Back to Journals » Clinical Interventions in Aging » Volume 13
Aortic stiffness and brain integrity in elderly patients with cognitive and functional complaints
Authors Tap L , van Opbroek A , Niessen WJ, Smits M , Mattace-Raso FUS
Received 25 July 2018
Accepted for publication 5 September 2018
Published 26 October 2018 Volume 2018:13 Pages 2161—2167
DOI https://doi.org/10.2147/CIA.S181437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Lisanne Tap,1 Annegreet van Opbroek,2 Wiro J Niessen,2,3 Marion Smits,4 Francesco US Mattace-Raso1
1Department of Internal Medicine, Section of Geriatric Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands; 2Department of Medical Informatics and Radiology, Biomedical Imaging Group Rotterdam, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands; 3Imaging Physics, Faculty of Applied Sciences, Delft University of Technology, Delft, the Netherlands; 4Department of Radiology and Nuclear Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands
Purpose: Cerebral white matter lesions (WML) and brain atrophy are frequent in older persons and are associated with adverse outcomes. It has been suggested that aortic stiffness plays a role in the pathogenesis of WML and gray matter (GM) loss. There is, however, little evidence on the association between aortic stiffness and brain integrity in older patients. In this study, we investigated whether aortic stiffness is associated with WML and GM volume in older patients with cognitive and functional complaints.
Patients and methods: Fazekas score was used to analyze WML on brain imaging data of 84 persons; in a subanalysis on 42 MRI scans, the exact volume of white matter hyperintensities (WMH) and GM was determined using a brain-tissue and WMH tool. Aortic stiffness, measured as aortic pulse wave velocity (aPWV) and central pulse pressure (cPP), and blood pressure levels were non-invasively measured by the Mobil-O-Graph.
Results: Mean age was 76.6 (±6.8) years. Age was correlated with cPP (Spearman’s ρ =0.296, P=0.008), aPWV (r²=0.785, P<0.001) and WMH volume (r²=0.297, P<0.001). cPP did not differ between categories of Fazekas, whereas aPWV increased with increasing Fazekas score (P for trend <0.001). After additional adjustment for age, levels of aPWV did not differ between categories. Both cPP and aPWV were associated with WMH volumes (lnB 0.025, P=0.055 and lnB 0.405, P<0.001, respectively); after additional adjustment for age, estimates were less consistent. Both cPP and aPWV were negatively associated with GM volumes in multivariate analysis (B=2.805, P=0.094 and B=111.052, P=0.032).
Conclusion: Higher aortic stiffness was partly associated with increased volume of WMH and decreased volume of GM and slightly influenced by blood pressure. Age also plays a role in this association in older patients.
Keywords: vascular aging, white matter hyperintensities, gray matter, older persons, cognitive complaints, functional decline
Introduction
Cerebral white matter lesions (WML) and gray matter (GM) volume loss are frequently seen in older persons.1,2 These age-related processes can be associated not only with gait disturbances and mood disorder but also with cognitive and functional decline and mortality.3–8 Known risk factors for brain abnormalities are hypertension, diabetes mellitus, and inflammation.9–12 Also genetic predisposition, such as Anderson–Fabry disease, can result in WML.13,14 It has been suggested that arterial stiffness also plays a role in the pathogenesis of WML.15 Increased arterial stiffness leads to an increased pulsatile pressure, which can affect the microcirculation in high-flow organs leading to cerebral small vessel disease (CSVD).15,16 A recent systematic review and meta-analysis was conducted on the association between arterial stiffness and CSVD.17 Most studies included in the systematic review found an independent association between arterial stiffness and different markers of CSVD.18–27 However, most of these studies investigated the effect of arterial stiffness on cerebral infarcts or cerebral microbleeds and not on WML.20–22,24,25,27 Several studies used brachial-ankle pulse wave velocity (PWV) instead of more reliable central measurements (ie, aortic stiffness) or included specific categories of patients at risk for cardiovascular disease.18,20–23,25,26 There is, however, little evidence on the possible association between aortic stiffness and WML in the older patient. Also, little is known on the association between arterial stiffness and GM volume, which has only been investigated in young adults and selected groups of patients.28–31 No previous study has investigated the possible association between aortic stiffness and GM volume in older persons. The aim of this study was to investigate the relationship between measures of aortic stiffness and both GM volume and the severity of cerebral WML load in a population of elderly patients with cognitive and functional complaints.
