Back to Journals » OncoTargets and Therapy » Volume 11
Apatinib combined with docetaxel as a salvage treatment for metastatic esophageal squamous cancer: a case report
Authors Liang LJ, Wen YX, Xia YY, Wang L, Fei JY, Jiang XD
Received 16 May 2018
Accepted for publication 10 August 2018
Published 13 September 2018 Volume 2018:11 Pages 5821—5826
DOI https://doi.org/10.2147/OTT.S174429
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Federico Perche
Li-Jun Liang,1,2,* Yi-Xuan Wen,1,2,* You-You Xia,1,* Lei Wang,1 Jia-Yan Fei,1,2 Xiao-Dong Jiang1,2
1Department of Radiation Oncology, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, People’s Republic of China; 2Tumor Laboratory, The Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, People’s Republic of China
*These authors contributed equally to this work
Abstract: The first-line treatment for metastatic esophageal squamous cell cancer (ESCC) is a platinum- or fluorouracil-based agent, followed by later treatment with taxanes or irinotecan. However, there is still no standard third-line treatment for patients with metastatic ESCC. We present a 62-year-old man initially diagnosed with locally advanced ESCC. After esophagectomy, the patient was administrated with six cycles of docetaxel and cisplatin combined with radiotherapy. After 8.0 months, computed tomography showed the left cervical lymph node metastasis. However, the metastatic lymph node was not significantly shrunk after locally palliative radiotherapy and the patient was intolerant of irinotecan as second-line systemic therapy. Then, the patient was rechallenged with six cycles of docetaxel combined with apatinib (an oral tyrosine kinase inhibitor to vascular endothelial growth factor receptor 2 [VEGFR2]), followed by single dose of apatinib as maintenance therapy. According to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standard, partial response was achieved in this case after treating with docetaxel combined with apatinib. Now, the progression-free survival of this patient has been 7.5 months. After administrating with apatinib for 2 weeks, hypertension (grade III) was observed. Thus, the dose of apatinib was decreased from 850 to 500 mg and then the adverse effects were controllable and tolerable. In conclusion, apatinib with concurrent docetaxel provided potential efficacy as a salvage treatment for patients with metastatic ESCC. To our knowledge, this is the first case of ESCC who responded to apatinib combined with docetaxel.
Keywords: metastatic esophageal cancer, apatinib, docetaxel, vascular endothelial growth factor receptor-2, anti-angiogenesis
Introduction
Esophageal cancer is the world’s eighth most common cancer and the sixth leading cause of cancer-related deaths.1 Different from the situation in western countries, esophageal squamous cell carcinoma (ESCC) is still the dominant pathological type in China, which accounts for more than 95% of clinical cases.2 The annual incidence of ESCC in China was about 260,000 and approximately half of the patients diagnosed with ESCC presented with metastatic diseases.2 The first-line treatment for metastatic esophageal cancer is a platinum or fluorouracil-based agent,3–5 followed by later treatment with taxanes6,7 or irinotecan.8,9 No treatment strategy has been defined for patients who have been failed or intolerant to current standard therapies.
Angiogenesis is a critical process for cell growth, especially for the tumor growth.10 The vascular epidermal growth factor (VEGF) could bind to vascular epidermal growth factor receptor (VEGFR) and activate the downstream pathway to stimulate the proliferation of vessel endothelium, then leading to the growth of tumor.11 Previous molecular pathology studies have revealed that VEGF overexpression could be detected in about 44.43% of esophageal cancer and the estimated mortality risk was 1.82-fold greater in patients with high VEGF expression,12,13 which indicated that antiangiogenic agents may have a therapeutic effect on esophageal cancer.
Apatinib, a novel small molecule tyrosine kinase inhibitor (TKI), exerts its anti-tumor effects by specifically acting on VEGFR-2.14 Phase II and III clinical trials suggested that apatinib could prolong overall survival (OS) of advanced gastric cancer patients who failed in the second-line treatment.15,16 In December 2014, China State Food and Drug Administration approved and launched apatinib as a treatment for patients with chemotherapy-refractory gastric cancer. Furthermore, many clinical studies have reported the satisfying efficacy of apatinib on various cancers including non-small-cell lung cancer,17 breast cancer,18,19 and pancreatic cancer.20 However, there is rare report to evaluate its efficacy and safety in patient with esophageal cancer. We herein report a unique case of ESCC receiving concurrent apatinib and docetaxel following failure of the second-line therapy. The progression-free survival (PFS) of this patient at least 7.5 months has been achieved now, demonstrating the potential of apatinib combined with chemotherapy in the treatment of ESCC. To our knowledge, this is the first case of ESCC who responded to apatinib combined with docetaxel.
