Back to Journals » Cancer Management and Research » Volume 11
Apatinib enhances chemosensitivity of gastric cancer to paclitaxel and 5-fluorouracil
Authors Xu Z, Hu C, Chen S, Zhang C, Yu J, Wang X, Lv H, Cheng X
Received 29 November 2018
Accepted for publication 6 April 2019
Published 29 May 2019 Volume 2019:11 Pages 4905—4915
DOI https://doi.org/10.2147/CMAR.S196372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Zhiyuan Xu,1,* Can Hu,2,* Shangqi Chen,2 Chunli Zhang,3 Jianfa Yu,4 Xiaofeng Wang,2 Hang Lv,5 Xiangdong Cheng1
1Department of Abdominal Surgery, Zhejiang Cancer Hospital, Hangzhou, Zhejiang, People’s Republic of China; 2First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 3Department of Pathology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 4Department of Gastrointestinal Surgery, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 5Key Laboratory of Integrated Traditional Chinese and Western Medicine for Diagnosis and Treatment of Digestive System Tumor, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Background and aim: Paclitaxel (PTX) plus 5-fluorouracil (5-Fu) has become the standard chemotherapy for advanced gastric cancer (GC). Apatinib, a small-molecule tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor-2, improves outcomes in GC patients as a third-line treatment. However, its impact on the chemosensitivity of GC remains to be determined. Hence, we aimed to assess the efficacy and safety of apatinib combined with chemotherapy in vivo and in vitro.
Methods: The MGC803 cell viability was determined by Cell Counting Kit-8 assay, and the interactions between apatinib and conventional cytotoxic agents revealed by combination index values were calculated using Calcusyn 2.0 software. We also used a zebrafish embryo xenograft model to validate the synergistic interactions. Furthermore, 4 patients with late-stage GC were enrolled to explore the efficacy and safety of PTX/Tegafur Gimeracil Oteracil Potassium (S1) (PS) chemotherapy plus apatinib in conversion surgery.
Results: Apatinib showed synergistic interactions with both PTX and 5-Fu in vivo. The zebrafish embryo xenograft model also demonstrated that apatinib significantly enhanced the antitumor activity of PTX and 5-Fu. Apatinib plus PS chemotherapy was well tolerated before surgery. Objective response to preoperative SPA treatment was achieved in all 4 patients. No postoperative bleeding events or wound-healing complications were observed. No postperative morbidity occurred and no morbidity was encountered. Pathological examination showed that all patients had grade Ib pathological response.
Conclusion: The experimental data suggested that apatinib improves the efficacy of PTX and 5-Fu both in vitro and in vivo. Clinical evidence showed that a combination of PS chemotherapy with apatinib may be an efficient and acceptable safety treatment for late-stage GC, especially in conversion surgery.
Keywords: gastric cancer, apatinib, conversion surgery, targeted therapy
Introduction
Gastric cancer (GC) is a common malignancy and has been estimated to account for the third highest cause of cancer deaths.1 Surgery is the optimal treatment for patients with GC and provides the best chance of long-term survival. However, many patients are initially diagnosed incurable due to locally advanced or metastatic GC; these patients are not considered surgical candidates and chemotherapy is the standard of care, indicating a very poor outcome.2 Combination of chemotherapy with target medicines or nanoparticles can enhance the cellular uptake of drug payload and reduce the side effects.3,4
Targeted therapy may offer new possibilities for improved outcome. High vascular endothelial growth factor (VEGF) expression is one of the characteristic features of GC,5 and targeting VEGF is therefore considered a promising therapeutic strategy for GC. The anti-vascular endothelial growth factor 2 receptor (anti-VEGFR-2) monoclonal antibody ramucirumab has shown a survival benefit as a second-line treatment option in those metastatic GC patients who progressed on fluoropyrimidine-based or platinum-based first-line chemotherapy.6 A recent phase III trial demonstrated that apatinib, the first oral receptor tyrosine kinase inhibitor selectively targeting VEGFR-2, could significantly improve the overall survival and the progression-free survival with an acceptable safety profile. Meanwhile, apatinib was approved by the China Food and Drug Administration CFDA in December 2014 for patients with pretreated metastatic GC.7 In this contest, apatinib has been regarded as an appealing agent in GC treatment.
