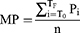Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Aortic Stiffness and Pulsatile Pressures as Potential Mediators of Chronic Kidney Disease Induced Impaired Diastolic Function
Authors Hsu HC, Tade G , Norton GR, Peters F, Robinson C, Dlongolo N, Teckie G, Woodiwiss AJ, Dessein PH
Received 25 October 2021
Accepted for publication 22 January 2022
Published 15 February 2022 Volume 2022:15 Pages 27—40
DOI https://doi.org/10.2147/IJNRD.S346074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pravin Singhal
Hon-Chun Hsu,1,2,* Grace Tade,1,* Gavin R Norton,1 Ferande Peters,1 Chanel Robinson,1 Noluntu Dlongolo,3 Gloria Teckie,4 Angela J Woodiwiss,1 Patrick H Dessein1,5
1Cardiovascular Pathophysiology and Genomics Research Unit, School of Physiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; 2Nephrology Unit, Milpark Hospital, Johannesburg, South Africa; 3Rheumatology Unit, Rosebank Hospital, Johannesburg, South Africa; 4Division of Nephrology, Department of Medicine, Chris Hani Baragwanath Hospital and Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa; 5Internal Medicine Department, University of the Witwatersrand, Johannesburg, South Africa
*These authors contributed equally to this work
Correspondence: Patrick H Dessein, Tel +27 662491468, Email [email protected]
Purpose: We assessed whether aortic stiffness and pulsatile pressures can mediate chronic kidney disease (CKD)-associated impaired diastolic function.
Participants and Methods: In 276 black Africans including 46 CKD (19 non-dialysis; 27 dialysis) and 230 control subjects, pulse wave velocity (PWV) estimated aortic stiffness and pulsatile pressures (forward and backward wave pressure, central systolic blood pressure (CSBP) and pulse pressure (CPP)) were determined by applanation tonometry; e’ as an index of left ventricular active relaxation and E/e’ as a measure of left ventricular filling pressure or passive relaxation were evaluated by echocardiography.
Results: In age, sex, traditional cardiovascular risk factor and mean arterial pressure (MAP) adjusted regression models, CKD was inversely associated with e’ (p = 0.03) and directly with E/e’ (p < 0.01). The CKD-e’ relationship was attenuated and no longer significant (p = 0.31) upon additional adjustment for aortic PWV but not pulsatile pressures (p = 0.03– 0.05). In product of coefficient mediation analysis, PWV accounted for 47.6% of the CKD-e’ association. CSBP (22.9%) and CPP (18.6%) but not PWV (11.3%) accounted for a significant and relevant proportion of the CKD-E/e’ relationship. However, CKD remained strongly associated with E/e’ independent of aortic function measures (p < 0.01). Treatable covariates that were or tended to be consistently associated with diastolic function included MAP (p < 0.01) and diabetes (p = 0.02– 0.07) for the CKD-e’ and CKD-E/e’ relations, respectively.
Conclusion: Aortic stiffness rather than pulsatile pressures mediates CKD-related impaired left ventricular active relaxation. By contrast, aortic pulsatile pressures (and not stiffness) contribute to CKD-related left ventricular filling pressures but do not fully account for the respective association.