Case report
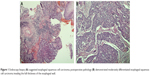
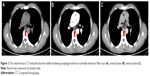
A 62-year-old man suffered from dysphagia for almost 1 month was admitted to our hospital. Endoscopy biopsy suggested ESCC 28–35 cm away from incisors (Figure 1A and B) and computed tomography (CT) indicated that there was a space-occupying lesion at the lower esophagus (Figure 2). Physical examination did not show syndromes of hoarse voice and cough during drinking water, and no swelling superficial lymph node felt by touch. The patient had no history of hypertension, heart disease, diabetes, and kidney-related diseases.
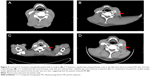
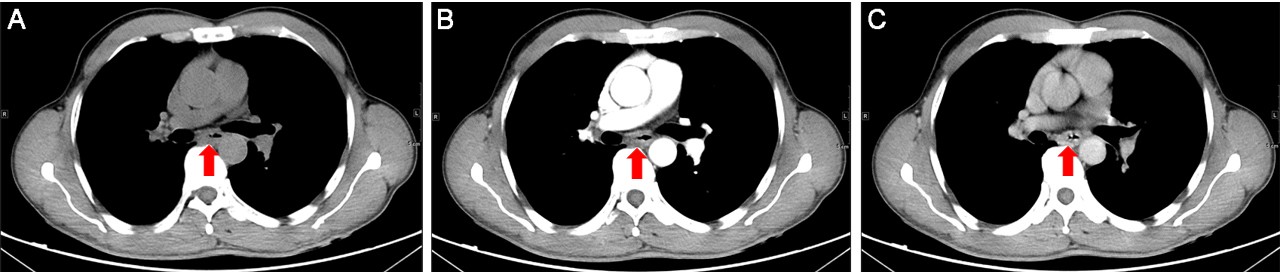
The esophagectomy was performed on September 2, 2016; postoperative pathological examination indicated moderately differentiated ESCC. The full thickness of the esophageal wall was invaded, with dimensions of 5 × 2 × 1.3 cm, and metastasis was observed in esophageal lymph nodes (3/7; Figure 1B) but not observed in the resection margin. According to the eighth edition Staging Guidelines of American Joint Committee on Cancer, the surgical-pathological staging was T3N2M0, stage IIIB. Since September 2016, the patient received six cycles of chemotherapy of docetaxel (75 mg/m2, day 1) and cisplatin (70 mg/m2, day 1) combined with radiation therapy (total dose=50 Gy/25 fractions). In the follow-up after therapy, the gastroscopy and imaging examination showed no tumor recurrence. Until May 1, 2017, a lump could be touched in the patient’s left neck, shown as left cervical lymph node metastasis by CT (Figure 3A and B). Subsequently, locally radiotherapy (total dose=50 Gy/25 fractions) and three cycles of irinotecan (CPT-11, 250 mg/m2, 21 days as one cycle) monotherapy were performed; however, the patient suffered intolerable myelosuppression (grade IV) and severe gastrointestinal disturbances. Since CT revealed that the left cervical lymph node was not narrowed, the treatment was terminated (Figure 3C). After symptomatic treatment, the patient’s leucocytes returned to normal level, and the gastrointestinal adverse reactions disappeared.
Since July 28, 2017, the patient received apatinib combined with six cycles of docetaxel (75 mg/m2, day 1, 21 days as one cycle) and then administrated apatinib as maintenance therapy. After treatment with apatinib for 1.4 months, CT examination showed cervical lymph node was decreased, without tumor recurrence or metastasis in other sites (Figure 3D). These results suggested that the patient achieved partial response (PR) according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standard. There was no sign of progression in the patient’s conditions until we reported this case and now the PFS was 7.5 months (until March 10, 2018). The treatment schedule is given in Figure 4.
  | Figure 4 The treatment schedule of the patient. |
After apatinib was administrated for two weeks, hypertension (grade III) appeared, so the dose of apatinib was adjusted from 850 to 500 mg. During the apatinib treatment cycles, the patient developed mild-to-moderate hypertension and hand-foot syndromes. After treating with appropriate agents, no other anti-angiogenesis-associated adverse event (AE) was reported. Toxicity was evaluated and graded according to the NCI-CTC for Adverse Events, version 4.0.
