Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Apelin and chemerin as promising adipokines in children with type 1 diabetes mellitus
Authors Elsehmawy AAEW , El-Toukhy SE , Seliem NMA , Moustafa RS , Mohammed DS
Received 30 September 2018
Accepted for publication 29 January 2019
Published 22 March 2019 Volume 2019:12 Pages 383—389
DOI https://doi.org/10.2147/DMSO.S189264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Asmaa Abd El Wakeel Elsehmawy,1 Safinaz Ebrahim El-Toukhy,2 Nora Mohamed Ahmed Seliem,3 Rehab Selim Moustafa,4 Doaa Sayed Mohammed5
1Department of Pediatrics, Faculty of Medicine, Al-Azhar University, Cairo, Egypt; 2Department of Medical Biochemistry, National Research Center, Cairo, Egypt; 3Biochemistry Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt; 4Child Health Department, National Research Center, Cairo, Egypt; 5Endocrine Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
Background: Type 1 diabetes mellitus (T1DM) is one of the most common chronic diseases in children that may be due to micro or macrovascular complications. Diabetic renal disease or nephropathy is a common complication of DM, clinically silent and the only detectable abnormality due to the presence of microalbuminuria.
Subjects and methods: This study was a case–control study. Participants were classified into two groups. The first group included 40 children with T1DM and the second group included 30 matched healthy controls. Serum apelin (APLN), chemerin, cholesterol, and triglycerides (TG) levels were measured for each case. Also, albumin/creatinine ratio was analyzed in random urine sample.
Results: Comparison between T1DM patients and controls revealed that serum apelin, chemerin, cholesterol, TG levels, and albuminuria were significantly increased in cases compared to their controls. Significant positive correlations were found between HbA1c% and albuminuria for APLN and chemerin in the diabetic group. Whereas significant negative correlations were found between apelin and glomerular filtration rate (GFR).
Conclusion: Increased levels of serum apelin and chemerin in T1DM patients may be considered as promising adipokines for the development of diabetic complication.
Keywords: apelin, chemerin, children, diabetes mellitus
Introduction
Type 1 diabetes mellitus (T1DM) is a common, chronic and metabolic disease characterized by hyperglycemia as a cardinal metabolic feature.1 Long-term damage, dysfunction and failure of various organs especially eyes, kidneys, nerves, heart and blood vessels are caused by chronic hyperglycemia of diabetes.2 Diabetic nephropathy (DN) is an insidious and a common complication of diabetes mellitus (DM). At its onset, DN is clinically silent, and the only detectable abnormality due to the presence of microalbuminuria, which is defined as urine albumin of 30–300 g/day (or also known as albuminuria).3
DN is believed to be the consequence of poorly controlled hyperglycemia. The mechanisms leading to renal injury in diabetes mellitus is evolving, may be caused by accumulation of injurious metabolic products, such as glycosylated compounds, involvement of the renin–angiotensin system, and endothelial and podocyte injury being involved in the pathogenesis of DN.3
Glomerular hypertrophy, thickening of glomerular basement membrane and mesangial expansion with the formation of Kimmelstiel–Wilson nodules are the classical changes that are seen in the glomerular compartment that are usually accompanied with glomerular lesions including tubulo-interstitium undergoing fibrosis, as well as arteriolar thickening and hyalinization in the vascular compartment of the kidney. There is an imbalance of extracellular matrix (ECM) synthesis and degradation, which is most likely responsible for the accumulation of excessive matrices in various compartments of the kidney. Thus, the genesis of Kimmelstiel–Wilson lesion, a diagnostic feature of DN, is not only due to the excessive synthesis of ECM glycoproteins but also due to a decreased degradation by matrix metalloproteinases.4
Hyperglycemia-induced hyperfiltration is an early observable abnormality in DM. At this stage, patients are asymptomatic, blood pressure is normal, there is no detectable microalbuminuria, and therefore it is considered as a latent stage of diabetic nephropathy. Presence of microalbuminuria (also known as albuminuria) in patients with DM1 or DM2 is recognized as an early marker of DN.5
Chemerin, a discovered adipocytokine, is a chemoattractant protein that acts as a ligand for the G-protein-coupled receptor CMKLR1 (also known as ChemR23). Chemerin is a 14 kDa protein secreted in an inactive form as prochemerin and is activated through cleavage of the C-terminus by inflammatory and coagulation serine proteases. It is involved in glucose and lipid metabolism. Elevated levels of this peptide have been associated with insulin resistance and systemic inflammation.6
Apelin (APLN), adipocytokine, is a peptide known as the endogenous ligand of the G-protein-coupled receptor Apelin. Apelin-13, the most active member of the APLN group, is expressed in a variety of tissues, including kidney and endothelial cells. In addition, increased body mass in type 2 diabetic patients might contribute to increase APLN levels in the blood.7
Aim of the study
The aim of the current study was to evaluate the level of serum chemerin and apelin in type 1 diabetic children and their correlation with glycated hemoglobin and proteinuria.