Keywords: chronic kidney disease, diastolic function, arteriosclerosis, aortic stiffness, pulsatile pressures
Introduction
Uraemic cardiomyopathy1 and marked premature arteriosclerosis2 are the most characteristic cardiovascular abnormalities that are experienced by patients with chronic kidney disease (CKD). The core feature of uraemic cardiomyopathy comprises impaired diastolic function, which results in inadequate left ventricular filling and ultimately often heart failure with preserved ejection fraction (HFpEF).1–5 Uremic cardiomyopathy also enhances the risk of ventricular arrhythmias and sudden cardiac death.6 Impaired diastolic function in CKD is engendered by (1) decreased active reuptake of calcium from the cytoplasm into the endoplasmic reticulum that leads to impaired active relaxation and (2) myocardial fibrosis that causes decreased passive relaxation and increased left ventricular filling pressures.1 Uraemic cardiomyopathy is also associated with pronounced compensatory left ventricular hypertrophy.1
The haemodynamic factors that are implicated in impaired diastolic function among CKD patients are increased preload and afterload.1,2 Increased preload is mostly due to volume overload and anaemia, whereas premature arteriosclerosis augments afterload in CKD.1,2
Premature arteriosclerosis increases aortic stiffness that enhances the forward and subsequent backward or reflected wave pressure.2 These changes account for increased central systolic blood pressure and pulse pressure.2 Aortic stiffness and pulsatile pressures thereby impact cardiac function, a phenomenon that is mostly referred to as ventricular-vascular coupling.7 However, aortic stiffness can also contribute to ventricular-vascular coupling in a mechanical way.7–9 Indeed, during early diastole, particularly proximal aortic stiffness impairs the rapid upward movement of the left ventricle around stationary blood in the left atrium that normally contributes markedly to left ventricular filling, this when the left ventricle fails to compensate for aortic stiffening.
The relative contributions of aortic stiffness and pulsatile pressures to CKD induced impaired active and passive left ventricular relaxation are currently unknown. In this study, we performed pulse wave analysis and echocardiography in black African CKD patients and matched healthy controls.10–12 We determined early diastolic mitral annulus displacement velocity (e’) and inflow velocity (E) by tissue and pulsed Doppler, respectively. E’ was used as a measure of active relaxation, and we calculated E/e’ as an index of left ventricular filling pressure or passive relaxation. We hypothesized that in traditional cardiovascular risk factor and mean arterial pressure adjusted analysis, both aortic stiffness and pulsatile pressures can mediate the relationship of CKD with impaired diastolic function.
Participants and Methods
Study Participants
This study was conducted in line with the principles of the Helsinki declaration. The Committee for Research on Human Subjects of the University of Witwatersrand approved the protocols for investigation of non-CKD and CKD participants (M02-04-72 and renewed as M07-04-69, M12-04-108 and M17-04-01 in non-CKD subjects and M15-08-43 in CKD patients). Participants gave informed, written consent. Our study design was previously reported.10–13 Briefly, 46 consecutive black CKD participants including 19 non-dialysis and 27 dialysis patients were enrolled at the Milpark Hospital in Johannesburg, South Africa. Patients with active infection or/and cancer were excluded. Non-dialysis patients had a Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate (eGFR)14 of <60mL/min/1.73m2 at enrolment; their mean (SD) eGFR was 26 (18) mL/min/1.73m2. Dialysis was performed thrice weekly for 4 hour sessions; measurements were made on the day prior to dialysis in the respective patients. Non-CKD controls comprised 230 age-, sex- and race-matched participants of a population study on cardiovascular disease risk in Johannesburg, which includes randomly recruited families of black African descent with siblings older than 16 years; their mean (SD) eGFR was 96 (18) mL/min/1.73m2. None of the study participants had atrial fibrillation at the time of the investigation. Data were missing in fewer than 3% of any of the recorded characteristics among study participants.
Baseline Characteristics Including Traditional Cardiovascular Risk Factors
We recorded demographic characteristics, traditional cardiovascular risk factors and cardiovascular events as previously reported10–13 and detailed in the Supplementary Data Methods. Mean or distending arterial blood pressure for the peripheral waveform was determined electronically by the SphygmoCor device (see below) and using the formula
where T0=start of the waveform; TF=end of waveform; Pi=pressure points and n=number of pressure points.
Arterial Function
Central haemodynamic assessments were determined from central arterial pressure recordings obtained from pulse wave analysis by applanation tonometry and SphygmoCor software as previously described10–13 and detailed in the Supplementary Data Methods. We recorded aortic pulse wave velocity (PWV) as an index of arterial stiffness, forward wave pressure (Pf), reflected wave pressure (Pb), central systolic blood pressure (CSBP) and central pulse pressure (CPP).