The study was approved by the Medical Ethics Committee of the Affiliated Lianyungang Hospital of Xuzhou Medical University and written informed consent was obtained from the patient. The patient also provided his written informed consent for the case details and accompanying images to be published in this case study.
Discussion
To date, there has been no effective therapy as standard third-line therapy for improving the survival of ESCC patients due to its aggressive nature. To the best of our knowledge, this is the first case report on apatinib combined with docetaxel for ESCC treatment achieving PFS of 7.5 months. In addition, the patient tolerated well to the treatment, and reported satisfactory quality of life.
As early as 1971, professor Folkman suggested that tumor growth relied on the formation of tumor blood vessels, thus “anti-tumor angiogenesis” would be a promising strategy for treating tumors.11 Based on previous researches, multiple antiangiogenic agents have been developed and studied in clinical trials. In the RAINBOW clinical trial, ramucirumab (a monoclonal antibody of VEGFR-2) combined with paclitaxel significantly increased OS compared with placebo combined with paclitaxel in advanced gastric or gastro-esophageal junction adenocarcinoma.21 This agent was also advocated in treatment guidelines for esophageal adenocarcinoma.22 Some clinical trials have demonstrated the effectiveness of bevacizumab as neoadjuvant therapy in ESCC.23,24 However, there are very few studies on anti-angiogenesis agents for metastatic ESCC.
Apatinib is a novel orally taken small molecular TKI, exerting its anti-angiogenesis function through highly and selectively competing with intracellular VEGFR-2’s adenosine triphosphate (ATP) binding sites. Thus, downstream signaling could be blocked to inhibit neovascularization in tumor tissue. The Phase II clinical trial of apatinib reported that median OS was increased to 4.83 months with apatinib 850 mg group, 4.27 months with apatinib 425 mg group, compared with 2.50 months with placebo group (P<0.001 and P=0.0017, respectively).15 Similar results were confirmed by the Phase III study (median OS was 6.5 months in the apatinib 850 mg vs 4.7 months in the placebo group, P=0.0149; HR=0.709, 95% CI 0.537–0.937, P=0.0156).16 The efficacy of apatinib on 62 advanced ESCC patients who failed the standard therapy was retrospectively analyzed,25 among which, 15 received PR, while 31 achieved stable disease, with a manageable safety profile. These results demonstrated the preliminary efficacy and safety of apatinib for advanced ESCC.
Although antiangiogenic therapies show remarkable antitumor activity, their efficacy is limited as monotherapy. Hence, these agents have been integrated with conventional chemotherapy or radiotherapy to enhance antitumor activity.26,27 In our case, the patient received the docetaxel plus cisplatin regimen as first-line treatment and a PFS of 8.0 months was achieved. After the failure of standard chemotherapy and local radiation therapy of metastatic lesion, the patient still showed performance status score of 1, with strong willingness to continue the treatment. Thus, the docetaxel plus apatinib was administrated as salvage treatment strategy. Beyond our expectation, for this patient, the PFS of third-line treatment can be favorably comparable with the first-line treatment, indicating a potentially synergistic effect between apatinib and docetaxel chemotherapy. Moreover, recent research showed that the cytotoxicity of paclitaxel could be significantly enhanced by apatinib in vitro and in vivo by reversing chemotherapy-resistance through blocking the function of multiple ATP-binding cassette transporters.28 Furthermore, apatinib can markedly increase the intracellular accumulation of conventional chemotherapy agents in side population cells sorted from K562 cells,29 and cisplatin-resistant non-small-cell lung carcinoma A549 cell could be resensitized through suppressing extracellular signal-regulated kinase signaling pathway.30 Currently, there are also multiple ongoing clinical trials investigating on apatinib for molecular targeted therapy in ESCC (Table 1).
  | Table 1 Selected ongoing trials with apatinib in metastatic ESCC |
The common AEs of apatinib were hypertension and hand-foot syndrome.31 According to the instruction of apatinib, we first prescribed the patient with apatinib of 850 mg daily. Then, grade III hypertension was observed, leading to dosage adjustment to 500 mg. The AEs were controllable and tolerable. The bioavailability of apatinib in ESCC patients was noteworthily higher than those in patients with gastric cancer, which might be attributed to the fact that most gastric cancer patient had a subtotal gastrectomy surgery history.32
In conclusion, apatinib with concurrent docetaxel had potential efficacy for metastatic ESCC patients as a salvage treatment and the prospective clinical trials are ongoing (Table 1).
