Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Apolipoprotein E e4 allele is associated with extrapyramidal symptoms in Alzheimer’s disease
Authors Chang YP , Chou MC , Lai CL, Chien I, Yang YH
Received 1 March 2019
Accepted for publication 14 May 2019
Published 9 July 2019 Volume 2019:15 Pages 1915—1919
DOI https://doi.org/10.2147/NDT.S207050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yang-Pei Chang,1 Mei-Chuan Chou,1–3 Chiou-Lian Lai,2,4–5 I Chien,1 Yuan-Han Yang1,2
1Department of Neurology, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan; 2Department of Neurology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan; 3Graduate Institute of Clinical Medicine, Kaohsiung Medical University, Kaohsiung City, Taiwan; 4Department of Neurology, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan; 5Department of Neurology, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
Background: Extrapyramidal symptoms (EPS) are not uncommon in Alzheimer’s disease (AD). As apolipoprotein E(APOE) e4 allele is a major risk factor for late-onset AD, we intend to examine the association between APOE genotype and the development of EPS in AD.
Method: This study describes two hundred and fifty-five clinically diagnosed AD patients aged 72 to 80 years from 2010 to 2014. We reviewed the medical charts to determine the development of EPS. APOE genotypes were also confirmed.
Results: APOE e4 allele was detected in 74 patients (29%) and rigidity was among the most common EPS (61%). After adjusting the age, gender, baseline clinical dementia rating, we found AD patients carrying APOE e4 allele are more likely to develop EPS (OR: 4.515, p=0.033).
Conclusion: This study demonstrates the higher coexistence of EPS in AD patients with APOE e4 allele. Furthermore, the identification of APOE e4 allele in the development of EPS in AD patients supports the hypothesis that EPS may be partially attributed to AD pathology.
Keywords: Alzheimer’s disease, extrapyramidal symptoms, apolipoprotein e e4
Introduction
Alzheimer’s disease (AD) patients may develop EPS, alternatively termed parkinsonian-like symptoms or motor symptoms. This heterogeneous array of symptoms affects an estimated one-third of patients with clinically diagnosed AD; the symptoms include hypokinesia, rigidity, tremor, and gait disturbance.1,2,3 As mild cognitive impairment may be associated with an increased risk of AD, poorer lower-limb performance or gait disturbance may also increase the risk of AD in patients with mild cognitive impairment.4 In addition, EPS are associated with more a rapid development of AD, a heightened risk of poor prognosis, and earlier institutionalization.5 Pathological evidence has disclosed that the presence of EPS was postulated to represent neurofibrillary tangles (NFTs) or Lewy body accumulation across the neural systems.6,7 The presence of the APOE e4 allele may be also associated with the onset of AD. AD may be linked to amyloid trafficking and plague clearance.8,9,10 however, the few studies that have investigated AD with APOE e4 allele have converged to reveal an estimated duration from disease diagnosis to the occurrence of EPS. In this study, we explore the influence of APOE e4 allele on the occurrence of EPS in a hospital-based cohort of AD patients.
Materials and methods
The data used in our analysis came from a study of 255 patients aged 70–90 years old, recruited from the outpatient clinic at Kaohsiung Ta-Tung Municipal Hospital. This investigation into the relationship between genes and AD was carried out from 2010 to 2014 and was approved by the Kaohsiung Ta-Tung Municipal Hospital Institutional Review Board (IRB-KMTTH-E(I)-20160142). All patients provided written informed consent, and that this study was conducted in accordance with the Declaration of Helsinki. Information on current medical conditions and any medications being taken by the patients was obtained. Two specialists (Yang YH, Chou MC) performed complete neurological examinations and cognitive function assessments on all patients. The motor symptoms of bradykinesia, rigidity, tremor, and gait disturbance were determined from a complete chart review (Chang YP). We also ascertained and excluded patient histories of neuroleptic, antidepressant, or other medications known to cause EPS. Patients were given a battery of neuropsychological tests, and individuals showing evidence of clinically significant cognitive impairment were determined as having AD by both our specialists (Yang YH, Chou MC) and neuropsychologist (Hsu CL).
APOE genotyping
Peripheral blood leukocytes were extracted for genomic DNA testing using a QIAamp blood kit (QIAGEN). A polymerase chain reaction (PCR) amplified exon 4 of the APOE gene with an upstream primer, 50 - TCGCGGGCCCCCGGGCCTGGTACA-30, and a downstream primer, 50 - ACAGAATTCGCCCCGGCCTGGTACACTGCCA-30. The PCR products were digested with HhaI, and the fragments were separated by electrophoresis on a 6% polyacrylamide gel and ethidium bromide staining. The DNA fragments were visualized by UV illumination. The determination of APOE genotypes was carried out in a blinded fashion by scoring for a special combination of fragment sizes. Allele frequencies were estimated by counting the alleles and calculating sample proportions.
