Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Aortic Stiffness Index And Carotid Intima-Media Thickness Are Independently Associated With The Presence Of Microalbuminuria In Patients With Type 2 Diabetes Mellitus
Received 19 July 2019
Accepted for publication 10 September 2019
Published 19 September 2019 Volume 2019:12 Pages 1889—1896
DOI https://doi.org/10.2147/DMSO.S223880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Yaşar Turan,1 Elif Turan2
1Department of Cardiology, Medical School of Bozok University, Yozgat, Turkey; 2Department of Endocrinology and Metabolic Disease, Medical School of Bozok University, Yozgat, Turkey
Correspondence: Yaşar Turan
Bozok University, Cardiology, Yozgat 66200, Turkey
Tel +903542127050
Fax +903542126201
Email [email protected]
Purpose: Microalbuminuria is a premature and widely used indicator of diabetic nephropathy and is reported to be related with a higher cardiovascular risk in diabetic patients. We aimed to examine whether the echocardiographic parameters, such as epicardial fat thickness (EFT), carotid intima-media thickness (CIMT) and aortic stiffness index (ASI) are associated with microalbuminuria in patients with diabetes mellitus type 2 (T2DM).
Patients and methods: A total of 272 consecutive patients were enrolled and after the exclusion criteria, the data of 180 patients with T2DM were used in this cross-sectional study. Patients were divided into two groups: 82 patients with microalbuminuria and 98 patients without microalbuminuria (normoalbuminuria). The laboratory results and echocardiographic EFT, CIMT and ASI parameters were noted.
Results: Compared with the normoalbuminuria group, EFT, CIMT and ASI were significantly higher in the microalbuminuria group (p<0.05 for all). In logistic regression analysis; CIMT (OR: 3.15, p=0.024) and ASI (OR: 4.19, p=0.016) were independently associated with microalbuminuria in patients with T2DM.
Conclusion: In addition to CIMT, as a novel finding, ASI which is an indicator for the elastic properties of the aortic root was independently associated with microalbuminuria. CIMT and ASI measurement by echocardiography may be helpful in identifying the accompanying factors in the development of nephropathy.
Keywords: aortic stiffness, carotid intima-media thickness, microalbuminuria
Introduction
Diabetes mellitus (DM) is a major risk factor for cardiovascular disease.1 Cardiovascular complications and events are more common in patients with diabetic nephropathy (DNP) than in patients without.2 Microalbuminuria is a premature and widely used indicator of DNP and is reported to be related with cardiovascular risk.3
Epicardial fat thickness (EFT) indicates visceral adipose tissue around the heart. This adipose tissue is located between the pericardial and myocardial layer. It has endocrine effects by secreting some cytokines. These cytokines are related with endothelial dysfunction, oxidative stress, inflammation and atherosclerosis.4,5 Ultrasonographic assessment of carotid intima-media thickness (CIMT) is a marker of subclinical atherosclerosis and may predict future cardiovascular disease.6 It is widely used in clinical practice because of its advantages such as low cost, reliability and reproducibility.
Distension during systole and elastic recoil during the diastole phase, primarily in elastic arteries such as the aorta and the carotid, is fundamental to maintain optimal blood flow in a cardiac cycle. The elasticity of these major arteries enables the pressure wave to be dampened. A decrease in the elasticity leads to the loss of this compensatory mechanism. Aortic elastic properties may give additional information about the future cardiovascular events.7 An impaired aortic stiffness is a known cardiovascular risk factor and is related with other cardiovascular risk factors like age, hyperlipidemia, hypertension, diabetes, and smoking.8,9 Echocardiographic measurement of aortic stiffness index (ASI) is a simple and low-cost method that can provide satisfactory information about the aortic elasticity.10,11 ASI can be calculated by using the simple parameters such as blood pressure and systolic-diastolic aortic root diameters.12
We aimed to examine whether the echocardiographic parameters, such as EFT, CIMT and ASI, are associated with microalbuminuria in patients with diabetes mellitus type 2 (T2DM).