The optimal use of angiogenic targeted drugs in GC has yet to be established. Considering the limited benefit of apatinib as a single agent, studies are investigating whether its combination with chemotherapy should be initiated. Interestingly, a combination of antiangiogenic targeted therapies and chemotherapy in metastatic GC appeared to show a synergistic effect without excessive toxicity. The AVAGAST trial, which was designed to evaluate the efficacy of adding bevacizumab to capecitabine–cisplatin chemotherapy in metastatic GC, showed that the combination led to significant improvement with regard to the response rate and progression-free survival.8 Another phase III clinical trial, the RAINBOW study, found that overall survival was significantly prolonged with the addition of ramucirumab to paclitaxel (PTX) chemotherapy.9 Moreover, the chosen backbone of chemotherapy may further influence the response to antiangiogenic treatment.10,11
PTX plus 5-fluorouracil (5-Fu) has become the standard chemotherapy for advanced GC. Considering apatinib and ramucirumab share the same target with a different mechanism of action, the combination of apatinib and PTX-based chemotherapy promises to be an attractive strategy leading to a greater magnitude of clinical benefit.12 In this study, we conducted experiments to investigate whether apatinib can enhance the antitumor efficacy of PTX and 5-Fu in vitro/in vivo. In addition, we further evaluated the administration of apatinib with PTX plus S1 (an orally administered 5-Fu analog) in 4 GC patients with different metastatic disease who received conversion surgery; both the safety and efficacy of this combination were assessed.
Materials and methods
Reagents
Apatinib was obtained from Hengrui Medicine Co. (Jiangsu, People's Republic of China). The Cell Counting Kit-8 (CCK-8) and BCA Protein Assay Kit were purchased from cwbiotech. FBS was from Gibco BRL (Thermo Fisher Scientific, Waltham, MA, USA). RPMI 1640, PBS, and 0.25% trypsin were from Life Technologies, Inc. (Thermo Fisher Scientific). PTX was obtained from Pfizer, Inc. (New York, NY, USA). 5-Fu, cisplatin, and irinotecan were obtained from Sigma-Aldrich Co. (St Louis, MO, USA).
Cell line
The MGC803 cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, People's Republic of China. MGC803 cells were cultured in RPMI-1640 basal medium with a supplementation of 10% FBS at 37°C in a humidified atmosphere of 5% CO2. Cells were grown in drug-free culture medium for >2 weeks before assay.
Cell proliferation and toxicity test (Cell Counting Kit-8)
The cells were seeded at a concentration of 1500 cells in each well of a 96-well plate. After 24 h, the medium was replaced by fresh medium with or without various concentrations of drugs. A cell viability assay was carried out using the Cell Counting Kit-8 according to the manufacturer’s instructions. Six wells were used for each drug concentration and the experiment was replicated three times. Optical density values were measured at 450 nm. The IC50 values were defined as the concentrations resulting in a 50% reduction in growth as compared with control cell growth. Drug interactions were analyzed using the median effect principle (Talalay–Chou method). The combination index (CI) was calculated using CompuSyn software, where CI=1 is indicative of an additive effect, CI<1 is indicative of a synergistic effect, and CI>1 is indicative of an antagonistic effect.
In vivo assays of antitumor activity using the zebrafish model
The MGC803 cells were labeled with CM-Dil (Thermo Fisher Scientific) as the protocol described. CM-Dil cells were washed in PBS and resuspended at 10,000 cells/mL. Embryos were anesthetized by placement in 0.04 mg/mL ethyl 3-aminobenzoate methanesulfonate tricaine 48 h post fertilization. CM-Dil cells (100 cells in 10 nL, 30 nL injection volume per embryo) were injected into the perivitelline space with a pneumatic injector using glass microinjection needles. Groups of larvae (n=30) with red fluorescence at the injection site were moved to 6-well plates (30 embryos per well). Larvae were treated at 5 days post infection and incubated at 35°C during treatment.