Statistics
A survival analysis model was constructed using the occurrence of EPS in all AD patients as an endpoint. In the analysis, we controlled age, gender, clinical dementia rating (CDR), and at least one copy of APOE allele. Beyond the descriptive analysis, we also performed a comparative analysis using the Cox regression model to assess the effects of these criteria on the occurrence of EPS in AD patients.
Results
For the present study, we selected 255 patients with a mean MMSE of 16.6, and a mean number of seven years spent in education. Among these patients, 29% (n=74) were found to carry at least one E4 allele. (Table 1) Based on the definition of the EPS of AD from previous published articles,2 the percentages of hypokinesia, rigidity, gait disturbance, and tremor were 53%, 61%, 46%, and 15%, respectively. The percentage of patients found to have at least 1 e4 allele was 46%, which was higher in AD patients with EPS than the 29% found in AD patients without EPS. The Kaplan-Meier survival curve suggests that EPS occur three years after AD is clinically diagnosed. (Figure 1) Using the Cox regression model to control age, gender, and baseline CDR at the time of AD diagnosis, a significant increase of risk (odds ratio: 4.541, p=0.033) was discovered in the APOE genotype carrying e4 allele (Table 2).
 |
Table 1 Demographic data of alzheimer’s disease patients |
 |
Table 2 Adjusted factors contributing to the development of EPS in alzheimer’s disease patients |
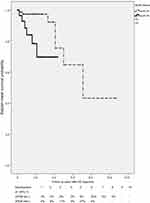 |
Figure 1 Kaplan-Meier survival curve of AD developing EPS. Log rank test (t=8.14, p=0.004). Abbreviations: APOE, Apolipoprotein E; AD, Alzheimer’s disease. |
Discussion
The current study examines the rate and frequency of APOE e4 allele carrier status in subjects with probable AD, who were separated into two groups: AD with EPS and AD without EPS. The distribution of EPS in our study is similar to that of previous reports.2
Several findings are of particular interest. First, the prevalence of EPS symptoms in our study (5%) were much lower than in previous reports (6–50%),11 and thus, our results should be interpreted with caution; however, three specialists and one psychologist verified all of our AD patients as being properly diagnosed according to the common AD criteria,12 and this allowed us to be confident with the accuracy of our diagnosis of AD, and EPS may be underestimated because of the relatively strict evaluations. Second, the AD patients carrying the APOE e4 allele may be more prone to developing EPS. The APOE e4 allele is associated with the development of NFTs in AD.13 Although the pathogenic role of NFTs in the development of EPS remains to be elucidated, neuropathological evidence suggests that AD patients with EPS may be subject to a different distribution of NFTs;6 and that the neuronal loss in substantia nigra, partly related to tau lesions, is a major pathological substrate of parkinsonian symptoms in AD patients.14,15 In contrast, pathological evidence shows the APOE e4 allele to be over-represented in Lewy body variants of AD patients; furthermore, coexisting Lewy body pathology has been found among AD patients.16,17 While previous studies show that APOE e4 alleles in AD were less likely to develop EPS,2 Iqbal et al reported that the presence of EPS may not only be useful in discriminating between AD and non-AD controls, but also exhibits a distinct distribution in a subgroup defined by late-onset AD with the presence of one or two APOE e4 alleles.18 Because the APOE e4 allele is also associated with higher frequencies of neuritic and diffuse amyloid plaques in AD patients, it is therefore reported in line with severity in AD pathological findings.20
Our study requires several caveats. First, our case numbers are relatively small compared to those of previous studies, and our study are based on clinical diagnosis of probable AD, without neuropathological examination. Second, although excluding the possibility of drug-induced events by analyzing detailed histories of previous neuroleptic or antiparkinsonian medications for all the patients in the present study, it should not be ruled out that EPS might be related to medication. Third, the possibility of Lewy body dementia cannot be excluded because of the lack of neuropathology data;19 however, our strict diagnosis of AD patients and Lewy body dementia was excluded if EPS like parkinsonian symptoms occurred within one year of initial dementia diagnosis, in which case this issue would be rendered obsolete. The presence of small-vessel cerebrovascular disease cannot be excluded because not every AD patient takes brain magnetic resonance imaging;21 however, history of previous cerebrovascular disease and brain-computed tomography in AD patients was reviewed at the diagnosis stage.
