Back to Journals » Cancer Management and Research » Volume 9
APF530 versus ondansetron, each in a guideline-recommended three-drug regimen, for the prevention of chemotherapy-induced nausea and vomiting due to anthracycline plus cyclophosphamide–based highly emetogenic chemotherapy regimens: a post hoc subgroup analysis of the Phase III randomized MAGIC trial
Authors Schnadig ID, Agajanian R, Dakhil C, Gabrail N, Vacirca J, Taylor C, Wilks S, Braun E, Mosier MC, Geller RB, Schwartzberg L , Vogelzang N
Received 30 November 2016
Accepted for publication 23 March 2017
Published 19 May 2017 Volume 2017:9 Pages 179—187
DOI https://doi.org/10.2147/CMAR.S129059
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Ian D Schnadig1, Richy Agajanian2, Christopher Dakhil3, Nashat Gabrail4, Jeffrey Vacirca5, Charles Taylor6, Sharon Wilks7, Eduardo Braun8, Michael C Mosier9, Robert B Geller10, Lee Schwartzberg11, Nicholas Vogelzang12
1Compass Oncology, US Oncology Research, Tualatin, OR, 2The Oncology Institute of Hope and Innovation, Whittier, CA, 3Cancer Center of Kansas, Wichita, KS, 4Gabrail Cancer Center, Canton, OH, 5North Shore Hematology Oncology, East Setauket, NY, 6Tulsa Cancer Institute, Tulsa, OK, 7Cancer Care Centers of South Texas, San Antonio, TX, 8Michiana Hematology Oncology, Westville, IN, 9Biostatistics, EMB Statistical Solutions, LLC, Overland Park, KS, 10Medical Affairs, Heron Therapeutics, Inc., San Diego, CA, 11West Cancer Center, Germantown, TN, 12Comprehensive Cancer Centers of Nevada, Las Vegas, NV, USA
Background: APF530, a novel extended-release granisetron injection, was superior to ondansetron in a guideline-recommended three-drug regimen in preventing delayed-phase chemotherapy-induced nausea and vomiting (CINV) among patients receiving highly emetogenic chemotherapy (HEC) in the double-blind Phase III Modified Absorption of Granisetron In the prevention of CINV (MAGIC) trial.
Patients and methods: This MAGIC post hoc analysis evaluated CINV prevention efficacy and safety of APF530 versus ondansetron, each with fosaprepitant and dexamethasone, in patient subgroup receiving an anthracycline plus cyclophosphamide (AC) regimen. Patients were randomized 1:1 to APF530 500 mg subcutaneously (granisetron 10 mg) or ondansetron 0.15 mg/kg intravenously (IV) (≤16 mg); stratification was by planned cisplatin ≥50 mg/m2 (yes/no). Patients were to receive fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1, then dexamethasone 8 mg orally once daily on day 2 and twice daily on days 3 and 4. Patients were mostly younger females (APF530 arm, mean age 54.1 years, female, 99.3%; ondansetron arm, 53.8 years, female 98.3%). The primary end point was delayed-phase (>24–120 hours) complete response (CR).
Results: APF530 versus ondansetron regimens achieved numerically better CINV control in delayed and overall (0–120 hours) phases for CR, complete control, total response, rescue medication use, and proportion with no nausea. APF530 trends are consistent with the overall population, although not statistically superior given the underpowered AC subgroup analysis. The APF530 regimen in this population was generally well tolerated, with safety comparable to that of the overall population.
Conclusion: APF530 plus fosaprepitant and dexamethasone effectively prevented CINV among patients receiving AC-based HEC, a large subgroup in whom CINV control has traditionally been challenging.