Materials and methods
From April 2015 to June 2016, all patients entering the outpatient clinic of geriatrics of the Erasmus MC, University Medical Center Rotterdam, were asked to participate in the study. Patients with (reliable) measurements of aortic stiffness and brain imaging were included. MRI and computed tomography (CT) scans were used for analysis when brain imaging was performed within 6 months before or after the study visit. The ethical committee of the hospital approved this study and all participants signed informed consent. During the study visit, biographical information was collected and measurements of aortic stiffness were obtained. Medical history, medication, and lifestyle factors were documented. Height and weight were measured, and body mass index was calculated as kg/m2. Global cognitive function was assessed with the Mini Mental State Examination score.32 Katz index and Lawton and Brody index were used for scoring activities of daily living (ADL) and instrumental ADL, respectively.33,34 Instrumental ADL dependency was defined as ≥1 limited activity.
Vascular measurements
Aortic stiffness was non-invasively measured with a validated method by the Mobil-O-Graph (Mobil-O-Graph 24 hours PWA Monitor; I.E.M. GmbH, Stolberg, Germany).35,36 Central and peripheral systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP) were also measured. Aortic pulse wave velocity (aPWV) and central pulse pressure (cPP) were used to reflect aortic stiffness.35,37 Mean arterial pressure (MAP) was calculated as DBP +1/3 (SBP–DBP).
Brain imaging
Brain imaging was performed as part of clinical work-up of patients with cognitive and/or functional complaints. The Fazekas scale was used by one experienced radiologist to score the severity of WML on MRI and CT scans.38 The Fazekas scale represents the sum of deep white matter corps and periventricular corps divided into three categories of increasing severity (0= absent, 1= punctuate foci, 2= beginning confluence of foci, and 3= large confluent area). For more quantitative estimates, the brain tissue and white matter hyperintensity (WMH) segmentation tool, as developed by Quantib B.V. (www.quantib.com), was applied to T1-weighted and FLAIR scans. This tool generates automatic segmentations of GM, white matter (WM), cerebrospinal fluid and WMH in the cerebrum.39 The algorithm used is available for clinical use in the Quantib Brain product (v1.2) and an improved version is available in Quantib ND (v1.5). From these segmentations, we computed volumes in milliliters of total GM and WMH.
Statistical analyses
All analyses were performed using SPSS statistics 24. Descriptive data for continuous variables were presented as mean ± SD or median and IQR. Number and percent prevalences were presented for dichotomous variables. Data of variables with a skewed distribution (WMH in mm3 and cPP) were log-transformed using the natural logarithm. Spearman’s correlation analysis was used to investigate the relationship between age and cPP. Pearson’s correlation analysis was performed to investigate the relationship between age (independent variable) and aPWV, the natural logarithm of WMH volume, and GM volume (dependent variables). In analysis of covariance (ANCOVA), mean cPP and aPWV were investigated across categories of Fazekas score in three different models: model A was unadjusted; model B was adjusted for MAP; model C was adjusted for MAP and age. Mean values of cPP were back transformed to original scale. The significant associations in ANCOVA analysis were re-evaluated using the Bonferroni method for multiple testing (P<0.0083). Linear regression analysis was performed to assess whether cPP and aPWV (determinant) were associated with WMH and GM volume, as a continuous variable (outcome). The same models as in the ANCOVA analyses were used. All tests were two-sided and a P-value <0.05 was considered as statistically significant.
Results
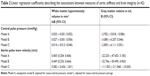
In total, 250 patients signed informed consent. A total of 166 patients had no reliable measurements of aPWV and/or brain imaging available. Therefore, the study population consisted of 84 patients. The characteristics of the population are shown in Table 1. Mean age was 76.6±6.8 years, 65.5% were men, and 65.5% lived with a partner. Most of the patients were ADL independent (65.5%) and 26.2% were iADL independent.
Median peripheral SBP was 138.0 mmHg [IQR 124.0–148.0] and DBP was 83.5 mmHg [IQR 77.0–93.0]. Median central SBP was 125.5 mmHg [IQR 114.3–136.0] and DBP was 85.0 mmHg [IQR 78.0–94.5]. Median cPP was 37.5 mmHg [IQR 31.0–45.8]. Mean aPWV was 11.6±1.65 m/s. Fourteen percent of the patients were classified as Fazekas 0, 23.8% as Fazekas 1, 17.9% as Fazekas 2, and 44.0% as Fazekas 3. Median volume of WMH was 3.1 mL [IQR 0.8–6.4]. Mean GM volume was 679.5±130.7 mL.
Age was associated with cPP (Spearman’s ρ =0.296, P=0.008), aPWV (Pearson’s r=0.886, P<0.001), and WMH volume (Pearson’s r=0.545, P<0.001), but not with GM volume (Pearson’s r=−0.179, P=0.255). Results are shown in Figure 1.