Materials And Methods
In this cross-sectional study, 272 consecutive patients who applied to the endocrinology outpatient clinic with the diagnosis of T2DM, according to the criteria of the American diabetes association,13 between November 2018 and June 2019 were enrolled. The study was performed to conform to the Declaration of Helsinki and the study protocol was approved by the ethics committee of Bozok University. Written and informed consent was taken from all the participants. Patients with known cardiovascular disease, heart failure, severe valvular disease, inflammatory disease, chronic renal failure with an estimated glomerular filtration rate (eGFR) <30 mL/min per 1.73 m2, liver insufficiency, acute infections, glucocorticoid therapy, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy and pregnant or nursing women were excluded. After the exclusion criteria, the data of 180 patients were used. Demographic data and anthropometric measurements of the participants were recorded. Body mass index (BMI) was calculated as weight (kg)/height (m)2.
Laboratory Analysis
Venous blood samples were taken for biochemical analyses, after at least 12 hrs of fasting. Fasting glucose, HbA1C, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), serum creatinine were studied for all patients. HbA1C was determined using HPLC-UV detector. The other parameters were measured using Abbott Architect c8000 analyzer (Abbott Laboratories, Abbott Park, Illinois) and original measurement kits. Microalbuminuria was determined by a commonly used clinical index (urinary albumin-to-creatinine ratio). Urinary albumin and creatinine were measured in morning spot urine twice with an interval of 3 months for all patients. The diabetic patients with an albumin-to-creatinine ratio ≤30 mg/g were categorized as normoalbuminuria group (N=98), whereas the diabetic patients with an albumin-to-creatinine ratio 30–300 mg/g were categorized as microalbuminuria group (N=82). Patients with macroalbuminuria (≥300 mg/g) were not included in our study. eGFR was calculated based on Modified Diet in Renal Disease formula which is recommended in patients with diabetes.14
Cardiac Evaluation
The participants included in the study were referred to the cardiology department for echocardiography and measurement of CIMT. Blood pressure (BP) was measured by using the standard mercury manometer. The patients underwent transthoracic echocardiography imaging conforming to the American Society of Echocardiography/European Association of Echocardiography recommendations.15 Echocardiographic examinations were performed using a Philips Affiniti 50 echocardiography device (Philips Healthcare, the Netherlands) and a broadband transducer with simultaneous electrocardiogram follow-up. Echocardiography was done by the same cardiologist who was unaware of the clinical data. Epicardial adipose tissue appears as a relatively echo-free area between the external surface of the myocardium and visceral layer of pericardium.16 EFT was measured from the long axis and apical four-chamber view, perpendicularly on the free wall of the right ventricle at end-diastole in three cardiac cycles.
Carotid intima-media thickness was measured from the far wall of the right carotid artery within 10 mm proximal to the bifurcation on two-dimensional ultrasound images with a 12–4 MHz linear array transducer (Logic Affiniti 50G; Philips, Amsterdam, Netherlands). Three measurements were obtained per scan and the mean CIMT was calculated from these measurements.
In the parasternal long-axis view, both systolic and diastolic inner diameters of the ascending aorta were measured via M-mode echocardiography 3 cm above the aortic annulus.11 Three measurements were obtained from 3 consecutive cardiac cycles and the mean values of aortic systolic diameter (AoSD) and aortic diastolic diameter (AoDD) were calculated.11 ASI was calculated by following formula:11,12
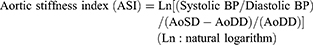
Ten patients were selected on a random basis for repeated echocardiographic EFT, CIMT and ASI measurement to assess intraobserver variability. Reproducibility of the measurements was significantly correlated for intraobserver agreement (p < 0.01 for all).
Statistical Analysis
Kolmogorov–Smirnov test was used for the distribution analysis. Categorical variables were compared by chi-square test. Comparisons among the groups were performed using independent samples t-test for variables distributed normally and Mann–Whitney U-test for non-normally distributed variables. Correlation of the ASI with the other study parameters was assessed by Pearson or Spearman correlation analysis, as appropriate.