Echocardiography
Echocardiography was performed as recommended by the American Society of Echocardiography convention.15 We employed a Philips XC50 POC Compact CompactXtreme ultrasound system (Philips Medical System (Philips Medical Systems (Pty) Ltd, USA) in CKD patients (performed by C. Robinson) and an Acuson SC2000 Diagnostic ultrasound system (Siemens Medical Solutions, USA, Inc.) in healthy control subjects (performed by A.J. Woodiwiss and C.D. Liebhaber). Measurement details were reported previously.10–13 Intra-observer echocardiographic measurement variability is low in our setting with Pearson’s correlation coefficients and variances (mean % difference (SD)) for left ventricular end-diastolic diameter, septal wall thickness, posterior wall thickness, E and e’ of 0.92, 0.72, 0.76, 0.88 and 0.93 (p < 0.0001 for all), and −0.41 (4.16), 0.45 (7.74), 1.74 (6.08), 0.16 (9.95) and −1.46 (8.58), respectively.16 Participants were examined in the partial left decubitus position. Left ventricular dimensions were determined by 2-dimensional directed M-mode echocardiography. Left ventricular mass (LVM) was determined using a standard formula.17 Left ventricular hypertrophy (LVH) was identified when the left ventricular mass index was >95 g/m2 in women and >115g/m2 in men. Left ventricular end diastolic and systolic volumes were determined using the Teichholz method.18 Left ventricular ejection fraction (EF) was calculated as [(left ventricular end diastolic volume – left ventricular end systolic volume)/left ventricular end diastolic volume] × 100. Left ventricular diastolic function was assessed by measuring the pulsed Doppler determined early mitral valve blood inflow velocity (E) and the tissue Doppler determined early mitral valve annulus upward displacement velocity (e’). Results were expressed as e’, which is a marker of active relaxation, and the E/e’ ratio, which is an index of left ventricular diastolic filling pressure that is mediated by passive relaxation or left ventricular stiffening.
Data Analysis
Results are expressed as mean (SD), median (interquartile range) of number (percentages) as appropriate. Non-normally distributed characteristics were logarithmically transformed upon entering them in multivariate regression models.
The recorded characteristics were compared between controls and CKD patients and between dialysis and non-dialysis patients in age and sex adjusted linear and logistic regression models with additional adjustments for antihypertensive and lipid lowering therapy for blood pressure and lipid variables, respectively.
Among all study participants, the association of CKD with e’ and E/e’ were first assessed in age and sex adjusted linear regression models. We subsequently adjusted additionally for established confounders in the present context (height, heart rate and mean arterial pressure)10–13 as well as those traditional cardiovascular risk factors that differed between control and CKD participants (waist–hip ratio, HDL-cholesterol concentrations, diabetes prevalence and current smoking status) (base model). Thereafter, we adjusted additionally for aortic stiffness (PWV), central pulsatile pressures (Pf, Pb, CSBP, CPP), LVEVD and LVM in separate models. For the latter models, associations of the respective factors with e’ and E/e’ were also illustrated by giving partial correlation coefficients and their 95% confidence intervals. Partial correlation coefficients comprise a measure of the strength and direction of a linear relationship between two continuous variables whilst controlling for the effect of one or more other variables (covariates or control variables).
Age, sex, established confounder and traditional cardiovascular risk factor adjusted product of coefficient mediation analysis that accounts for hierarchical causal structures,17–22 was then performed to determine the contribution of aortic stiffness, central pulsatile pressures, LVEVD and LVM to CKD-e’ and CKD-E/e’ relations. In this analysis, the fraction to which the respective factors contributed to the total effect on e’ and E/e’ was calculated by dividing the regression coefficient (estimate) for the factor-e’ and factor-E/e’ relationships by the regression coefficient for the total effect on e’ and E/e’.
Data analysis was performed on IBM SPSS statistical program (version 27.0 IBM, USA). Significance was set at p < 0.05.