Stock solutions of the drugs to be tested were made up to 100× working concentration in an appropriate solvent. Apatinib was then diluted to the final concentration with E3 medium, fresh drug containing E3 medium was replaced daily, and E3 was used as a no treatment control. PTX and 5-Fu at the concentrations to be tested were administrated through caudal vein microinjection. We evaluated tumor growth and fish survival at 5 days post treatment by fluorescence microscopy. Images were captured on a Nikon SMZ1500 microscope and the bioluminescence density (BD) from infected zebrafish larvae was measured with NIS-Elements D 3.10 software. The inhibition rates were calculated using the following formula: inhibition rate (%)=(BD of control group−BD of experimental group)/(BD of control group−BD of blank group)×100%.
Patients
We enrolled 4 patients with a clinical diagnosis of stage IV gastric adenocarcinoma at our institute between August 2015 and September 2015. The inclusion criteria were as follows: 1) newly diagnosed with gastric adenocarcinoma; 2) clinically diagnosed with one unresectable and/or metastatic lesion (case 1 with para-aortic lymphatic metastasis, case 2 with highly locally advanced lesion, case 3 with liver metastasis, and case 4 with peritoneal metastasis); 3) Eastern Cooperative Oncology Group performance status 0–1; 4) no prior chemotherapy, radiotherapy, or major surgical procedure; and 5) provision of signed written informed consent. Patient characteristics are listed in Table 1.
 | Table 1 Baseline characteristics of the 4 cases |
Treatment
Apatinib was administered 500 mg once a day continuously; PTX 130 mg/m2 was given on day 1 as a 2-h intravenous infusion with standard premedication for case 1, case 2, and case 3. Case 4 with peritoneal metastasis received intraperitoneal PTX at 70 mg/m2 as well as intravenous PTX at 60 mg/m2 on day1; S-1 was administered at 80 mg/m2 for 14 consecutive days, followed by 7 days of rest. Treatment was administered for 3–5 cycles, but the last cycle of therapy did not include apatinib.
Clinical assessment of surgery and histological evaluation of surgical specimen
Routine computed tomography (CT) scans and laparoscopic exploration were applied both before and after chemotherapy. The clinical response for measurable metastatic tumors was evaluated based on the Response Evaluation Criteria in Solid Tumors guidelines version 1.0. A multidisciplinary team of oncologists, liver surgeons, and radiologists confirmed whether the patient was eligible for surgery. In the present study, conversion gastrectomy was defined as surgery with curative intent aimed at leaving no macroscopic residual tumor.
Surgical complications were assessed according to the Clavien–Dindo classification. All resected specimens were examined by the same pathologist to assess the extent of residual disease, disease stage, and effect of chemotherapy according to the criteria of the Japanese Classification of Gastric Carcinoma 3rd edition.13 Tumors were graded as 0–3 based on the degree of necrosis or disappearance of the tumor in relation to the estimated total amount of the lesion.
Results
Synergistic analysis for the combined use of apatinib and cytotoxic agents in vitro
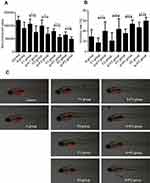
To explore the impact of apatinib on the cytotoxicity of PTX and 5-Fu in MGC803 GC cells, concentration–response curves of each agent alone were first assessed and then IC50 values were determined; both PTX and 5-Fu alone produced inhibition of cell viability with an IC50 value of 17.57±1.09 nM/L and 145.43±10.3 μM/L, respectively. The IC50 value of apatinib was 28.39±1.82 μM/L. Then, MGC 803 cells were treated with PTX or 5-Fu combined with apatinib at the ratios calculated based on their IC50 values (1000:1 for PTX and apatinib or 5:1 for 5-Fu and apatinib). The results showed a decrease in IC50 by 30% for PTX and 48% for 5Fu, respectively, with an obvious left shift of the concentration–response curves (Figure 1A and B). The synergy analysis using Calcusyn software showed that the combination of 5-Fu and apatinib could synergistically inhibit cell growth at high concentrations, while PTX and apatinib was found synergistic (<1) at all analyzed concentrations (Figure 1C and D).
