantly lower in the solution enriched in O2 by electrolysis than by injection, both with glutamate–malate (36.1±9.2 μmol/L versus 53.3±10.1 μmol/L, respectively; P<0.001) and TMPD–ascorbate as substrates (52.8±11.4 μmol/L and 90.3±10.7 μmol/L, respectively; P<0.001). The difference in Km O2 between the two waters cannot be computed for each preparation and, consequently, no statistical comparisons can be made. However, the average difference was larger with TMPD–ascorbate (37.5 μmol/L or a 41% difference) than with glutamate–malate (17.2 μmol/L, or a 32% difference).
No significant difference was observed for the apparent Km of mitochondria for ADP: 328±67 μmol/L and 303±70 μmol/L (P=0.4) between the water enriched in O2 by injection and electrolysis, respectively (Figure 2).
Discussion
Results from the present experiment show that, when compared to a solution enriched in O2 by injection, the apparent affinity for O2 of the mitochondria was higher in a solution enriched in O2 by the electrolytic process, the difference being larger with TMPD–ascorbate than glutamate–malate as electron donors. This observation is consistent with the hypothesis that O2 diffusion can be facilitated in water enriched in O2 by electrolysis.
As expected, Vmax increased with O2 concentration in a curvilinear fashion toward a plateau and was significantly higher with TMPD–ascorbate than with glutamate–malate, the difference decreasing slightly with O2 concentration (~14.3 μmol O2/min/g versus 7.8 μmol O2/min/g at 240 μmol/L, or a 75% difference; ~2.6 μmol O2/min/g versus 1.8 μmol O2/min/g at 10 μmol/L, or a 45% difference) (Figure 1A). The reduction in Vmax with O2 concentration was larger than that reported by Gnaiger and Kuznetsov27 with succinate and TMPD–ascorbate in isolated mitochondria because the apparent Km of cytochrome oxidase (COX) for O2 has been shown to be higher in saponin-skinned muscle fibers. As for the difference observed between glutamate–malate and TMPD–ascorbate, this phenomenon is known as the “cytochrome oxidase excess”16 and indicates that when O2 availability is very high, electron supply from glutamate–malate through the respiratory chain is much lower than could be processed by COX: when electrons are directly supplied to COX at a larger rate from TMPD–ascorbate, Vmax reaches higher values. However, the difference between the two electron donors decreased with O2 concentration. This observation is due to the fact that the control of O2 consumption in complex IV of the respiratory chain at any O2 concentration and over the range of electron supply by glutamate–malate and TMDP–ascorbate is shared by the availability of both O2 and electrons; when compared to glutamate–malate, in spite of the much larger electron flux from TMPD–ascorbate, the difference in Vmax between the two substrates decreases with O2 supply.
The main result from the present experiment is that for both electron donors, when compared to the water enriched in O2 by injection, a left shift in O2 consumption by the mitochondria was observed with the water enriched in O2 by electrolysis. Vmax plateau values were similar at the highest O2 concentration for a given electron donor (7.8 μmol O2/min/g for both water with glutamate–malate; 14.2 μmol O2/min/g and 14.7 μmol O2/min/g for water enriched in O2 by electrolysis and injection, respectively). However, for any given Vmax, consistently lower O2 concentrations were needed with the water enriched in O2 by electrolysis in the steepest portion of the curve, the difference between the two waters being slightly larger with TMPD–ascorbate than glutamate–malate as substrates. As a consequence, when compared to the water enriched in O2 by injection, the shift in the apparent Km O2 of the mitochondria with the water enriched in O2 by electrolysis was also larger.
The higher affinity of the mitochondria for O2 in water enriched in O2 by electrolysis was not associated with any increase in the sensitivity of the mitochondrial respiration to ADP since the apparent Km for ADP measured with glutamate–malate remained unaffected by the type of water used to prepare the solution. In addition, this phenomenon was observed with glutamate–malate, which provides electrons to COX through complex I to III of the respiratory chain complex, but also with TMDP–ascorbate, which directly provides electrons to COX. The reduction in the apparent Km of mitochondria in water enriched in O2 by injection thus suggests that for a given O2 concentration, O2 flux to COX is facilitated in the solution enriched in O2 with the electrolytic process. This phenomenon is not observed with any of the two electron donors at high O2 content because in these situations, the control of O2 consumption by COX depends mainly on the availability of electrons. It is only observed at lower O2 concentrations where the rate of O2 consumption by COX mainly depends on the availability of O2, and it was higher with TMPD–ascorbate, which supplies a larger flux of electrons than does glutamate–malate.
Conclusion
These findings are in line with our recent observation in pigs using intragastric administration of water enriched in O2 that, when compared to the water enriched in O2 by injection, the decrease in transcutaneous O2 pressure, which develops during anesthesia, was slower with the water enriched in O2 by electrolysis.2 Although the mechanisms behind these observations have to be elucidated, they are consistent with the hypothesis that O2 diffusion could be facilitated in water enriched in O2 by electrolysis.
Disclosure
François Péronnet and Ruddy Richard are occasional consultants for Danone Research. Alexis Klein and Liliana Jimenez are employees of Danone Research. The authors report no further conflicts of interest in this work.