Results
Baseline Characteristics Including Cardiovascular Risk Factors in Controls and CKD Patients
Recorded characteristics of CKD patients that formed part of this study were reported previously.12 In Table 1, baseline characteristics including traditional cardiovascular risk factors are compared between control and CKD participants and among non-dialysis and dialysis patients. Age and sex did not differ significantly in control compared to CKD participants.
 |
Table 1 Baseline Characteristics Including Traditional Cardiovascular Risk Factors in Control Subjects and Chronic Kidney Disease Patients |
CKD patients had a similar body mass index but larger waist circumference and waist–hip ratio compared to control subjects. Hypertension, dyslipidemia and diabetes prevalence were each larger in CKD patients than in controls. Antihypertensive drugs were used in all CKD patients with hypertension (n = 45 (97.8%)) and 60 (26.1%) controls; lipid lowering agents were employed by 21 (45.7%) CKD patients and none of the controls. Systolic blood pressure and peripheral pulse pressure were larger, whereas HDL-cholesterol concentrations were smaller in CKD compared to control participants. CKD patients smoked less frequently than did control subjects. Heart rate was larger in CKD patients compared to controls. The Framingham score was 2.9 fold larger in CKD compared to control participants.
Differences in baseline characteristics between patients and controls were overall consistent irrespective of CKD status. Cardiovascular disease was recorded in 17.4% of CKD patients. Baseline characteristics did not differ significantly in non-dialysis compared to dialysis patients with the exception of diabetes prevalence that was 1.5 larger in non-dialysis participants. However, the sample size of the 2 CKD subgroups was too small to reliably identify potentially clinically relevant differences amongst non-dialysis and dialysis patients through statistical analysis.
Aortic Function and Echocardiographic Characteristics in Controls and CKD Patients
Aortic function indices and echocardiographic findings are shown in Table 2. PWV, Pf, Pb, CSBP and CPP were each larger in CKD patients compared to control subjects.
 |
Table 2 Arterial Function Indices and Echocardiographic Characteristics in Controls and Chronic Kidney Disease Patients |
E’ was smaller, and E and the E/e’ ratio were larger in CKD compared to control participants. LVEDV, LVM and left ventricular hypertrophy prevalence were each larger in CKD patients than control subjects. EF did not differ among CKD patients and control subjects.
Differences in arterial function indices and echocardiographic findings between patients and controls were overall consistent irrespective of CKD status. Although aortic and diastolic function measures and LVEDV and LVM did not differ significantly among non-dialysis and dialysis patients, some of the numerical differences noted are likely to be clinically relevant.
Associations of CKD with E’ and E/e’
In age and sex adjusted analysis among all study participants, CKD was associated with e’ (partial R = −0.216, p < 0.01) and E/e’ (partial R = 0.392, p < 0.01).
Table 3 and Figure 1 give the associations of CKD with e’ in multivariable regression models before and after adjustment for PWV, pulsatile pressures (Pf, PB, CSBP and CPP), LVED or LVM. In the base model in which age, sex, other established confounders and traditional cardiovascular risk factors that differed between CKD patients and control subjects were adjusted for, CKD remained associated with e’ (SB (SEM)=−0.121 (0.055), p = 0.03). Upon additional adjustment for pulsatile pressures, LVEDV and LVM, CKD remained (p < 0.05) or tended to remain (p = 0.05) associated with e’. However, upon additional adjustment for PWV, CKD was no longer associated with e’ (SB (SEM)=−0.064), p = 0.31). Among the covariates in the respective models, age and mean arterial pressure were consistently or tended to be consistently associated (p < 0.01 to 0.05) with e’.