Apatinib enhanced paclitaxel-mediated and 5-Fu-mediated antitumor efficacy in vivo
We used transparent Casper zebrafish early-stage embryos to investigate the antitumor effects of apatinib plus PTX or 5-Fu. The maximum tolerated dose of every agent was determined before examination (data not shown). MGC803 xenografts in Casper zebrafish embryos were treated with PTX (0.75 and 1.5 ng/fish) or 5-Fu (25 and 50 ng/fish) either alone or in combination with apatinib (0.25 µg/mL). Tumor sizes were evaluated by fluorescence microscopy 5 days post injection (Figure 2C). The inhibitory rates of every group are summarized in Table 2. Except for group F1 (5-Fu 25 ng/fish), the bioluminescent signal decreased after treatment with 5-Fu and PTX, either alone or in combination with 0.25 µg/mL apatinib, compared to the negative control (p<0.05). The results also indicated that apatinib plus PTX or 5-Fu is more efficient than single treatment in suppressing tumor growth (Figure 2A and B). No obvious edema and death of zebrafish were observed in any of the treatment groups, suggesting the therapies were well tolerated.
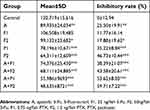 | Table 2 The bioluminescent signal and inhibitory rates of every group |
Tolerance and adverse events of preoperative chemotherapy
The combination of apatinib plus PTX/S1 (PS) chemotherapy was relatively well tolerated. Three patients received 3 cycles of treatment and 1 patient who was diagnosed with peritoneal metastasis received 5 cycles of treatment before surgery. No patient had a dose reduction of either apatinib or PS chemotherapy. No more than a 3-day chemotherapy delay due to toxicity was observed in all patients. The incidence of adverse events (AEs) for preoperative treatment was high: 4 patients developed a total of 21 AEs, but only 4 of these (19%) were grade III or higher according to the Clavern system (Table 3). The main AEs that may be exclusively associated with apatinib occurred in 3 patients, including hypertension in 2 patients and proteinuria in 2 patients. The most common AE was neutropenia and could be managed with standard treatment. There was no preoperative mortality. The incidence of hematologic and nonhematologic AEs is shown in Table 3.
 | Table 3 Incidence of hematologic and nonhematologic adverse events |
Assessment of tumor response to preoperative chemotherapy
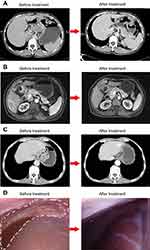
All patients received both a CT scan and magnetic resonance diffusion-weighted imaging (MRI) for volumetric or functional evaluation of the tumor response before resection. Laparoscopic exploration was also conducted perioperatively in the case with peritoneal metastasis. With regard to the primary tumor, no complete response was observed and all patients enrolled had a partial response. As for the evaluation of metastatic lesions, the patient who showed apparent shrinkage of his peritoneal nodules as well as negative peritoneal cytology at the second laparoscopy was considered a complete response. The other 3 patients had a partial response in their metastatic lesion. After preoperative treatment, all 4 patients who were initially considered unresectable were regarded as potentially resectable, which represented a conversion rate of 100%. Tumor comparison between before and after treatment assessed by CT, MRI, or laparoscopic exploration is demonstrated in Figure 3.