Figure 2 shows the mean values and 95% CI of cPP and aPWV across Fazekas categories. No differences were found in mean cPP values across categories of Fazekas in unadjusted analysis (Figure 2A). Mean values and 95% CI were 35.3 (29.2–42.7) mmHg, 36.2 (31.2–41.9) mmHg, 35.2 (29.7–41.7) mmHg, and 42.4 (37.8–47.5) mmHg from lowest to highest Fazekas categories, respectively. Mean values of cPP in model B slightly differed from univariate analysis (Figure 2B). Mean values and 95% CI were 37.6 (31.4–44.9) mmHg, 36.3 (31.7–41.6) mmHg, 36.3 (31.0–42.6) mmHg, and 40.7 (36.6–45.4) mmHg from lowest to highest Fazekas categories, respectively. In model C, no differences were found in mean values of cPP across categories of Fazekas (Figure 2C). Mean values and 95% CI were 39.5 (32.8–47.5) mmHg, 37.6 (32.7–43.2) mmHg, 36.2 (31.0–42.3) mmHg, and 39.2 (35.0–44.0) mmHg from lowest to highest Fazekas categories, respectively.
Mean values of aPWV increased from lowest to highest categories of Fazekas (test for trend P<0.001) in unadjusted analysis (Figure 2D). Mean values and 95% CI were 10.4 (9.6–11.2) m/s, 10.9 (10.3–11.5) m/s, 11.5 (10.7–12.2) m/s, and 12.6 (12.1–13.1) m/s, respectively. A significant difference in aPWV was found between categories 0 and 3 (mean difference 2.2 m/s, P<0.001) and 1 and 3 (mean difference 1.7 m/s, P<0.001). In model B, aPWV was higher in those in Fazekas 3 than those in Fazekas 0 (mean difference 1.8 m/s, P<0.001) and in Fazekas 1 (mean difference 1.5 m/s, P<0.001). Furthermore, aPWV increased from lowest to highest categories of Fazekas (test for trend P<0.001) comparable with unadjusted analysis (Figure 2E). Mean values and 95% CI of aPWV from lowest to highest categories were 10.7 (9.9–11.4) m/s, 10.9 (10.3–11.5) m/s, 11.6 (10.9–12.3) m/s, and 12.4 (12.0–12.9) m/s, respectively. In model C, aPWV did no longer differ between categories of Fazekas. Mean values and 95% CI of aPWV were 11.7 (11.4–11.9) m/s, 11.6 (11.4–11.8) m/s, 11.5 (11.3–11.7) m/s, and 11.7 (11.5–11.8) m/s from lowest to highest Fazekas categories, respectively (Figure 2F).
Levels of cPP showed a trend toward linear association with (natural logarithm of) WMH (lnB =0.025, 95% CI −0.001; 0.052) and this trend only slightly changed in model B (lnB =0.027, 95% CI −0.001; 0.056). Nonetheless, in model C, no association was found between cPP and WMH (lnB =0.014, 95% CI −0.013; 0.040) (Table 2). Levels of aPWV were linearly associated with (natural logarithm of) WMH (lnB =0.405, 95% CI 0.204; 0.606) and remained significant in model B (lnB =0.449, 95% CI 0.233; 0.664). In model C, however, no association was found between aPWV and WMH (lnB =0.382, 95% CI −0.436; 1.201).
Levels of cPP were negatively associated with GM volume in univariate analysis (B=−2.952, 95% CI −5.818; −0.086). In models B and C, a negative trend toward association was found between cPP and WMH (B=−3.080, 95% CI −6.207; 0.046; B=−2.805, 95% CI −6.111; 0.501, respectively). A negative trend was also found between aPWV and GM volume in univariate analysis (B=−22.235, 95% CI −47.653; 3.183). In model B, estimates were slightly less consistent (B=−22.766, 95% CI −50.495; 4.963). In model C, a negative association was found between aPWV and GM volume (B=−111.052, 95% CI −211.840; −10.265).
Discussion
In this study, we found that higher aortic stiffness was partly associated with a higher load of cerebral WML and lower GM volume in patients with cognitive and functional complaints. This association was slightly influenced by blood pressure. Higher aortic stiffness was found to be associated with a higher Fazekas score. The association was strongly mediated by age.