A multivariate logistic regression analysis was used to identify factors that independently associated with microalbuminuria in the study population. The variables associated with the presence of microalbuminuria in the univariate analysis were included in the multivariable models. All analyses were performed by SPSS version 18.0 (SPSS for Windows, Chicago, IL). P values <0.05 were accepted as significant.
Results
A total of 180 patients participated in this study (112 females, 68 males), patients were divided into two groups; 82 diabetic patients with microalbuminuria and 98 diabetic patients without microalbuminuria (normoalbuminuria). Baseline demographic and clinical characteristics are summarized in Table 1. Compared with the normoalbuminuria group, diabetes duration, HbA1C, fasting glucose and serum creatinine levels were significantly higher, while eGFR and HDL-C were significantly lower in the microalbuminuria group (p<0.05 for all). Analysis of echocardiographic parameters showed that; compared with the normoalbuminuria group, EFT (5.91±1.43 vs. 6.45±1.39; p=0.014), CIMT (0.71±0.12 vs. 0.76±0.11; p=0.001) and ASI (3.16±0.23 vs. 3.28±0.30; p=0.002) were significantly higher in the microalbuminuria group (Figure 1).
 |
Table 1 Demographic And Clinical Data Of The Participants |
 |
Figure 1 Comparison of aortic stiffness index (A), carotid intima-media thickness (B) and epicardial fat thickness (C) among the normoalbuminuria and microalbuminuria groups. |
A correlation analysis was performed in order to determine the factors associated with the albumin-to-creatinine ratio in patients with T2DM. The albumin-to-creatinine ratio was positively correlated with age (r=0.169, p=0.023), diabetes duration (r=0.160, p=0.032), HbA1C (r=0.198, p=0.008), fasting glucose (r=0.196, p=0.008), serum creatinine (r=0.175, p=0.019), EFT (r=0.179, p=0.016), CIMT (r=0.206, p=0.005) and ASI (r=0.226, p=0.002) (Figure 2) as well as negatively correlated with eGFR (r=−0.181, p=0.015) in patients with T2DM (Table 2).
 |
Table 2 The Correlation Between Albumin-To-Creatinine Ratio And Clinical/demographic Variables In T2DM Patients |
 |
Figure 2 Scatter plot showing correlation of albumin-to-creatinine ratio with carotid intima-media thickness (A) and aortic stiffness index (B). |
The results of the logistic regression analysis showed that CIMT [odds ratio (OR): 3.15; 95% confidence interval (CI): 1.5 to 36.3; P=0.024] and ASI (OR: 4.19; 95% CI: 1.3 to 13.4; P=0.016) was independently associated with the presence of microalbuminuria in patients with T2DM.
Discussion
The main findings of the present study were that EFT, CIMT and ASI were significantly higher in diabetic patients with microalbuminuria compared to the diabetic patients without microalbuminuria. Furthermore, logistic regression analysis revealed that CIMT and ASI were independently associated with the presence of microalbuminuria in patients with T2DM.
In diabetic patients, DNP is a major complication, and it is an important cause of end-stage renal disease. Several pathways are activated during the development of DM. Main pathways, considered as responsible for the pathogenesis of DNP are; oxidative stress, inflammation, fibrosis and cell death. These pathways also regulate the progression of DNP. DM is accompanied by chronic inflammation affecting the kidneys and the whole body. DN is traditionally considered as nonimmune disease, but there is an accumulating data indicating a significant role of inflammatory processes in the pathophysiology of DNP.17,18 Microalbuminuria can be defined as the earliest clinical marker of DNP and it is a well-documented predictor of DNP development in diabetic patients.19 Detection of microalbuminuria is beneficial because starting the treatment early may be preventive for overt nephropathy.