Keywords: APF530, extended release granisetron, chemotherapy-induced nausea and vomiting (CINV), highly emetogenic chemotherapy (HEC), anthracycline, cyclophosphamide
Introduction
Chemotherapy-induced nausea and vomiting (CINV) associated with highly emetogenic chemotherapy (HEC) adversely affects the quality of life of patients; it is especially challenging to manage in the delayed phase (24–120 hours after chemotherapy)1 and affects chemotherapy compliance.2,3 HEC includes chemotherapeutic agents with the potential to cause emesis in >90% of patients in the absence of prophylaxis.4 For patients receiving HEC, antiemesis guidelines from the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), the European Society of Medical Oncology (ESMO), and Multinational Association of Supportive Care in Cancer (MASCC) recommend the use of a three-drug regimen consisting of a 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist (RA), a neurokinin 1 (NK-1) RA, and a corticosteroid.3,5,6 Despite these comprehensive treatment guidelines, there is still an unmet clinical need for improved prevention of delayed CINV in patients receiving HEC regimens.7,8
Anthracycline and cyclophosphamide (AC)–based regimens represent a distinct class of emetogenic chemotherapy. Previously classified as moderately emetogenic chemotherapy (MEC) according to the Hesketh emetogenicity criteria, developed in 1997,4 AC-based regimens were subsequently reclassified as HEC in the ASCO 2011 emetogenicity guidelines to recognize their high emetogenic risk.9 Updated MASCC and ESMO guidelines have also recommended classification of AC-based regimens as HEC and recommend the above-mentioned three-drug regimen for CINV prevention in this setting.5 AC-based regimens are most commonly administered to women with breast cancer, a population at higher risk for CINV, based on both female gender and typically younger age.10–15 Therefore, CINV in this population is influenced by both chemotherapy- and patient-related risk factors. CINV prevention in patients receiving AC-based HEC6 is challenging and remains an urgent therapeutic need.
Granisetron, a first-generation serotonin (5-HT3) RA, is commonly used to treat CINV but has a short half-life (9 hours).16 APF530 is a new formulation of 2% granisetron in a viscous bioerodible Biochronomer® tri(ethylene glycol) poly(orthoester) (TEG-POE) polymer.17 Following subcutaneous (SC) administration of APF530 in the upper arm or abdomen, the polymer undergoes slow, controlled hydrolysis, maintaining therapeutic concentrations of granisetron for ≥5 days.18,19 A single dose of APF530 (granisetron 10 mg) provides extended release of granisetron for the prevention of both acute (0–24 hours) and delayed (24–120 hours) CINV.17 In a Phase III noninferiority trial, APF530 (500 mg SC) was noninferior to palonosetron (0.25 mg IV) in the control of acute CINV in patients receiving MEC or HEC and in the prevention of delayed CINV in patients receiving MEC.17,20 APF530 is approved by the US Food and Drug Administration (FDA) for use in conjunction with other antiemetics to prevent acute or delayed CINV following initial or repeat courses of MEC or AC combination regimens.21
The Phase III Modified Absorption of Granisetron In the prevention of CINV (MAGIC) trial compared delayed-phase complete response (CR; no emesis [vomit or retch] and no rescue medication use) achieved by using APF530 with that using ondansetron, each in a three-drug regimen with an NK-1 RA and dexamethasone. Ondansetron, the active comparator, has been used in large-scale CINV studies as the positive comparator for other 5-HT3 RAs22,23 and is indicated for the prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including high-dose cisplatin.24 In MAGIC, the APF530 arm demonstrated superior CR versus ondansetron in delayed CINV following HEC (64.7% vs 56.6%; P=0.014; 8% absolute improvement).25 APF530 is the first and only 5-HT3 RA to demonstrate superiority over another 5-HT3 RA in a three-drug versus three-drug pivotal Phase III efficacy trial. This exploratory post hoc analysis evaluated the efficacy and safety of an APF530 regimen versus an ondansetron regimen in MAGIC trial patients who received AC-based chemotherapy regimens.
Patients and methods
Details of the MAGIC trial design and methodology have been presented previously,25 whereas a brief overview is presented in this report. This prospective, multicenter, randomized, double-blind, double-dummy, parallel-group Phase III trial was conducted at 77 sites (see Table S1 for the list of study investigators list) in the United States (clinicaltrials.gov identifier: NCT02106494). The protocol was approved by the institutional review board at all sites and conducted according to the International Conference on Harmonisation E6 Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written consent. Access to the data for this post hoc analysis was provided by Heron Therapeutics, Inc. (San Diego, CA, USA), who funded the original trial.
Adult patients who had histologically or cytologically confirmed malignancy and were scheduled to receive their first cycle of single-day HEC, according to ASCO 2011 emetogenicity criteria,9 were enrolled. Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients with current or prior significant cardiac disease, including QT interval prolongation, were excluded.
Patients were randomly assigned 1:1 to receive either APF530 500 mg SC (granisetron 10 mg) and ondansetron placebo IV or ondansetron 0.15 mg/kg IV (to a maximum of 16 mg) and APF530 placebo SC (containing the Biochronomer TEG-POE vehicle); stratification was by planned cisplatin ≥50 mg/m2 (yes/no). In addition, all patients received fosaprepitant 150 mg IV and dexamethasone 12 mg IV on day 1 and were scheduled to receive dexamethasone 8 mg orally once daily on day 2 and twice daily on days 3 and 4. Rescue medication was permitted at the investigator’s discretion.