Surgical outcome
All 4 patients underwent conversion surgery with curative intent after preoperative treatment. The median time from the last dose of apatinib to surgery was 4.3 weeks. The patient with peritoneal metastasis underwent primary tumor resection only and the other 3 patients had a synchronous primary tumor and metastatic resection. No increase of bleeding events, compared to our historical data, could be observed during operation; only 1 patient required a blood transfusion because of severe preoperative anemia. Since it has been suggested that antiangiogenic targeted drugs can interfere with wound healing, an anastomotic leak and wound-healing delay were considered apatinib-related morbidities. There was no apatinib-related morbidity observed in the 4 patients. No patient died during the study or experienced grade III or higher complications according to the Clavien–Dindo system. The surgical procedures and outcomes are shown in Table 4.
 | Table 4 Surgical outcome of patients |
Pathological examination
All 4 patients were staged initially as T4 by imaging studies according to the Japanese Classification of Gastric Carcinoma. However, only 2 patients were pathologically diagnosed to invade to the serosa or adjacent structures and staged to T4. Tumor-downstaging was observed. When patients were stratified according to the magnitude of tumor viability, all 4 primary lesions had grade 2 pathological response, with no complete pathologic response (ie, no identifiable viable tumor cells in any tumor nodules) observed. With regard to metastasis, however, the degree of pathologic response was more significant. There was no identifiable viable tumor cell in the lymphatic nodes, liver metastasis, or omentum of case 1, case 2, and case 4, respectively.
Discussion
This study aimed to explore the feasibility and effectiveness of apatinib combined with chemotherapy in conversional therapy. Our results demonstrated that the combination of apatinib and chemotherapy may have high efficiency and acceptable safety profiles. The synergistic effect of apatinib with both PTX and 5-Fu was observed by in vitro study. In GC patients with a single site of metastasis, the addition of apatinib to the PS regimen chemotherapy also led to a superior response rate and resectability. The overall occurrence of toxicity of this combination was high; however, the expected adverse effect associated with apatinib was mild. Of the 4 patients included in this study, 1 patient experienced proteinuria and another presented with hypertension which required further medication. No bleeding, gastrointestinal perforation, arterial thromboembolism, or would healing complication occurred. In addition, a high pathological response rate was observed in this study.
Angiogenesis plays a major role in GC development and progression; targeting angiogenesis represents a new era for the treatment of advanced GC. Apatinib, an orally bioavailable agent targeting VEGFR-2, has shown efficacy and safety in preclinical and clinical studies involving GC patients. In vitro, apatinib inhibited proliferation and migration of human umbilical vein endothelial cells through suppression of the kinase activities of VEGFR-2, c-kit, and c-src.14 In vivo, apatinib showed clear antitumor effects on various tumor xenograft models including colon cancer and GC. According to the recently published results of a phase III study, apatinib alone showed good safety, tolerance, and treatment efficacy in patients with chemotherapy-refractory metastatic GC, prolonging the median overall survival by 55 days and the median progression-free survival by 25 days compared with placebo. These data confirm that apatinib represents a new effective treatment option for GC. However, monotherapy with antiangiogenesis drugs appears to be effective only in selected highly VEGF-dependent tumors; combinations with chemotherapy have otherwise proven necessary.15 So far, clinical experience has confirmed that the addition of antiangiogenic target therapy to conventional chemotherapy may lead to improved response rates in several solid tumors, including colon cancer,16 breast cancer,17 and ovarian cancer;18 however, the impact of apatinib on the efficiency of conventional chemotherapeutic agents remains to be determined. Previous studies in vivo demonstrated that apatinib significantly improved the efficacy of traditional chemotherapy by stimulating the ATPase activity of P-gp.19 In this study, we further found that apatinib could significantly improve the efficacy of chemotherapy including PTX and 5-Fu both in vivo and in vitro.