Epicardial fat is an indicator of visceral adiposity, which has a role in the pathogenesis of inflammation, endothelial dysfunction and cardiovascular diseases.20–22 Visceral adiposity has been implicated in the pathogenesis of DNP. Hanai K. reported that visceral adiposity was independently associated with microalbuminuria in Japanese adult patients with T2DM.23 Data regarding the relationship between microalbuminuria and EFT are very limited. Akbaş et al have reported that EFT was independently associated with microalbuminuria in diabetic patients.24 EFT was higher in the microalbuminuria group in our study. However, possibly because the other parameters related to atherosclerosis were also involved in regression analysis, the independent relationship was not detected.
Albuminuria is reported to be associated with endothelial dysfunction and subclinical atherosclerosis.25,26 Atherosclerosis has a long asymptomatic period and CIMT measurement is a useful method for the assessment of atherosclerosis in the asymptomatic period.27 In this context, we aimed to investigate the relationship between CIMT and microalbuminuria. CIMT measurements were significantly higher in the microalbuminuria group in the present study. Additionally, CIMT was independently associated with microalbuminuria in patients with T2DM. Our results were consistent with the previous data probing the relationship between CIMT and microalbuminuria.25,28 However, the possible mechanism of this relation in patients with T2DM remains unclear. With these results, we cannot conclude whether this is a cause or a common result of diabetes complications.
The usefulness of CIMT measurement in cardiovascular risk stratification is a current issue and it is still controversial whether CIMT measurement could add predictive value to the Framingham risk score and associate with subsequent cardiovascular events.29–32 In this manner, considering the relation of microalbuminuria and cardiovascular risk,6 the current results may provide a proof for the relationship between CIMT and risk of cardiovascular events. We can conclude that CIMT measurement may be useful for predicting future cardiovascular events.
Noninvasive detection of premature vascular changes related to the cardiovascular risk is becoming increasingly important in daily clinical practice. Subclinical atherosclerosis may alter vascular functions by causing arterial stiffness. Arterial stiffness can be evaluated in various sections of the vascular system by using different methods. Pulse wave velocity may be simply described as the speed of an arterial pulsation when it is propagating through the arterial tree and the current-recognized gold standard for the assessment of arterial stiffness is the carotid-femoral pulse wave velocity (cfPWV).33 However, the availability of appropriate equipment and experienced operators is essential to assess cfPWV. In addition, considering the required exposure of the inguinal area and the extended examination time, this method is mostly used in research settings rather than daily clinical practice.34 Echocardiography-based analysis of aortic deformation may be a widely available and relatively low-cost option to investigate the biomechanical properties of the ascending aorta. The image quality is the most important factor for capturing a sufficient sequence of digital images over a cardiac cycle and may vary depending on the patient, operator and device. A good parasternal view clearly depicting the ascending aorta may be considered pivotal and insufficient image quality may adversely affect the results of this analysis.34
When the groups were compared in terms of aortic elastic properties, the microalbuminuria group was found to have a stiffer aorta. The ASI was significantly higher in the microalbuminuria group. As a novel finding, ASI was independently associated with the presence of microalbuminuria. There is an accumulated data concerning the relation of microalbuminuria and arterial stiffness assessed by cfPWV method.35,36 However, there is no data probing the relation of microalbuminuria and elastic properties of the aortic root. Proximal aorta with a normal elasticity has a protective function and may store 50% of the systolic left ventricular stroke volume.37 An impaired aortic elasticity may be associated with the progression of microvascular and macrovascular complications of diabetes. Echocardiography, which has a widespread availability compared to above-mentioned methods, may be quite helpful in determining aortic stiffness and the risk of microalbuminuria and DNP in diabetic patients. It is doubtful whether aortic elasticity causes microalbuminuria or vice versa. An unknown mechanism may underlie the association between microalbuminuria and aortic elasticity, but clear evidence for this is lacking.
Echocardiographic measurements of ASI and CIMT were independently associated with the presence of microalbuminuria in the present study. Diabetes is associated with chronic low-grade inflammation and subclinical atherosclerosis. Compared with other study parameters, both CIMT and ASI may be interpreted as the indicators of long-term cardiovascular impact of diabetes. In this context, echocardiography-based evaluation of CIMT and ASI in patients with T2DM may help us to detect the patients who are more exposed to the harmful effects of diabetes, likewise, in identifying patients with a greater risk of developing diabetic nephropathy. We can conclude that our results may be a proof for the importance of multidisciplinary approach to diabetes and its complications.