Materials and methods
Participants
Seventy children of both genders were enrolled for this study and divided into two groups.
Study group: included 40 patients with T1DM were randomly selected from the outpatient pediatric clinic.
Control group: included 30 apparently healthy children.
The study was carried out in Alzhraa University Hospital, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt: Pediatric and Biochemistry Departments.
Design
Participants were evaluated during a hospital visit to determine family medical history, current medical conditions, medication use, self-report of the presence of any smokers in the household and demographic information.
In addition, physical examinations, including height and weight measurements and laboratory testing using blood samples for lipid profile, were performed. Children with renal diseases or urological problems, presence of urinary tract infection or taking any medication rather than insulin or those with chronic devastating diseases were excluded from this study.
The body weight was measured using Seca scale to the nearest 0.1 kg barefoot and in light clothes, after emptying the urinary and gastrointestinal apparatuses (Seca Model 770, Hamburg, Germany).
Investigations
In the morning, after 12–14 hours fasting, 7 mL of blood was collected from all participants and divided into two: one half was taken in a plain tube to separate serum and another half was taken in a tube containing EDTA. Serum was separated to determine fasting blood glucose, urea, and creatinine levels and with the another half of blood sample following analysis were performed.
- Glycosylated hemoglobin (HbA1c%)
- Lipid profile and serum creatinine
- Albumin/creatinine ratio (ACR) screening for microalbuminuria was assessed for fresh morning urine samples by measuring ACR using ELISA kit provided by Orgentec Diagnostika, GmbH (Mainz, Germany).9
- Serum APLN and chemerin
HbA1c% was measured using a spectrophotometer (Clinical Chemistry Analyzer 7; Germany).
Lipid profile was measured using an autoanalyzer (Beckman Coulter Synchron CX 9; Beckman Coulter Inc., Brea, CA, USA). For cholesterol measurements, serum total cholesterol was determined by a commercial kit (Boehringer-Mannheim, Germany).8 The concentration of triglycerides (TG) was measured using a TechnoCon AutoAnalyzer II (TechnoCon Instruments, Tarrytown, NY, USA).
Serum apelin and chemerin were assayed using human ELISA kit (Cusabio, Wuhan, Hubei Province, China).
Serum chemerin
The human chemerin ELISA, standards and samples were incubated in microtitration wells pre-coated with polyclonal antihuman chemerin antibody. Incubation was followed by washing, and then biotin-labeled polyclonal antihuman chemerin antibody was added and incubated with the captured chemerin. After another washing, streptavidin-HRP conjugate was added. After the last washing step, the remaining conjugate was allowed to react with the substrate solution. The reaction was stopped by addition of acidic solution, and absorbance of the resulting yellow product was measured. The absorbance was proportional to the concentration of chemerin. A standard curve was constructed by plotting absorbance values against chemerin.10,11
Serum APLN
This assay employs the competitive inhibition enzyme immunoassay technique. A monoclonal antibody specific to apelin has been pre-coated into a microplate. A competitive inhibition reaction was launched between biotin-labeled APLN and biotin-unlabeled APLN (standards or samples) with the pre-coated antibody specific to APLN. After incubation the unbound conjugate was washed off, avidin conjugated to horseradish peroxidase (HRP) was added to each microplate well and incubated. The amount of bound HRP conjugate was inversely proportional to the concentration of APLN in the sample. After addition of the substrate solution, the intensity of color developed was inversely proportional to the concentration of APLN in the sample. Detection range was 0.1–1000 ng/mL.12,13
Statistics
Data were collected, revised, coded, and entered on the Statistical Package for Social Science (IBM SPSS) version 20 statistical analysis software (IBM Corporation, Armonk, NY, USA). The statistical tools used include mean, SD, and t-test.