The choice of chemotherapy regimen may be key to maximizing the response rate with the apatinib combination.12 There is now both in vivo and in vitro evidence that PTX displays innate antiangiogenic properties, mediated through direct effects on endothelial cell function as well as inhibition of VEGF-induced neovascularization.11 In the RAINBOW trial,9 the anti-VEGFR-2 monoclonal antibody ramucirumab demonstrated a considerable effect on the efficacy of PTX in metastatic GC after first-line chemotherapy failure. Considering the combination of ramucirumab with platinum-based backbone chemotherapy (FOLFOX) in the first-line setting did not meet its survival endpoint, an improved synergy between PTX and ramucirumab may contribute to the positive results seen in the RAINBOW trial. Since ramucirumab and apatinib share the same target of action with a different mechanism, we believed that the combination of apatinib and PTX has promising efficacy in patients with metastatic GC. In addition, PTX and S-1 chemotherapy (PS regimen) has been regarded as a promising choice and recently received considerable attention from researchers in GC. S-1 monotherapy for advanced GC has shown a high overall response rate of 44–54%. According to the JCOG9912 Trial,20 S1 has been used as a good alternative to continuous infusion of 5-Fu in unresectable GC. The response rate of PTX monotherapy is optimal, reported as 20–25%.21 Furthermore, it has been confirmed that fluoropyrimidine is synergistic with taxane.22 Fukuchi et al23 also noted that the PS regimen has similar efficacy but lower toxicity than S-1 plus cisplatin. Given its high efficiency and possible synergy with antiangiogenic drugs, the PS regimen was considered a promising combination treatment with apatinib. In this study, we enrolled 4 advanced GC patients with different noncurable factors, including 1 liver metastasis, 1 peritoneal metastasis, 1 para-aortic node metastasis, and 1 highly local advanced disease. Patients were treated with apatinib plus PS for 3–5 cycles, the last cycle including only PS, leaving at least 4 weeks before surgery without apatinib. After treatment, all patients achieved a significant response and underwent potentially curative gastrectomy. These results indicated that apatinib together with the PS regimen may improve the response rate and facilitate a secondary resection with curative intent in metastatic GC.
Our results showed that the addition of apatinib to the PS regimen did not substantially alter the safety profile of chemotherapy in patients with GC. The incidence of hematological and gastrointestinal AEs compared well with results from other trials in metastatic GC. The most common grade 3 or 4 AE was neutropenia which could be treated with symptomatic therapy. Hypertension and proteinuria were other common AEs and were readily managed with standard antihypertensives such as angiotensin converting enzyme inhibitors and diuretics. No other classical toxicity of antiangiogenic drugs, in particular gastrointestinal perforation, venous or arterial thromboembolic events, or bleeding, was observed during preoperative treatment. The safety profile of apatinib in this study was acceptable, and no dose interruptions or reductions occurred due to intolerable toxicity.
Apatinib is a potent inhibitor of VEGFR activity, and thus it has been suggested that use of apatinib in the neoadjuvant treatment could potentially impact on intraoperative bleeding, anastomotic healing, and postsurgical wound healing. Based on early clinical data/experience of bevacizumab in the neoadjuvant and conversion treatment of metastatic colorectal cancer, it is recommended that the antiangiogenic targeted agent should be stopped at least 5–8 weeks before surgery and should not be restarted until 28 days after surgery or when all incisions have healed completely.24 Our data demonstrated that a break of 4 weeks between the last dose of apatinib and radical gastrectomy may not increase the rate of complications in patients with metastatic GC; no increased intraoperative bleeding compared to our historical data and no delayed wound-healing complication or anastomotic dehiscence occurred. These results provided the clinical information to indicate that apatinib in combination with chemotherapy does not affect perioperative morbidity after appropriate management.
However, the present study has limitations. First, this study is a preclinical study aiming to explore the possible effect of a combination of apatinib and chemotherapy. Also, only 4 patients were included in the study, so the conclusion needs to be further confirmed by a large-sample, randomized study. Therefore, we have initiated a phase II trial of apatinib plus PS regimen chemotherapy in the conversion therapy of metastatic GC (NCT02529878), which will be completed with detailed data to evaluate the clinical efficacy of adding apatinib to the PS regimen in conversional therapy of unresectable GC. Second, the mechanism for the combination of apatinib and chemotherapy is unknown, and we are looking forward to exploring the mechanism by which the combination of apatinib and chemotherapy displays enhanced activity.
Conclusion
A combination of chemotherapy and apatinib may be an efficient and acceptable safety treatment for late-stage GC. Apatinib may be an option to improve the chemosensitivity of a standard chemotherapy regimen in the treatment of tumors.