 |
Table 3 Associations of CKD with e’ in Base Model and Multivariate Regression Models |
 |
Figure 1 Partial correlations (95% CI) for the associations of chronic kidney disease with e’ in the base model (adjusted for age, sex, height, waist–hip ratio, HDL-cholesterol concentration, diabetes, smoking, heart rate and mean arterial pressure; see Table 3) and after additional adjustment for PWV, Pf, Pb, CSBP, CPP, LVEDV and LVM. Abbreviations: CKD, chronic kidney disease; PWV, pulse wave velocity; Pf, forward wave pressure; Pb, backward wave pressure; CSBP, central systolic blood pressure; CPP, central pulse pressure; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; SB, standard regression coefficient; SEM, standard error of the mean; MAP, mean arterial pressure. |
Table 4 and Figure 2 show the associations of CKD with E/e’ in multivariable regression models before and after adjustment for PWV, pulsatile pressures, LVEDV or LVM. In the base model, CKD remained strongly associated with E/e’ (SB (SEM)=0.351 (0.060), p < 0.01). Upon additional adjustment for LVEDV or LVM, CKD remained equally strongly associated with E/e’ (p < 0.01). Upon additional adjustment for PWV and central pressures, particularly CSBP and CPP, the CKD-E/e’ relationship was attenuated but remained strongly significant (p < 0.01). Among the covariates in the respective models, height and diabetes were consistently or tended to be consistently associated (p < 0.01 to 0.07) with E/e’.
 |
Table 4 Associations of CKD with E/e’ in Base Model and Multivariate Regression Models |
 |
Figure 2 Partial correlations (95% CI) for the associations of chronic kidney disease with E/e’ in the base model (adjusted for age, sex, height, waist–hip ratio, HDL-cholesterol concentration, diabetes, smoking, heart rate and mean arterial pressure; see Table 4) and after additional adjustment for PWV, Pf, Pb, CSBP, CPP, LVEDV and LVM. Abbreviations: CKD, chronic kidney disease; PWV, pulse wave velocity; Pf, forward wave pressure; Pb, backward wave pressure; CSBP, central systolic blood pressure; CPP, central pulse pressure; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; SB, standard regression coefficient; SEM, standard error of the mean; MAP, mean arterial pressure. |
Potential Mediators of the Confounder Adjusted Associations of CKD with e’ and E/e’
Table 5 gives the potential mediating effects of PWV, pulsatile pressures, LVEDV and LVM in the confounder adjusted association of CKD with e’. In this regard, PWV mediated 47.6% of the CKD-e’ relationship. By contrast, Pf and LVEDV strengthened the CKD-e’ association by 26.3% and 14.3%, respectively.
 |
Table 5 Potential Mediators of the Confounder Adjusted Associationa of CKD with e’ |
Table 6 shows the potential mediating effects of PWV, pulsatile pressures, LVEDV and LVM in the confounder adjusted association of CKD with E/e’. CSBP and CPP mediated the CKD-E/e’ relationship by 22.9% and 18.6%, respectively. None of the other characteristics made a relevant contribution to the association of CKD with E/e’.
 |
Table 6 Potential Mediators of the Confounder Adjusted Associationa of CKD with E/e’ |
We previously reported that determinants of diastolic function may differ in dialysis and non-dialysis CKD patients.9 In this regard, in the present study, the associations of PWV with e’ and CSBP with E/e’ did not differ by CKD status (interaction p = 0.1 and interaction p = 0.7, respectively, in multivariate models).