There are some limitations in the present study. First, it is a cross-sectional study. Therefore, we could make assumptions about only possible etiological relationships. Longitudinal studies may be designed to determine the long-term influence of these parameters. Second, the sample size of this study population was relatively small. Third, it reflects a single-center experience. Fourth, the exclusion criteria were applied for many situations and patients with macroalbuminuria and established cardiovascular disease were also excluded; therefore, the validity of the results for different populations and settings is potentially limited.
Conclusion
In addition to CIMT, as a novel finding, ASI which is an indicator for the elastic properties of the aortic root was independently associated with the presence of microalbuminuria. Our results may be evidence of a complex mechanism between subclinical atherosclerosis, aortic elasticity and nephropathy, and may constitute proof for the importance of multidisciplinary approach to diabetes and its complications. Our findings need to be confirmed by prospective studies with larger populations and long-term follow-up. The causal effect of these parameters can be demonstrated with longitudinal studies evaluating them in diabetic patients without microalbuminuria.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors received no financial support for the research, authorship, and/or publication of this article.
References
1. Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290(7):891–897. doi:10.1001/jama.290.7.891
2. Palsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–280. doi:10.1053/j.ackd.2014.03.003
3. Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813–1821. doi:10.1681/ASN.2008121270
4. Balta S, Demirkol S, Kurt O, Sarlak H, Akhan M. Epicardial adipose tissue measurement: inexpensive, easy accessible and rapid practical method. Anadolu Kardiyol Derg. 2013;13(6):611.
5. Katsiki N, Mikhailidis DP, Wierzbicki AS. Epicardial fat and vascular risk: a narrative review. Curr Opin Cardiol. 2013;28(4):458–463. doi:10.1097/HCO.0b013e3283605fba
6. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467. doi:10.1161/CIRCULATIONAHA.106.628875
7. Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7. doi:10.1016/j.vph.2015.11.083
8. Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140(8):669–682. doi:10.1093/oxfordjournals.aje.a117315
9. Sutton-Tyrrell K, Newman A, Simonsick EM, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38(3):429–433. doi:10.1161/01.hyp.38.3.429
10. Vitarelli A, Giordano M, Germano G, et al. Assessment of ascending aorta wall stiffness in hypertensive patients by tissue doppler imaging and strain doppler echocardiography. Heart. 2010;96(18):1469–1474. doi:10.1136/hrt.2010.198358
11. Sciatti E, Vizzardi E, Castiello A, et al. The role of type 2 diabetes mellitus on hypertensive-related aortic stiffness. Echocardiography. 2018;35(6):798–803. doi:10.1111/echo.13841
12. Cho JY, Kim KH. Evaluation of arterial stiffness by echocardiography: methodological aspects. Chonnam Med J. 2016;52(2):101–106. doi:10.4068/cmj.2016.52.2.101
13. Marathe PH, Gao HX, Close KL. American diabetes association standards of medical care in diabetes 2017. J Diabetes. 2017;9(4):320–324. doi:10.1111/1753-0407.12524
14. Rognant N, Lemoine S, Laville M, Hadj-Aissa A, Dubourg L. Performance of the chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in diabetic patients. Diabetes Care. 2011;34(6):1320–1322. doi:10.2337/dc11-0203
15. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi:10.1016/j.echo.2014.10.003
16. Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11(2):304–310. doi:10.1038/oby.2003.45