Acknowledgments
We thank the patients and all investigators involved in this study. The first authors of this manuscript are Li-Jun Liang, Yi-Xuan Wen, and You-You Xia.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, et al. CA: a cancer journal for clinicians. 2015;65(2):87–108. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Hayashi K, Ando N, Watanabe H, et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol. 2001;31(9):419–423. | ||
Bleiberg H, Conroy T, Paillot B, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997;33(8):1216–1220. | ||
Huang J, Zhou Y, Zhang H, et al. A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Med Oncol. 2013;30(1):343. | ||
Kato K, Tahara M, Hironaka S, et al. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol. 2011;67(6):1265–1272. | ||
Muro K, Hamaguchi T, Ohtsu A, et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. 2004;15(6):955–959. | ||
Burkart C, Bokemeyer C, Klump B, Pereira P, Teichmann R, Hartmann JT. A phase II trial of weekly irinotecan in cisplatin-refractory esophageal cancer. Anticancer Res. 2007;27(4C):2845–2848. | ||
Mühr-Wilkenshoff F, Hinkelbein W, Ohnesorge I, et al. A pilot study of irinotecan (CPT-11) as single-agent therapy in patients with locally advanced or metastatic esophageal carcinoma. Int J Colorectal Dis. 2003;18(4):330–334. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15(6):385–403. | ||
Luz CCF, Noguti J, Araújo L, et al. Expression of VEGF and Cox-2 in Patients with Esophageal Squamous Cell Carcinoma. Asian Pac J Cancer Prev. 2018;19(1):171–177. | ||
Chen M, Cai E, Huang J, Yu P, Li K. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1126–1134. | ||
Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. | ||
Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. | ||
Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34(13):1448–1454. | ||
Zhang L, Shi M, Huang C, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. Journal of Clinical Oncology. 2012;30(15_suppl):7548. | ||
Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135(8):1961–1969. | ||
Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14:820. | ||
Li CM, Liu ZC, Bao YT, Sun XD, Wang LL. Extraordinary response of metastatic pancreatic cancer to apatinib after failed chemotherapy: A case report and literature review. World J Gastroenterol. 2017;23(41):7478–7488. | ||
Wilke H, Muro K, et al; RAINBOW Study Group, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. | ||
Ajani JA, D’Amico TA, et al. National comprehensive cancer network, et al. Esophageal and esophagogastric junction cancers, version 1. 2015. J Natl Compr Canc Netw. 2015;13(2):194–227. | ||
Idelevich E, Kashtan H, Klein Y, et al. Prospective phase II study of neoadjuvant therapy with cisplatin, 5-fluorouracil, and bevacizumab for locally advanced resectable esophageal cancer. Onkologie. 2012;35(7–8):427–431. | ||
Bendell JC, Meluch A, Peyton J, et al. A phase II trial of preoperative concurrent chemotherapy/radiation therapy plus bevacizumab/erlotinib in the treatment of localized esophageal cancer. Clin Adv Hematol Oncol. 2012;10(7):430–437. | ||
Li J, Wang L. Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma. Onco Targets Ther. 2017;10:3965–3969. | ||
Cheng X, Xu Z, Chen J. Apatinib to enhance chemosensitivity of gastric cancer to paciltaxel and 5-fluorouracil. J Clin Oncol. 2017;35(15_suppl):e15545. | ||
Zhou F, Feng S, Zhang J, Aa J, Wang G. Combined treatment of apatinib with docetaxel in non-small-cell lung cancer mice and its material basis of pharmacokinetics. Journal of Clinical Oncology. 2017;35(15_suppl):e14069. | ||
Yj M, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70(20):7981–7991. | ||
Tong XZ, Wang F, Liang S, et al. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol. 2012;83(5):586–597. | ||
Liu ZL, Jin BJ, Cheng CG, et al. Apatinib resensitizes cisplatin-resistant non-small cell lung carcinoma A549 cell through reversing multidrug resistance and suppressing ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(23):5370–5377. | ||
Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529. | ||
Yu M, Gao Z, Dai X, et al. Population Pharmacokinetic and Covariate Analysis of Apatinib, an Oral Tyrosine Kinase Inhibitor, in Healthy Volunteers and Patients with Solid Tumors. Clin Pharmacokinet. 2017;56(1):65–76. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.