In a community-based cohort of 668 participants who were between the age of 69 and 93, higher levels of carotid-femoral pulse wave velocity were associated with diffuse microvascular brain lesions, which included subcortical infarcts and higher volumes of WMH.24 Carotid pulse pressure was associated with increased risk of silent subcortical infarcts. However, this study was performed in an apparently healthy population of older individuals, and those with a history of stroke, transient ischemic attack, or dementia were excluded. Considering these differences in the inclusion, the results of the two studies are not completely comparable. Also, previous studies were most often performed in specific categories of patients such as young hypertensives and diabetics.18,23 In these populations, higher aortic stiffness was associated with a greater volume of WMH. In addition, Saji et al found that increased brachial-ankle PWV was associated with WML in healthy adults in Japan.40 Since brachial-ankle PWV is not a measure of aortic stiffness, these results could not be compared with our findings. A recent systematic review made an overview of all studies investigating the association between arterial stiffness and CSVD, summing up the results in diverse study populations using diverse methods of defining arterial stiffness and CSVD.17 Out of the 15 cross-sectional studies included, 73% showed an association between greater arterial stiffness and CSVD.17
A few studies found an inverse relation between aortic stiffness and GM volume.28–30 However, these studies were conducted in young and healthy adults, in type 2 diabetics, and in patients with manifest arterial disease. No previous study has investigated the potential role of aortic stiffness in determining brain integrity in older persons with cognitive and functional complaints.
The mechanisms underlying the association between aortic stiffness and CSVD have previously been described as when aortic stiffness increases the pulsatile pressure.15,16 High pulsatile pressure increases the flow load and can cause damage in high-flow organs. This damage can be seen as WML, but also as cerebral microbleeds and lacunar infarcts, which are all markers of CSVD. The high pressure can affect the brain directly resulting in microvascular damage. Besides the direct damage that increased flow load can cause, it may also induce an indirect remodeling response by elevating the vascular resistance to protect the microvascular system from high pressures. This response might lead to ischemia in the long term.17 Moreover, GM atrophy may be the result of damages to the small cerebral arteries caused by vascular disease in advanced stage.28 The role of age in vascular processes is attributed to degenerative changes. At a molecular level, vascular aging is characterized by breaks in elastin fibers, accumulation of collagen, fibrosis, inflammation, and calcifications. All these processes result in a decline of the elastic properties of the central large arteries.41 Also cardiovascular diseases contribute to the decline of these elastic properties due to vascular remodeling.9 Interestingly, in this study population, the majority have had or still had cardiovascular diseases. However, the role of age still seems to be very important. We cannot exclude that associations between aortic stiffness and brain integrity would be different in a study population with a wider range of age and therefore a different time of exposure to risk factors. Moreover, we are aware that participants of the present study have an elevated load of degenerative vascular alterations due to age and comorbidities and possibly patients with elevated vascular stiffness might not be included in the study due to morbidity or mortality.
This study has some limitations. First, the cross-sectional study design did not allow us to draw conclusions about causality. Second, the low number of available MRI scans can limit the possibility to add further adjustments in analysis on WMH and GM. Third, we included older patients within a relatively small range of age and with an elevated load of degenerative vascular alterations. These “ceiling effects” in a relatively small sample of elderly could limit the ability to assess the role of aortic stiffness in brain integrity.
This study also has strengths. First, we used both graded scores (Fazekas) and more quantitative and automatic methods for assessing the cerebral WML load in order to investigate the association between aortic stiffness and WML. Moreover, the Fazekas score was scored by one experienced radiologist, which makes the results less susceptible to variability as a result of different raters. Second, we used two parameters of central arterial stiffness. cPP and aPWV both reflect aortic stiffness.35,37 Aortic stiffness is known to play a fundamental role in the development and progression of disease in end organs.42 Thus, the use of aortic stiffness as a parameter to assess brain integrity makes the outcomes of this study clinically relevant.
Conclusion
We found that higher levels of aortic stiffness were partly associated with increased cerebral WML load and decreased GM volume in elderly patients with cognitive and functional complaints. This association seems to be slightly influenced by blood pressure and strongly driven by age. In this population of older patients, majority of whom were exposed to cardiovascular disease, age is still an important factor, most likely due to a cumulative exposure to risk factors and age-related degenerative processes. Prospective investigations in a larger geriatric population are recommended to investigate the independent role of aortic stiffness in the development and progression of WML and loss of GM over time.
Acknowledgments
The authors would like to thank (former) medical students at Erasmus MC, University Medical Center Rotterdam, Antine Flikweert and Linda Kannegieter, who both contributed to the inclusion of patients during the period of their MD thesis. The authors would also like to thank Wave Medical BV Heerenveen, the Netherlands, for logistic support for performing this study (Mobil-o-graph device).
Disclosure
The authors report no conflicts of interest in this work.