The primary objective was to demonstrate the superiority of APF530 500 mg SC in achieving delayed-phase CR, compared with ondansetron 0.15 mg/kg IV, in patients receiving HEC in cycle 1. Secondary end points included CR in the overall (0–120 hours) phase and two, more stringent end points that measure additional effects on nausea: complete control (CC; CR and no more than mild nausea) in delayed and overall phases. Other end points included CR and CC in the acute phase and total response (TR; CR and no nausea) in acute, delayed, and overall phases; the number of nausea episodes and rescue medication use, results for which were based on observed data without imputation for missing data, were also included. Patients recorded daily, up to 120 hours following chemotherapy, the number of nausea, retching and/or vomiting episodes, and instances of rescue medication use; these data were used to evaluate the above-mentioned efficacy measures. Response rates were compared using 95% confidence intervals for treatment differences using a modified intent-to-treat (mITT) population (all patients who received HEC and the study drug and had postbaseline efficacy measures); P-values between treatment arms were based on the chi-square test. This post hoc analysis was conducted on the subgroup of patients who received an AC-containing HEC regimen; however, the study was not powered to detect statistically significant treatment differences between the arms with this subgroup.
Safety was assessed by adverse events, physical examinations, vital signs, and clinical laboratory values in the safety population, comprising all patients who received the study drug. The type of treatment-emergent adverse event (TEAE) and its duration, severity, and relationship to the study drug were evaluated. TEAEs, serious adverse events (SAEs), treatment-related TEAEs, and treatment-related SAEs were assessed. TEAEs were defined as adverse events that either began within 8 days of study drug administration or prior to and increased in severity within 8 days of study drug administration. Treatment-related TEAEs included TEAEs with possible, probable, or definite relationship to study drug treatment or events with unknown or missing causality. Severity of TEAEs was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. All injection site reactions (ISRs) were collected by patient diary entries in addition to investigators’ evaluation. ISRs were conservatively considered treatment related. The severity of most ISRs was based on prespecified criteria of size and appearance only, rather than functional impairment.
Results
Patient disposition and characteristics
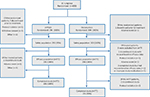
Of the 942 patients randomized in the entire study between March 31, 2014, and May 15, 2015, 600 patients received AC-based HEC (APF530 arm, 296; ondansetron arm, 304) (Figure 1). Of the 296 randomized patients in the APF530 arm, 3 discontinued prior to treatment (1 due to a protocol violation, 1 due to withdrawn consent, and 1 due to other reason); of the 304 randomized patients in the ondansetron arm, 1 discontinued prior to treatment because of an adverse event. The safety population consisted of 293 patients in the APF530 arm and 303 patients in the ondansetron arm. Of the 293 patients in the APF530 arm safety population, 2 discontinued the study prior to day 6 (1 due to an adverse event and 1 due to other reason). Among the 303 patients in the ondansetron arm safety population, 5 were excluded from the mITT population: 2 discontinued the study without postbaseline efficacy data (1 due to protocol violation and 1 due to an adverse event) and 3 completed the study, but 2 had no postbaseline efficacy data and 1 had improper study medication (Figure 1). All patients in the APF530 arm mITT population completed the study; among the ondansetron arm mITT population, 2 patients discontinued the study because of a protocol violation (Figure 1).
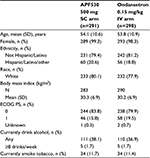
Of the 902 patients in the mITT population of the entire study, 589 (65%) received AC-based HEC (APF530 arm, n=291; ondansetron arm, n=298). Baseline demographics were generally balanced between the treatment arms in the AC subgroup (Table 1). Most patients in the AC subgroup were female (APF530, 99.3%; ondansetron, 98.3%), white (APF530, 80.1%; ondansetron, 77.9%), and had an ECOG performance status of 0 (APF530, 83.8%; ondansetron, 79.9%). The mean age of patients in the APF530 arm was 54.1 years and that in the ondansetron arm was 53.8 years. The most common AC-based chemotherapy regimen in both treatment arms was cyclophosphamide <1500 mg/m2 and doxorubicin (APF530 arm, 87.3%; ondansetron arm, 89.3%) (Table 2).