17. Nazir N, Siddiqui K, Al-Qasim S, Al-Naqeb D. Meta-analysis of diabetic nephropathy associated genetic variants in inflammation and angiogenesis involved in different biochemical pathways. BMC Med Genet. 2014;15:103. doi:10.1186/s12881-014-0134-1
18. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124(3):139–152. doi:10.1042/CS20120198
19. Parving HH, Chaturvedi N, Viberti G, Mogensen CE. Does microalbuminuria predict diabetic nephropathy? Diabetes Care. 2002;25(2):406–407. doi:10.2337/diacare.25.2.406
20. Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther. 2014;4(6):416–429. doi:10.3978/j.issn.2223-3652.2014.11.05
21. Maurovich-Horvat P, Kallianos K, Engel LC, et al. Relationship of thoracic fat depots with coronary atherosclerosis and circulating inflammatory biomarkers. Obesity (Silver Spring). 2015;23(6):1178–1184. doi:10.1002/oby.21080
22. Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12(1):31–42. doi:10.1089/met.2013.0107
23. Hanai K, Babazono T, Nyumura I, et al. Involvement of visceral fat in the pathogenesis of albuminuria in patients with type 2 diabetes with early stage of nephropathy. Clin Exp Nephrol. 2010;14(2):132–136. doi:10.1007/s10157-009-0245-8
24. Akbas EM, Demirtas L, Ozcicek A, et al. Association of epicardial adipose tissue, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with diabetic nephropathy. Int J Clin Exp Med. 2014;7(7):1794–1801.
25. Zhang YH, Gao Y, Mao X, Shang J, Su BL. Assessment of carotid atherosclerosis in type 2 diabetes mellitus patients with microalbuminuria by high-frequency ultrasonography. Int J Endocrinol. 2013;2013:819584. doi:10.1155/2013/819584
26. Deckert T, Yokoyama H, Mathiesen E, et al. Cohort study of predictive value of urinary albumin excretion for atherosclerotic vascular disease in patients with insulin dependent diabetes. BMJ. 1996;312(7035):871–874. doi:10.1136/bmj.312.7035.871
27. van den Oord SC, Sijbrands EJ, ten Kate GL, et al. Carotid intima-media thickness for cardiovascular risk assessment: systematic review and meta-analysis. Atherosclerosis. 2013;228(1):1–11. doi:10.1016/j.atherosclerosis.2013.01.025
28. Nand N, Jain R, Seth S, Sen J, Sharma M. A new marker of carotid atherosclerosis in middle aged adults: cystatin C or microalbuminuria. Indian Heart J. 2010;62(4):320–323.
29. Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379(9831):2053–2062. doi:10.1016/S0140-6736(12)60441-3
30. Lorenz MW, Price JF, Robertson C, et al. Carotid intima-media thickness progression and risk of vascular events in people with diabetes: results from the PROG-IMT collaboration. Diabetes Care. 2015;38(10):1921–1929. doi:10.2337/dc14-2732
31. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7(10):1025–1038. doi:10.1016/j.jcmg.2013.11.014
32. Nambi V, Chambless L, He M, et al. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012;33(2):183–190. doi:10.1093/eurheartj/ehr192
33. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi:10.1093/eurheartj/ehl254
34. Sabia L, Avenatti E, Cesareo M, et al. Evaluation of aortic stiffness by a new simplified 2D speckle tracking analysis. Int J Cardiovasc Imaging. 2018;34(11):1753–1760. doi:10.1007/s10554-018-1400-7
35. Miao R, Wu L, Ni P, Zeng Y, Chen Z. The comorbidity of increased arterial stiffness and microalbuminuria in a survey of middle-aged adults in China. BMC Cardiovasc Disord. 2018;18(1):83. doi:10.1186/s12872-018-0939-5
36. Zhang X, Low S, Sum CF, et al. Arterial stiffness is an independent predictor for albuminuria progression among Asians with type 2 diabetes – a prospective cohort study. J Diabetes Complications. 2017;31(6):933–938. doi:10.1016/j.jdiacomp.2017.02.004
37. Zoppini G, Bergamini C, Trombetta M, et al. Increased aortic stiffness index in patients with type 1 diabetes without cardiovascular disease compared to controls. J Endocrinol Invest. 2019. doi:10.1007/s40618-019-01032-7
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