Discussion
To our knowledge, this is the first study that assessed the potential mediating effects of aortic stiffness and pulsatile pressures to CKD induced impaired left ventricular active relaxation as indexed by e’ and filling pressure or passive relaxation as estimated by E/e’. Our main findings are 2-fold. Firstly, we found that CKD was inversely associated with e’ independent of non-modifiable and modifiable traditional cardiovascular risk factors including mean arterial pressure; this relationship was markedly attenuated and no longer significant after additional adjustment for aortic stiffness but not pulsatile pressures; accordingly, in product of coefficient mediation analysis, PWV accounted for 47.6% of the CKD-e’ relationship. Secondly, CKD was directly associated with E/e’ independent of traditional cardiovascular risk factors; however, CSBP (22.9%) and CPP (18.6) but not arterial stiffness (11.3%) accounted for a significant and relevant proportion of the CKD-E/e’ relationship, and CKD remained strongly associated with E/e’ independent of any of the aortic function measures; this indicates that non-traditional or renal cardiovascular risk factors are likely to mediate, at least in part, CKD induced impaired left ventricular passive relaxation.1,2 Such risk factors include anaemia, compromised bone mineral metabolism, inflammation and oxidative stress in CKD induced left ventricular fibrosis and stiffness with consequent impaired diastolic function.1,2
During systole, contraction of the left ventricle results in longitudinal downward stretching of the ascending aorta towards its apex.7,8 This translates into energy storage in the aorta that causes its elastic recoil during early diastole. The aorta thereby pulls left ventricle around the blood in the left atrium, which contributes to left ventricular filling. It follows that when the left ventricle fails to compensate for aortic stiffening, reduced aortic recoil can impair its early diastolic filling.7 Our findings of a potential mediating effect of arterial stiffness and not central pulsatile pressures on the CKD-e’ relationship are in line with this recently proposed hypothesis.7 More specifically, previously reported data in non-CKD persons7–9 together with our current results suggest that impaired left ventricular active relaxation is mediated by mechanical rather than hemodynamic effects of increased aortic stiffness on ventricular-vascular coupling in CKD. Notably, although not significant, Pf was directly related to e’ and accounted for 26.3% of the CKD-e’ relationship in product of coefficient mediation analysis. Since this concurred with a significant direct association between cardiac preload as indexed by LVEDV,10,11 with e’, increased Pf was likely flow mediated and a consequence rather than a cause of left ventricular relaxation.
Although blood pressure lowering with angiotensin-renin-aldosterone system inhibitors can reduce operating arterial stiffness,23,24 the structural changes that underlie aortic stiffness are considered to be largely untreatable at present.23 In this regard, mean arterial pressure comprised the one covariate and treatable cardiovascular risk factor that was consistently associated with e’ in multivariate regression models (Table 3). The potential impact of adequate blood pressure control on the development of impaired left ventricular active relaxation in the present context merits further longitudinal study.
In sharp contrast to our findings on e’, we found that the relationship of CKD with E/e’ was mediated, at least in part, by CSBP and CPP and not PWV. Whereas this result may further argue towards blood pressure control, perhaps more importantly, the CKD-E/e’ association in the present study remained consistently strong after adjusting for aortic function measures in addition to traditional cardiovascular risk factors. This finding indirectly substantiates the potential importance of non-traditional or renal cardiovascular risk factors.
Diabetes is strongly associated with increased arterial stiffness among CKD patients25 as well as an established risk factor for not only CKD26 but also HFpEF27 in the population at large. Our finding that diabetes was the one treatable covariate that related or tended to relate (p = 0.02 to p = 0.07) to E/e’ in multivariate regression models (Table 4) is in line with these reported findings.
A small body height reportedly associates with increased risk of cardiovascular disease including heart failure.28,29 This relationship was previously attributed to arterial stiffness, enhanced wave reflection and pulse pressure in relation to short stature.29 In this regard, Montero et al29 recently documented an inverse association between height and left ventricular end-diastolic pressure in patients with HFpEF. However, arterial function was not evaluated in the Montero study. It is therefore of interest that in our multivariate models in Table 4, body height was associated with increased E/e’ independent of PWV, Pb, CPP and CSBP (p < 0.01 to p = 0.01) as well as LVEDV and LVM (p < 0.01 to p = 0.05). In this regard, genetic factors, nutritional and social deprivation during early life may also contribute to the observed relationship between short stature and increased cardiovascular disease risk.28,30 However, in the present investigation, body height was larger in CKD compared to healthy control participants. This may be because CKD patients and healthy controls were enrolled in a private healthcare setting (a marker of sociodemographic advantage in Africa)30 and the community at large, respectively. Taken together, the potential mechanisms underlying the association of small body height with diastolic function and the assessment and therapeutic implications thereof require further elucidation in future studies.