Results
The descriptive statistics of the study groups are shown in Table 1. Patients and healthy volunteers were matched for the mean of both age and gender. Significant increase in blood level of urea, creatinine, fasting blood sugar, cholesterol, TG and HbA1c% was shown in diabetic children in comparison to the control. Increased ACR and GFR in diabetic children was shown in comparison to healthy controls.
  | Table 1 Demographic, anthropometric, and chemical data of the studied groups Abbreviations: BMI, body mass index; HbAlc%, glycosylated hemoglobin; APLN, apelin; GFR, glomerular filtration rate. |
Both APLN and chemerin showed a statistically significant increase in diabetic children when compared to their healthy counterparts (Table 1). Both APLN and chemerin showed a statistically significant increase in non-controlled diabetic children (Table 2).
  | Table 2 Chemerin and APLN levels in both controlled and non-controlled diabetic children Abbreviations: HbAlc%, glycosylated hemoglobin; APLN, apelin. |
The cutoff point for APLN and chemerin was >126.45 and 92.18, respectively, with 100% sensitivity and specificity for APLN, and 74% and 100% for chemerin in the prediction of nephropathy in diabetic children (Table 3).
  | Table 3 The cutoff point, sensitivity, specificity of APLN and chemerin as an early marker in detection of diabetic nephropathy Abbreviations: APLN, apelin; AUC, area under the curve. |
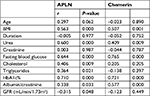
We found a positive correlation of APLN and chemerin with BMI, urea, fasting blood glucose, glycosylated hemoglobin and ACR. In addition, we also found a positive correlation of APLN with cholesterol and triglyceride as shown in Table 4.
Correlation analysis between GFR in diabetic children and the laboratory data revealed that there were negative correlations between GFR and fasting blood sugar, urea, creatinine, cholesterol, triglycerides, HbA1c%, and APLN (Table 5).
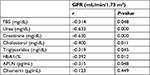  | Table 5 Correlation between GFR and the laboratory data Abbreviations: GFR, glomerular filtration rate; HbAlc%, glycosylated hemoglobin; APLN, apelin. |
Discussion
The present study was a controlled study that was carried out on 70 children of both genders and divided into two groups. The diabetic group included 40 children with T1DM who were randomly selected from the outpatient pediatric clinic; there were 21 (52.5%) females and 19 (47.5%) males, with age ranging from 8 to 13.5 years. The control group included 30 apparently healthy children including 12 (40%) females and 18 (60%) males with age ranging from 8.5 to 14 years.
The aim of this study was to evaluate the level of serum chemerin and APLN in patients with type 1 diabetes and their correlation with proteinuria and HbA1c%.
In the present study there were significant increases in BMI, blood urea, creatinine, fasting blood sugar, cholesterol, TG, and HbA1c% in diabetic children in comparison to the control. Increased ACR was shown in diabetic children in comparison to heathy controls.
In our study there was significant increase in serum chemerin in diabetic children in comparison to the controls, and this was in agreement with previous studies on T2DM.14,15 Moreover, in adolescent type 1 diabetic patients, Abd El Dayema et al16 showed that chemerin was significantly higher in diabetic group than the controls.
In our study there was a significant increase in serum APLN in diabetic children in comparison to the controls and this is in agreement with previous studies of APLN in type 1 diabetic patients.17–20
In the present study, we reported that serum cholesterol and TG were significantly increased in T1DM patients than in healthy controls. From the previous results, we considered patients with T1DM are at high risk of developing premature vascular affection because of hyperlipidemia and thus should be screened well for this serious complication; we found a positive correlation of APLN with cholesterol and TG. In support of these results, another study found statistically significant differences between diabetic and non-diabetic groups related to serum levels of TG and cholesterol.21 Few studies have described the effects of APLN on lipid metabolism; one stated that APLN was shown to inhibit lipolysis.22 This was ensured by Than et al23 who found that APLN increases the stability of lipid vacuoles making them more resistant to lipases.