In conclusion, APOE e4 allele may be associated increased risk of EPS in AD patients. Further large-scale longitudinal study may be performed to confirm the linkage.
Acknowledgments
We are thankful for the suggestions of Miss Yu-Han Chang on the statistical analysis. This work was supported by the grant from Kaohsiung Municipal Ta-Tung Hospital. (KMTTH-102-006).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Clark CM, Ewbank D, Lerner A, et al. The relationship between extrapyramidal signs and cognitive performance in patients with Alzheimer’s disease enrolled in the CERAD study. Neurology. 1997;49:70–75. doi:10.1212/wnl.49.1.70
2. Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, et al. Motor signs during the course of alzheimer disease. Neurology. 2004;63:975–982. doi:10.1212/01.WNL.0000138440.39918.0C
3. Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonianlike signs and risk of incident alzheimer disease in older persons. Arch Neurol. 2003;60:539–544. doi:10.1001/archneur.60.4.539
4. Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident alzheimer disease. Arch Neurol. 2006;63:1763–1769. doi:10.1001/archneur.63.12.1763
5. Stern Y, Albert M, Brandt J, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in alzheimer’s disease: prospective analyses from the predictors study. Neurology. 1994;44:2300–2307. doi:10.1212/wnl.44.9.1715
6. Hulette C, Mirra S, Wilkinson W, Heyman A, Fillenbaum G, Clark C. The consortium to establish a registry for alzheimer’s disease (CERAD) part IX. A prospective cliniconeuropathologic study of Parkinson’s features in alzheimer’s disease. Neurology. 1995;45:1991–1995. doi:10.1212/WNL.45.11.1991
7. Liu WK, Le TV, Adamson J, et al. Relationship of the extended tau haplotype to tau biochemistry and neuropathology in progressive supranuclear palsy. Ann Neurol. 2001;50:494–502. doi:10.1002/ana.1159
8. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in alzheimer disease. Arch Neurol. 2005;62:1601–1608. doi:10.1001/archneur.62.10.1601
9. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi:10.1126/science.8346443
10. Sperling R, Mormino E, Johnson K. The evolution of preclinical alzheimer’s disease: implications for prevention trials. Neuron. 2014;84:608–622. doi:10.1016/j.neuron.2014.10.038
11. Ellis RJ, Caligiuri M, Galasko D, Thal LJ. Extrapyramidal motor signs in clinically diagnosed alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:103–114. doi:10.1097/00002093-199601020-00008
12. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984;34:939–944. doi:10.1212/WNL.34.7.939
13. Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi:10.1038/nrneurol.2012.263
14. Attems J, Quass M, Jellinger KA. Tau and alpha-synuclein brainstem pathology in alzheimer disease: relation with extrapyramidal signs. Acta Neuropathol. 2007;113:53–62. doi:10.1007/s00401-006-0146-9
15. Liu Y, Stern Y, Chun MR, Jacobs DM, Yau P, Goldman JE. Pathological correlates of extrapyramidal signs in Alzheimer’s disease. Ann Neurol. 1997;41:368–374. doi:10.1002/(ISSN)1531-8249
16. Galasko D, Saitoh T, Xia Y, et al. The apolipoprotein E allele epsilon 4 is overrepresented in patients with the lewy body variant of alzheimer’s disease. Neurology. 1994;44:1950–1951. doi:10.1212/wnl.44.9.1715
17. Kobayashi S, Tateno M, Park TW, et al. Apolipoprotein E4 frequencies in a Japanese population with Alzheimer’s disease and dementia with lewy bodies. PLoS One. 2011;6:e18569. doi:10.1371/journal.pone.0018569
18. Iqbal K, Flory M, Soininen H. Clinical symptoms and symptom signatures of alzheimer’s disease subgroups. J Alzheimers Dis. 2013;37:475–481. doi:10.3233/JAD-130212
19. Kaur B, Harvey DJ, DeCarli CS, Zhang L, Sabbagh MN, Olichney JM. Extrapyramidal signs by dementia severity in alzheimer disease and dementia with lewy bodies. Alzheimer Dis Assoc Disord. 2013;27:226–232. doi:10.1097/WAD.0b013e31826f040d
20. Monsell SE, Besser LM, Heller KB, Checkoway H, Litvan I, Kukull WA. Clinical and pathologic presentation in parkinson’s disease by apolipoprotein e4 allele status. Parkinsonism Relat Disord. 2014;20:503–507. doi:10.1016/j.parkreldis.2014.02.001
21. Yang Y, Fuh J, Mok VCT. Vascular contribution to cognition in stroke and alzheimer’s disease. Brain Sci Adv. 2018;4:39–48. doi:10.26599/BSA.2018.9050001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