Efficacy analyses
In the AC subgroup, delayed-phase CR was numerically higher in the APF530 arm versus the ondansetron arm, approaching statistical significance (63.6% vs 56.0%; P=0.062) (Table 3). Similarly, in the overall phase, a trend favoring the APF530 arm versus the ondansetron arm was observed, although it was not statistically significant. No appreciable efficacy difference was observed in the acute phase in the APF530 arm compared with the ondansetron arm (Table 3).
For CC and TR, numerically higher rates were observed in the APF530 arm versus the ondansetron arm in the delayed and overall phases, although the treatment differences were not statistically significant. Minor differences were observed in CC and TR rates in the acute phase (Table 3).
A numerically higher proportion of patients in the APF530 arm versus the ondansetron arm reported no rescue medication use in the delayed (68.9% vs 61.7%; P=0.069) and overall phases (63.3% vs 56.9%; P=0.116) (Table 4). The proportion of patients with no rescue medication use was numerically higher in the APF530 arm compared with the ondansetron arm across all phases (Figure S1). Clinical results also favored the APF530 arm versus the ondansetron arm in the proportion of patients reporting no nausea in the delayed and overall phases (Table 5).
Safety analyses
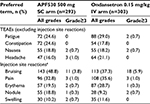
The APF530 regimen was generally well tolerated in this AC subgroup; no new safety signals were identified (Table 6). Most patients experienced at least one TEAE (APF530 arm, 93.5%; ondansetron arm, 91.1%). Excluding ISRs, the most frequently reported TEAEs were fatigue, constipation, nausea, and headache, occurring with a similar frequency in each treatment arm. Serious TEAEs were experienced by 4.1% of the patients in the APF530 arm and 2.0% of the patients in the ondansetron arm; no TEAEs led to death.
Excluding ISRs, the most common treatment-related TEAEs in the APF530 and ondansetron arms were constipation (8.2% vs 5.9%, respectively) and headache (7.2% vs 5.9%, respectively). A treatment-related SAE occurred in one patient in the APF530 arm (0.3%, injection-site infection) 14 days after APF530 administration and recovered within 9 days with antibiotic use. A treatment-related SAE occurred in one patient in the ondansetron arm (0.3%, dehydration) and subsequently resolved.
The most frequently reported TEAEs were ISRs (Table 6), which occurred in 66.9% of patients in the APF530 arm and 60.7% in the ondansetron arm. All ISRs were conservatively considered treatment related. The severity of most ISRs was based on prespecified criteria of size and appearance only, rather than functional impairment. These were generally mild or moderate, most resolved by the end of the study, and no ISR led to death or study discontinuation.
Discussion
The use of AC-based chemotherapy remains highly prevalent because of its effectiveness in patients with breast cancer and the relatively high breast cancer incidence.26–29 Placebo-controlled studies have shown that this combination induces emesis in a sufficient number of patients to warrant its reclassification, in 2011, from MEC to HEC. The MAGIC trial compared APF530 versus ondansetron in the context of a guideline-recommended three-drug regimen in CINV prevention following HEC regimens, including AC. The use of ondansetron was appropriate because it has been used in pivotal CINV studies as the active comparator for other 5-HT3 RAs22,23 and is indicated for the prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including high-dose cisplatin.24
No previous pivotal efficacy trial involving patients receiving AC-based regimens has compared two 5-HT3 RAs within a three-drug regimen. The MAGIC trial design was consistent with current antiemesis guideline recommendations and was conducted in US community practices, so the results are likely to be representative of this clinical practice setting.