APLN has a role in energy metabolism: it improves sensitivity of insulin in insulin-resistant obese mice, and it is related to an increase in glucose uptake in skeletal muscle.24 Synthesis of APLN is affected by insulin and plasma APLN levels is increased in obesity in association with hyper insulinemia.25 Furthermore, clinical studies demonstrated that chemerin modulates inflammation. Mechanisms linking chronic inflammation to the development of diabetes remain elusive, but it can be assumed that any effects may be exerted via insulin secretion, insulin resistance, or both.26
In our study both apelin and chemerin levels are more increased in non controlled diabetic children compared the controlled one (table 2) and both chemerin and APLN were positively correlated with fasting blood glucose and glycosylated hemoglobin and this is in agreement with previous studies that showed a correlation between serum APLN and HbA1c% in children with T1DM.17,18,20
ACR is used to screen people with chronic conditions, such as diabetes that put them at an increased risk of developing kidney disease. Studies have shown that identifying individuals in the very early stages of kidney disease helps people and healthcare providers adjust treatment. Controlling diabetes by maintaining tight glycemic control delays or prevents the progression of kidney disease.27
Both APLN and chemerin, novel adipocytokines, were increased in response to inflammation. In vitro studies have revealed that APLN and the APLN receptor can induce the sprouting of endothelial cells in an autocrine or paracrine manner, thus suggesting a role for APLN in angiogenesis.28 Moreover, it was found that chemerin induced functional angiogenesis in human endothelial cells, by promoting migration, capillary tube formation, activation of endothelial gelatinase (matrix metalloproteinases-2/matrix metalloproteinases-9) and activation of phosphatidylinositol 3-kinase/Akt and MAPKs pathways, which is a key mechanism for angiogenesis and cell survival. Therefore, the high level of serum chemerin possibly promotes the development of diabetic complication and diabetic nephropathy.29
The activation of the immune system and chronic inflammation are both involved in pathogenesis of DM and its complications as a result of DN. Some studies have demonstrated that cytokines, chemokines, growth factors, adhesion molecules, nuclear factors as well as immune cells such as monocytes, lymphocytes and macrophages are all involved in DM pathogenesis and of course play an important role in DM complications.30
In the present study there were increases in ACRs in diabetic children in comparison to the healthy controls and there were positive correlations between APLN and chemerin blood levels with alp/creatinine ratio. Correlation of serum APLN with microalbuminuria was confirmed by another study by Guo et al,31 which reported that APLN and chemerin may induce glomerular endothelial cell proliferation and then nephropathy, also there was a negative correlation between serum APLN and GFR, which was stated in the study on type 2 diabetes.32 The cutoff point of APLN and chemerin was >126.45 and 92.18, respectively, with 100% sensitivity and specificity for APLN and 74%, 100% for chemerin in the prediction of nephropathy in diabetic children (Table 3, Figure 1). To our knowledge this is the first study to mention the cutoff for both markers in diabetic children. We can state that increased serum APLN and chemerin in diabetic children may contribute to development of diabetic complication.
  | Figure 1 ROC curve demonstrates apelin and chemerin sensitivity and specificity in predicting nephropathy risk in TIDM children. |
Conclusion
In conclusion, increased levels of serum APLN and chemerin were increased in T1DM patients, which may be considered as promising adipokines in diabetic complication development, and hence measuring serum APLN and chemerin in diabetic children is of benefit for detection of diabetic complications.
Limitations
One of the limitations of the study was small sample size as it was a single-center study and we included all children only referred to our hospital during the period from January to June 2018.
Acknowledgments
This work was supported by the Department of Pediatrics, Faculty of Medicine for Girls – Al-Aazhar University and Biochemistry Department of the national Research center. The authors thank the families of the children who agreed to participate in this work.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Svoren BM, Jospe N. Diabetes mellitus in children. In: Kliegman RM, Stanton BF, St Geme JW, Score NF, Behrman RE editors. Nelson’s Textbook of Pediatrics. 20th ed. Elsevier, Inc. Chapter 589 2016:2760–2782. | ||
American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;35(1):64–71. | ||