As this study was cross-sectional in design, the direction of causality could not be inferred. All study participants were black Africans. Whether our findings apply to potential CKD induced impaired diastolic function among other populations requires further investigation. Also, whether our findings apply to peritoneal dialysis patients awaits further study. Additionally, whether the timing of measurement during the interdialytic period influences findings in relation to the impact of aortic function on diastolic function in CKD merits future research. As applies to previously reported studies by us,10,11,13 our aim was to determine relationships of recorded characteristics with e’ and E/e’, which necessitates including low as well as high values. In this regard, since we did not determine atrial volume index and tricuspid regurgitation velocity, we could not apply suggested algorithms for the identification of diastolic dysfunction as recently reported by Nagueh et al.31 Our study was designed prior to Nagueh report.31 However, since 45.7% of CKD participants in the present investigation had left ventricular hypertrophy, it is likely that a considerable proportion of them had diastolic dysfunction. We did not measure vitamin D concentrations, which are known to be reduced in CKD and are associated with arterial stiffness in heart failure32 and several other disorders.33 Strengths of the present study are the identification of relationships in comprehensively adjusted regression models and the application of product of coefficient mediation analysis that accounts for hierarchical causal structures.19–21
Conclusion
This study demonstrates that aortic stiffness rather than pulsatile pressures mediates CKD related impaired left ventricular active relaxation. By contrast, aortic pulsatile pressures (and not stiffness) contribute to CKD related left ventricular filling pressures but do not fully explain the respective association.
Ethics Statement
This study was conducted in line with the principles of the Helsinki declaration. The Committee for Research on Human Subjects of the University of Witwatersrand approved the protocols for investigation of non-CKD and CKD participants (M02-04-72 and renewed as M07-04-69, M12-04-108 and M17-04-01 in non-CKD subjects and M15-08-43 in CKD patients). Participants gave informed, written consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by the South African National Research Foundation.
Disclosure
The authors declare no conflicts of interest.
References
1. Wang X, Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol. 2019;15(3):159–175. doi:10.1038/s41581-018-0101-8
2. Zanoli L, Lentini P, Briet M, et al. Arterial stiffness in the heart of CKD. J Am Soc Nephrol. 2019;30:918–928. doi:10.1681/ASN.2019020117
3. Kim MK, Kim B, Lee JY, et al. Tissue Doppler-derived E/e’ ratio as a parameter for assessing diastolic heart failure and as a predictor of mortality in patients with chronic kidney disease. Korean J Intern Med. 2013;28(1):35–44. doi:10.3904/kjim.2013.28.1.35
4. Farshid A, Pathak R, Shadbolt B, Arnolda L, Talaulikar G. Diastolic function is a strong predictor of mortality in patients with chronic kidney disease. BMC Nephrol. 2013;14:280. doi:10.1186/1471-2369-14-280
5. Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99(3):393–398. doi:10.1016/j.amjcard.2006.08.042
6. Mozos I. Laboratory markers of ventricular arrhythmia risk in renal failure. BioMed Res Int. 2014;2014:509204. doi:10.1155/2014/509204
7. Bell V, Mitchell GF. Influence of vascular function and pulsatile hemodynamics on cardiac function. Curr Hypertens Rep. 2015;17:68. doi:10.1007/s11906-015-0580-y
8. Bell V, Sigurdsson S, Westenber JJM, et al. Relations between aortic stiffness and left ventricular structure and function in older participants in the age, gene/environment susceptibility-Reykjavik study. Circ Cardiovasc Imaging. 2015;8:e003039. doi:10.1161/CIRCIMAGING.114.003039
9. Kaess BM, Rong J, Larson MG, et al. Relations of central haemodynamics and aortic stiffness with left ventricular structure and function: the Framingham Heart Study. J Am Heart Assoc. 2016;5:e002693.