In contrast to other HEC, AC-based regimens are administered mostly to younger female patients; both female sex and young age constitute patient-related CINV risk factors.30,31 Accordingly, in the MAGIC trial, most patients receiving AC-based regimens were female and aged <55 years, and so at higher risk for CINV.31,32 The interaction of high emetogenicity of AC-based regimens with patient-related risk factors highlights the distinct CINV prevention needs in this patient group. In two large Phase III trials, CR rates were lower in patients receiving AC-based than in those receiving non–AC-based regimens across all phases, suggesting greater CINV prevention challenges associated with AC-based regimens.13,33 Furthermore, patients receiving HEC or MEC regimens continue to experience delayed-phase CINV, highlighting the need for new therapies.34
Although this exploratory post hoc AC subgroup analysis was not powered for statistical significance, trends favoring the APF530 versus the ondansetron arm in delayed and overall phases were observed in multiple measures of CINV control: CR, CC, TR, rescue medication use, and proportion of patients with no nausea. The Biochronomer technology underlying APF530 provides sustained therapeutic granisetron concentrations across ≥5 days following a single APF530 injection and was expected to provide better delayed-phase CINV control than ondansetron in the MAGIC trial. Therefore, this trial was designed to detect treatment differences in the delayed phase. The lack of any appreciable differences in efficacy in the acute phase is consistent with the trial design. Acknowledging the limits of cross-trial comparisons, the control arm of the MAGIC AC subgroup showed results consistent with those from a previous study in patients with breast cancer who received AC-based regimens.15
Similar to findings in the entire study population, APF530 was generally well tolerated in this patient subgroup receiving AC-based HEC, and no new safety signals were observed.25 The incidences of ISRs were similar in both the arms, and most resolved by the end of the study. Consistent with the treatment-related TEAEs observed with other 5-HT3 RAs,35 constipation and headache were the most commonly reported TEAEs with APF530. Trial results suggest that APF530 is a safe and effective CINV management option in this particularly challenging clinical setting. Consequently, the FDA approved APF530 for the prevention of both acute and delayed CINV following initial and repeat courses of MEC or AC combination regimens.21
The findings of the present study from the AC subgroup are concordant with the superior delayed-phase CR rate observed in the entire MAGIC study population.25 The superiority of APF530 versus ondansetron, in the presence of an NK-1 RA and dexamethasone, in achieving delayed-phase CR in the MAGIC trial patients suggests that APF530 provided benefit in addition to that of the NK-1 RA. Overall, these results demonstrate the benefit of the extended-release design of the APF530 formulation, whereby a single SC dose provides therapeutic granisetron concentrations for ≥5 days.18
Limitations of this analysis include being an exploratory post hoc analysis with a relatively small number of patients in each arm. In addition, use of the double-dummy design resulted in an increased incidence of ISRs in the ondansetron arm due to the presence of the viscous TEG-POE vehicle in the APF530 placebo injection.
In a recently published randomized, double-blind Phase III trial, olanzapine was compared with placebo, when added to a three-drug regimen of 5-HT3 RA, NK-1 RA, and dexamethasone in patients receiving cisplatin or AC-based HEC.36 In that study, the addition of olanzapine resulted in a significant improvement in nausea control. It will therefore be of interest to determine whether the addition of olanzapine to a three-drug regimen of APF530 may similarly further improve CINV control in patients receiving AC-based regimens.
Conclusion
The MAGIC trial demonstrated the superiority of APF530 versus ondansetron, each in a guideline-recommended three-drug regimen with fosaprepitant and dexamethasone, in the prevention of delayed-phase CINV following HEC. The findings of this post hoc subgroup analysis in patients receiving AC-based HEC and at a high risk of experiencing CINV are consistent with the results from the entire study population, particularly in control of delayed CINV. The APF530 regimen was generally well tolerated in the AC subgroup, as in the entire population. APF530, in conjunction with other antiemetics, is approved for preventing CINV in both acute and delayed phases in patients receiving initial or repeat courses of MEC or AC-based regimens.21 Thus, APF530 may be a convenient antiemetic option for female patients with breast cancer receiving AC-based chemotherapy, a population in which preventing nausea and vomiting is particularly difficult.
Acknowledgments
Research support and study drug was provided by Heron Therapeutics, Inc. Medical writing assistance was provided by Doyel Mitra, PhD, of SciStrategy Communications, supported by Heron Therapeutics, Inc. Selected data in this article were previously presented at 2015 San Antonio Breast Cancer Symposium (SABCS), Abstract 851257, P1-10-17, poster presentation.
Disclosure
IDS, LS, and NV have acted in consultant/advisory roles for Heron Therapeutics, Inc. EB has received research funding and honoraria from Heron Therapeutics, Inc. MCM has received honoraria from EMB Statistical Solutions, LLC. RBG is employed by and has ownership interests in Heron Therapeutics, Inc. The other authors report no conflicts of interest in this work.