Kher K, Mietus-Snyder M. Kidney disease associated with diabetes mellitus and metabolic syndrome. In: Kher KK, Schnaper HW, Greenbaum LA, editors. Clinical Pediatric and Nephrology. 3rd ed. Boca Raton, FL: CRC Press; 2017, Part E:533–540. | ||
Kolset SO, Reinholt FP, Jenssen T. Diabetic nephropathy and extracellular matrix. J Histochem Cytochem. 2012;60(12):976–986. | ||
Demirel F, Tepe D, Kara O, Esen I. Microvascular complications in adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2013;5(3):145–149. | ||
Li Y, Shi B, Li S. Association between serum chemerin concentrations and clinical indices in obesity or metabolic syndrome: a meta-analysis. PLoS One. 2014;9(12):e113915. | ||
Huang Z, Wu L, Chen L. Apelin/APJ system: a novel potential therapy target for kidney disease. J Cell Physiol. 2018;233(5):3892–3900. | ||
Flegg HM. Ames Award Lecture 1972. An investigation of the determination of serum cholesterol by an enzymatic method. Ann Clin Biochem. 1973;10(1–6):79–84. | ||
Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310(6):356–360. | ||
Takahashi M, Takahashi Y, Takahashi K, et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582(5):573–578. | ||
Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry. 2nd ed. Philadelphia: WB Saunders; 1994:1017–1089. | ||
Kaliora AC, Kanellos PT, Gioxari A, Karathanos VT. Regulation of GIP and ghrelin in healthy subjects fed on sun-dried raisins: a pilot study with a crossover trial design. J Med Food. 2017;20(3):301–308. | ||
Vehapoglu A, Ustabas F, Ozgen TI, Terzioglu S, Cermik BB, Ozen OF. Role of circulating adipocytokines vaspin, apelin, and visfatin in the loss of appetite in underweight children: a pilot trial. J Pediatr Endocrinol Metab. 2015;28(9–10):1065–1071. | ||
Khaled Y, Rashed L. Serum chemerin levels and chemerin rs17173608 genotypes in the susceptibility of diabetic nephropathy in Egyptian diabetic patients. Egypt J Obes Diabetes Endocrinol. 2016;2(1):18–22. | ||
El-Gohary IE, Abedl-Karima, Hashad DI. Serum chemerin level: does it have a role in progression of diabetic nephropathy. Am J Intern Med. 2016;4(2–1):13–17. | ||
El Dayem SMA, Battah AA, El Bohy AEM, El Shehaby A, El Ghaffar EA. Relationship of plasma level of chemerin and vaspin to early atherosclerotic changes and cardiac autonomic neuropathy in adolescent type 1 diabetic patients. J Pediatr Endocrinol Metab. 2015;28(3–4):265–273. | ||
Dayem SMAE, Battah AA, Bohy AEME, Yousef RN, Ahmed AM, Talaat AA. Apelin, nitric oxide and vascular affection in adolescent type 1 diabetic patients. Open Access Maced J Med Sci. 2017;5(7):934–939. | ||
Meral C, Tascilar E, Karademir F, et al. Elevated plasma levels of apelin in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2010;23(5):497–502. | ||
Habchi M, Duvillard L, Cottet V, et al. Circulating apelin is increased in patients with type 1 or type 2 diabetes and is associated with better glycaemic control. Clin Endocrinol. 2014;81(5):696–701. | ||
Al-Suhaimi EA, Al-Kulaifi FM, Ravinayagam V, Al-Qahtani MH. Serum adipocytokines, metabolic and immunological correlations in type 1 diabetes mellitus (T1DM) children. Open Endocrinol J. 2012;6(1):110–116. | ||
Nascimento AMMAD, Sequeira IJ, Vasconcelos DF, Gandolfi L, Pratesi R, Nóbrega YKM. Endothelial dysfunction in children with type 1 diabetes mellitus. Arch Endocrinol Metab. 2017;61(5):476–483. | ||
Yue P, Jin H, Xu S, et al. Apelin decreases lipolysis via G(q), G(i), and AMPK-dependent mechanisms. Endocrinology. 2011;152(1):59–68. | ||
Than A, Cheng Y, Foh L-C, et al. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol Cell Endocrinol. 2012;362(1-2):227–241. | ||
Yue P, Jin H, Aillaud M, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298(1): E59–E67. | ||
Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19(11):1574–1580. | ||
Weigert J, Neumeier M, Wanninger J, et al. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol. 2010;72(3):342–348. | ||
American Diabetes Association Position Statement: Standards of Medical Care in Diabetes --2015. Diabetes Care. 2015;38(Suppl. 1):S1–S94. | ||
Kidoya H, Ueno M, Yamada Y, et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. Embo J. 2008;27(3):522–534. | ||
Bozaoglu K, Curran JE, Stocker CJ, et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010;95(5):2476–2485. | ||
Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19(3):433–442. | ||
Guo C, Liu Y, Zhao W, et al. Apelin promotes diabetic nephropathy by inducing podocyte dysfunction via inhibiting proteasome activities. J Cell Mol Med. 2015;19(9):2273–2285. | ||
Dawood A, Abdelraof M, El Ghobashy Y. The relationship between serum apelin level and different grades of diabetic nephropathy in type 2 diabetic patients. Egypt J Obes Diabetes Endocrinol. 2017;3(1):32–37. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.