10. Hsu H-C, Norton GR, Robinson C, Woodiwiss AJ, Dessein PH. Potential determinants of the E/e’ ratio in non-dialysis compared with dialysis patients. Nephrology (Carlton). 2021;26:988–998. doi:10.1111/nep.13948
11. Hsu H-C, Norton GR, Peters F, et al. Association of post transplantation anaemia and persistent secondary hyperparathyroidism with diastolic function in stable kidney transplant recipients. Int J Nephrol Renovasc Dis. 2021;14:211–223. doi:10.2147/IJNRD.S314313.eCollection2021
12. Hsu H-C, Robinson C, Woodiwiss AJ, Norton GR, Dessein PH. Cardiovascular risk factor profiles and disease in black compared to other Africans with chronic kidney disease. Int J Nephrol. 2021;2021:8876363. doi:10.1155/2021/8876363.eCollection2021
13. Bello H, Norton GR, Peterson VR, et al. Hemodynamic determinants of age versus left ventricular diastolic function relations across the full adult age range. Hypertension. 2020;75:1574–1583. doi:10.1161/HYPERTENSIONAHA.119.14622
14. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi:10.7326/0003-4819-150-9-200905050-00006
15. Sahn DJ, DeMaria A, Kisslo J, Weyman A; American Society of Echocardiography. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi:10.1161/01.CIR.58.6.1072
16. Millen AME, Libhaber CD, Majane OHI, et al. Relative impact of blood pressure as compared to an excess adiposity on left ventricular diastolic dysfunction in a community sample with high prevalence of obesity. J Hypertens. 2014;32:2457–2464. doi:10.1097/HJH.0000000000000330
17. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(7):233–271. doi:10.1093/ehjci/jev014
18. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;37:7–11. doi:10.1016/0002-9149(76)90491-4
19. MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi:10.1146/annurev.psych.58.110405.085542
20. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Personal Soc Psych. 1986;51:1173–1182. doi:10.1037/0022-3514.51.6.1173
21. Krull JL, MacKinnon DP. Multilevel modeling of individual and group level mediated effects. Multivariate Behav Res. 2001;36:249–277. doi:10.1207/S15327906MBR3602_06
22. Normand ST. Some old and new statistical tools for outcomes research. Circulation. 2008;118:872–884. doi:10.1161/CIRCULATIONAHA.108.766907
23. Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease. JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1237–1261. doi:10.1016/j.jacc.2019.07.012
24. Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of stiffness attenuation on survival of patients in end-stage renal disease. Circulation. 2001;103:987–992. doi:10.1161/01.CIR.103.7.987
25. Townsend RR. Arterial stiffness in CKD: a review. Am J Kidney Dis. 2018;73:240–247. doi:10.1053/j.ajkd.2018.04.005
26. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. doi:10.1016/S0140-6736(16)32063-5
27. Eaton CB, Pettinger M, Rossouw J, et al. Risk factors for incident heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Cir Heart Fail. 2016;9:e002883. doi:10.1161/CIRCHEARTFAILURE.115.002883
28. Bourgeois B, Watts K, Thomas DM, et al. Associations between height and blood pressure in the United States population. Medicine. 2017;96(50):e9233. doi:10.1097/MD.0000000000009233
29. Montero D, Diaz-Canestro C. Body height is inversely associated with left ventricular end-diastolic pressure in heart failure with preserved ejection fraction. Eur J Prev Cardiol. 2020;27:1116–1118. doi:10.1177/2047487319873453
30. Dessein PH, Christian BF, Woodiwiss AJ, Norton GR, Solomon A. Public healthcare attendance associates with enhanced conventional and non-conventional atherosclerotic cardiovascular disease risk burden in established rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:230–237.
31. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–310. doi:10.1016/j.echo.2016.01.011
32. Buleu FN, Luca CT, Tudor A, et al. Correlations between vascular stiffness indicators, OPG, and 25-OH vitamin D3 status in heart failure conditions. Medicina (Kaunas). 2019;55(6):309. doi:10.3390/medicina55060309
33. Mozos I, Stoian D, Luca CT. Crosstalk between vitamins A, B12, D, K, C, and E status and arterial stiffness. Dis Markers. 2017;2017:8784971. doi:10.1155/2017/8784971
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.