References
de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70(1):9–14. | ||
Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23(8):1327–1333. | ||
Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol. 1995;52(10):970–974. | ||
Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1380–1388. | ||
Greenwald BS, Kramer-Ginsberg E, Krishnan KR, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke. 1998;29(3):613–617. | ||
Breteler MM, van Amerongen NM, van Swieten JC, et al. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke. 1994;25(6):1109–1115. | ||
Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2004;23(2):708–716. | ||
Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. | ||
de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(Pt 4):765–772. | ||
Tiehuis AM, van der Graaf Y, Visseren FL, et al. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke. 2008;39(5):1600–1603. | ||
Tuttolomondo A, di Sciacca R, di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol. 2011;151(3):318–322. | ||
di Raimondo D, Tuttolomondo A, Buttà C, Miceli S, Licata G, Pinto A. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des. 2012;18(28):4385–4413. | ||
Tuttolomondo A, Pecoraro R, Simonetta I, et al. Neurological complications of Anderson–Fabry disease. Curr Pharm Des. 2013;19(33):6014–6030. | ||
Tuttolomondo A, Pecoraro R, Simonetta I, Miceli S, Pinto A, Licata G. Anderson-Fabry disease: a multiorgan disease. Curr Pharm Des. 2013;19(33):5974–5996. | ||
Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105(5):1652–1660. | ||
Poels MM, Zaccai K, Verwoert GC, et al. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke. 2012;43(10):2637–2642. | ||
van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;53:121–130. | ||
Laugesen E, Høyem P, Stausbøl-Grøn B, et al. Carotid-femoral pulse wave velocity is associated with cerebral white matter lesions in type 2 diabetes. Diabetes Care. 2013;36(3):722–728. | ||
Brisset M, Boutouyrie P, Pico F, et al. Large-vessel correlates of cerebral small-vessel disease. Neurology. 2013;80(7):662–669. | ||
Seo WK, Lee JM, Park MH, Park KW, Lee DH. Cerebral microbleeds are independently associated with arterial stiffness in stroke patients. Cerebrovasc Dis. 2008;26(6):618–623. | ||
Song TJ, Kim J, Kim YD, et al. The distribution of cerebral microbleeds determines their association with arterial stiffness in non-cardioembolic acute stroke patients. Eur J Neurol. 2014;21(3):463–469. | ||
Hashimoto J, Aikawa T, Imai Y. Large artery stiffening as a link between cerebral lacunar infarction and renal albuminuria. Am J Hypertens. 2008;21(12):1304–1309. | ||
Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52(6):1120–1126. | ||
Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility – Reykjavik study. Brain. 2011;134(Pt 11):3398–3407. | ||
Ochi N, Kohara K, Tabara Y, et al. Association of central systolic blood pressure with intracerebral small vessel disease in Japanese. Am J Hypertens. 2010;23(8):889–894. | ||
Saji N, Kimura K, Shimizu H, Kita Y. Association between silent brain infarct and arterial stiffness indicated by brachial-ankle pulse wave velocity. Intern Med. 2012;51(9):1003–1008. | ||
Tsao CW, Seshadri S, Beiser AS, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81(11):984–991. | ||
Maillard P, Mitchell GF, Himali JJ, et al. Effects of arterial stiffness on brain integrity in young adults from the Framingham Heart Study. Stroke. 2016;47(4):1030–1036. | ||
Katulska K, Wykrętowicz M, Minczykowski A, et al. Gray matter volume in relation to cardio-vascular stiffness. J Neurol Sci. 2014;343(1–2):100–104. | ||
Climie RE, Srikanth V, Beare R, et al. Aortic reservoir characteristics and brain structure in people with type 2 diabetes mellitus; a cross sectional study. Cardiovasc Diabetol. 2014;13:143. | ||
Jochemsen HM, Muller M, Bots ML, et al. Arterial stiffness and progression of structural brain changes: the SMART-MR study. Neurology. 2015;84(5):448–455. | ||
Folstein MF, Folstein SE, Mchugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. | ||
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. | ||
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. | ||
O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15(5):426–444. | ||
Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit. 2013;18(3):173–176. | ||
Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197–203. | ||
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. | ||
de Boer R, Vrooman HA, van der Lijn F, et al. White matter lesion extension to automatic brain tissue segmentation on MRI. Neuroimage. 2009;45(4):1151–1161. | ||
Saji N, Shimizu H, Kawarai T, Tadano M, Kita Y, Yokono K. Increased brachial-ankle pulse wave velocity is independently associated with white matter hyperintensities. Neuroepidemiology. 2011;36(4):252–257. | ||
Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45(6):1050–1055. | ||
Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S. Central artery stiffness in hypertension and aging: a problem with cause and consequence. Circ Res. 2016;118(3):379–381. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.