References
Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer. 2012;20(1):107–117. | ||
Vidall C, Dielenseger P, Farrell C, et al. Evidence-based management of chemotherapy-induced nausea and vomiting: a position statement from a European cancer nursing forum. Ecancermedicalscience. 2011;5:211. | ||
NCCN Clinical Practice Guidelines in Oncology: Antiemesis—v2.2016. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed September 23, 2016. | ||
Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997;15(1):103–109. | ||
Aapro M, Gralla R, Herrstedt J, Molassiotis A, Roila F. MASCC/ESMO antiemetic guideline 2016. Available from: http://www.mascc.org/antiemetic-guidelines. Accessed September 23, 2016. | ||
Hesketh PJ, Bohlke K, Lyman GH, et al. Antiemetics: American Society of Clinical Oncology Focused Guideline Update. J Clin Oncol. 2015;34(4):381–386. | ||
Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100(10):2261–2268. | ||
Van Laar ES, Desai JM, Jatoi A. Professional educational needs for chemotherapy-induced nausea and vomiting (CINV): multinational survey results from 2388 health care providers. Support Care Cancer. 2015;23(1):151–157. | ||
Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29(31):4189–4198. | ||
Grunberg SM, Dugan M, Muss H, et al. Effectiveness of a single-day three-drug regimen of dexamethasone, palonosetron, and aprepitant for the prevention of acute and delayed nausea and vomiting caused by moderately emetogenic chemotherapy. Support Care Cancer. 2009;17(5):589–594. | ||
Hutton B, Clemons M, Mazzarello S, Kuchuk I, Skidmore B, Ng T. Identifying an optimal antiemetic regimen for patients receiving anthracycline and cyclophosphamide-based chemotherapy for breast cancer—an inspection of the evidence base informing clinical decision-making. Cancer Treat Rev. 2015;41(10):951–959. | ||
Roscoe JA, Morrow GR, Colagiuri B, et al. Insight in the prediction of chemotherapy-induced nausea. Support Care Cancer. 2010;18(7):869–876. | ||
Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol. 2015;16(9):1071–1078. | ||
Sekine I, Segawa Y, Kubota K, Saeki T. Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci. 2013;104(6):711–717. | ||
Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23(12):2822–2830. | ||
Kytril (granisetron) injection, for intravenous use [prescribing information]. South San Francisco: Genentech Inc; 2011. Available from: http://www.gene.com/download/pdf/kytril_injection_prescribing.pdf. Accessed September 23, 2016. | ||
Ottoboni T, Gelder M, O’Boyle E. Biochronomer™ technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15–21. | ||
Gabrail N, Yanagihara R, Spaczynski M, et al. Pharmacokinetics, safety, and efficacy of APF530 (extended-release granisetron) in patients receiving moderately or highly emetogenic chemotherapy: results of two phase 2 trials. Cancer Manag Res. 2015;7:83–92. | ||
Morrison D, Anderson A, Slama M, et al. Phase 1 bioavailability study comparing 2 different subcutaneous routes of administration for APF530. Support Care Cancer. 2015;23(Suppl): Abstract 11-16-P. | ||
Raftopoulos H, Cooper W, O’Boyle E, Gabrail N, Boccia R, Gralla RJ. Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double-blind, noninferiority phase 3 trial. Support Care Cancer. 2015;23(3):723–732. | ||
Sustol (granisetron) extended-release injection, for subcutaneous use [prescribing information]. Redwood City: Heron Therapeutics; 2016. Available from: http://www.herontx.com/sustol. Accessed September 23, 2016. | ||
Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17(9):1441–1449. | ||
Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol—EASE. J Clin Oncol. 2011;29(11):1495–1501. | ||
Zofran (ondansetron hydrochloride) injection for intravenous use [prescribing information]. Research Triangle Park: GlaxoSmithKline; 2014. Available from: http://www.pharma.us.novartis.com/product/pi/pdf/zofran_inj.pdf. Accessed September 23, 2016. | ||
Schnadig ID, Agajanian R, Dakhil S, et al. APF530 (granisetron injection extended release) in a three drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12(12):1469–1481. | ||
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer—v1.2016. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed September 23, 2016. | ||
Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30(18):2232–2239. | ||
Serrurier K. Chemotherapy treatment patterns for early stage breast cancer population (ESBC): decreasing use of anthracycline-based regimens. J Clin Oncol. 2012;30(Suppl 27): Abstract 141. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Georgy A, Neceskas J, Goodin S. Antiemetic care for patients with breast cancer: focus on drug interactions and safety concerns. Am J Health Syst Pharm. 2007;64(21):2227–2236. | ||
Doherty KM. Closing the gap in prophylactic antiemetic therapy: patient factors in calculating the emetogenic potential of chemotherapy. Clin J Oncol Nurs. 1999;3(3):113–119. | ||
Rapoport BL. Efficacy of a triple antiemetic regimen with aprepitant for the prevention of chemotherapy-induced nausea and vomiting: effects of gender, age, and region. Curr Med Res Opin. 2014;30(9):1875–1881. | ||
Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer. 2010;18(4):423–431. | ||
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478. | ||
Navari RM. Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting—two new agents. J Support Oncol. 2003;1(2):89–103. | ||
Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375(2):134–142. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.