Ethical approval
All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. This study was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University.
Acknowledgments
An abstract of this paper was published in “Poster Abstracts” in the Journal of Clinical Oncology, as “Apatinib to enhance chemosensitivity of gastric to paclitaxel and 5-fluorouracil”.
This work was supported by grants from the Natural Science Foundation of Zhejiang Province (LY16H280011 and LY18H290006), the Key Research Program of Traditional Chinese Medical Science and Technology Plan of Zhejiang Province (2016ZZ012), and the Medical Science and Technology Project of Zhejiang Province (WKJ-ZJ-1728 and 2016KYB220).
Disclosure
The authors reported no conflicts of interest in this work.
References
1. Hu C, Zhu HT, Xu ZY, et al. Novel abdominal approach for dissection of advanced type II/III adenocarcinoma of the esophagogastric junction: a new surgical option. J Int Med Res. 2019 Jan;47(1):398410;300060518802923.
2. Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14(12):e535–e547. doi:10.1016/S1470-2045(13)70436-4
3. Alamzadeh Z, Beik J, Pirhajati Mahabadi V, et al. Ultrastructural and optical characteristics of cancer cells treated by a nanotechnology based chemo-photothermal therapy method. J Photochem Photobiol B Biol. 2019;192:19–25. doi:10.1016/j.jphotobiol.2019.01.005
4. Keshavarz M, Moloudi K, Paydar R, et al. Alginate hydrogel co-loaded with cisplatin and gold nanoparticles for computed tomography image-guided chemotherapy. J Biomater Appl. 2018;33(2):161–169. doi:10.1177/0885328218782355
5.
6. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (London, England). 2014;383(9911):31–39. doi:10.1016/S0140-6736(13)61719-5
7. Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett. 2016;372(2):187–191. doi:10.1016/j.canlet.2016.01.014
8. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–3976. doi:10.1200/JCO.2011.36.2236
9. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-–blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi:10.1016/S1470-2045(14)70420-6
10. Roviello G, Petrioli R, Marano L, et al. Angiogenesis inhibitors in gastric and gastroesophageal junction cancer. Gastric Cancer. 2016;19(1):31–41. doi:10.1007/s10120-015-0537-5
11. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481–492.
12. Roviello G, Roviello F, Polom K, Generali D. Apatinib in metastatic gastric cancer: can paclitaxel make the difference? Anticancer Drugs. 2016;27(8):809. doi:10.1097/CAD.0000000000000380
13.
14. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. doi:10.1111/j.1349-7006.2011.01939.x
15. Garrido M. The safety and efficacy of ramucirumab in combination with paclitaxel for the treatment of advanced gastric or gastro-esophageal junction adenocarcinoma. Expert Rev Anticancer Ther. 2016;16(10):1005–1010. doi:10.1080/14737140.2016.1231576
16. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi:10.1056/NEJMoa032691
17. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi:10.1056/NEJMoa072113
18. Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–2496. doi:10.1056/NEJMoa1103799
19. Tong XZ, Wang F, Liang S, et al. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol. 2012;83(5):586–597. doi:10.1016/j.bcp.2011.12.007
20. Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10(11):1063–1069. doi:10.1016/S1470-2045(09)70259-1
21. Liu H, Chen X, Sun J, et al. The efficacy and toxicity of paclitaxel plus S-1 compared with paclitaxel plus 5-FU for advanced gastric cancer: a PRISMA systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2014;93(25):e164. doi:10.1097/MD.0000000000000164
22. Kodera Y, Fujiwara M, Yokoyama H, et al. Combination of oral fluoropyrimidine and docetaxel: reappraisal of synergistic effect against gastric carcinoma xenografts. In Vivo. 2005;19(5):861–866.
23. Fukuchi M, Ishiguro T, Ogata K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015;22(11):3618–3624. doi:10.1245/s10434-015-4422-6
24. Gruenberger B, Tamandl D, Schueller J, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26(11):1830–1835. doi:10.1200/JCO.2007.13.7679
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.