References
Lacoste C, Brunner S, Jimenez L, Klein A, inventors; Lacoste C, Brunner S, Jimenez L, Klein A, assignees. Method for enriching water with oxygen by an electrolytic process, oxygen enriched water or beverage and uses thereof. United States Patent US 20110064824 A1. 2011 Mar 17. | ||
Charton A, Péronnet F, Doutreleau S, et al. Effect of administration of water enriched in O2 by injection or electrolysis on transcutaneous oxygen pressure in anesthetized pigs. Drug Des Devel Ther. 2014;8:1161–1167. | ||
Chaplin MF. A proposal for the structuring of water. Biophys Chem. 2000;83(3):211–221. | ||
Ozeki S, Otsuka I. Transient oxygen clathrate-like hydrate and water networks induced by magnetic fields. J Phys Chem B. 2006;110(41):20067–20072. | ||
Wu HF, Chin CC, Liu BM, et al. Self-assembly formation of the magic ion of (H2O)20O+: observation of nanoscale cages of oxygenated water clusters induced from iron nanoparticles. Rapid Commun Mass Spectrom. 2011;25(3):410–414. | ||
German S, Ghosh S, Mega TL, et al. Isotonic saline subjected to Taylor-Couette-Poiseuille flow demonstrates anti-inflammatory activity in vitro. J Allergy Clin Immunol. 2011;127(2 Suppl):AB260. | ||
Ghosh S, Mega TL, German S, et al. Isotonic saline subjected to Taylor-Couette-Poiseuille flow demonstrates anti-inflammatory activity in a rat model of allergic asthma. J Allergy Clin Immunol. 2011;127(2 Suppl):AB84. | ||
Ghosh S, Jana A, Watson R, Pahan K. Inhibition of tau phosphorylation by modified saline containing charge-stabilized nanostructures: implications for Alzheimer’s disease. Neurology. 2011;76(9):SA229. | ||
Khasnavis S, Jana A, Roy A, et al. Suppression of nuclear factor-κB activation and inflammation in microglia by physically modified saline. J Biol Chem. 2012;287(35):29529–29542. | ||
Khasnavis S, Roy A, Ghosh S, Watson R, Pahan K. Protection of dopaminergic neurons in a mouse model of Parkinson’s disease by a physically-modified saline containing charge-stabilized nanobubbles. J Neuroimmune Pharmacol. 2014;9(2):218–232. | ||
Mondal S, Martinson JA, Ghosh S, Watson R, Pahan K. Protection of Tregs, suppression of Th1 and Th17 cells, and amelioration of experimental allergic encephalomyelitis by a physically-modified saline. PLoS One. 2012;7(12):e51869. | ||
Larkin KA, Borsa PA. Consumption of a novel, carbohydrate-free recovery beverage attenuates strength loss after high intensity resistance exercise. Med Sci Sports Exerc. 2012;44(5S):595. | ||
Cooper E, Feutz B, Dudley B, Burke LM, Watson RL. Consumption of Revalesio sports beverage alters markers of exercise performance and cardio-respiratory fitness in healthy males. Med Sci Sports Exerc. 2011;43(5):644. | ||
Borsa PA, Kaiser KL, Martin JS. Oral consumption of electrokinetically modified water attenuates muscle damage and improves postexercise recovery. J Appl Physiol (1985). 2013;114(12):1736–1742. | ||
Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3(6):965–976. | ||
Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol. 1998;201(Pt 8):1129–1139. | ||
Jacobs EE, Sanadi DR. Phosphorylation coupled to electron transport mediated by high potential electron carriers. Biochim Biophys Acta. 1960;38:12–34. | ||
Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987;892(2):191–196. | ||
Saks VA, Veksler VI, Kuznetsov AV, et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem. 1998;184(1–2):81–100. | ||
Richmond KN, Burnite S, Lynch RM. Oxygen sensitivity of mitochondrial metabolic state in isolated skeletal and cardiac myocytes. Am J Physiol. 1997;273(5 Pt 1):C1613–C1622. | ||
Lide DR, Frederikse HPR. Solubility of selected gases in water. In: CRC Handbook of Chemistry and Physics. 77th ed. New York, NY: CRC Press; 1997:6-3–6-4. | ||
Richardson RS, Noyszewski EA, Leigh JS, Wagner PD. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol (1985). 1998;85(2):627–634. | ||
Saks VA, Vasil’eva E, Belikova YuO, et al. Retarded diffusion of ADP in cardiomyocytes: possible role of mitochondrial outer membrane and creatine kinase in cellular regulation of oxidative phosphorylation. Biochim Biophys Acta. 1993;1144(2):134–148. | ||
De Sousa E, Veksler V, Bigard X, Mateo P, Ventura-Clapier R. Heart failure affects mitochondrial but not myofibrillar intrinsic properties of skeletal muscle. Circulation. 2000;102(15):1847–1853. | ||
Sanchez H, Zoll J, Bigard X, et al. Effect of cyclosporin A and its vehicle on cardiac and skeletal muscle mitochondria: relationship to efficacy of the respiratory chain. Br J Pharmacol. 2001;133(6):781–788. | ||
Risson V, Mazelin L, Roceri M, et al. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol. 2009;187(6):859–874. | ||
Gnaiger E, Kuznetsov AV. Mitochondrial respiration at low levels of oxygen and cytochrome c. Biochem Soc Trans. 2002;30(2):252–258. |
Supplementary materials
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.