Back to Journals » Journal of Multidisciplinary Healthcare » Volume 9
Anxiety mediates the effect of smoking on insomnia in people with asthma: evidence from the HUNT3 study
Authors Andenæs R, Schwartz CE
Received 21 October 2015
Accepted for publication 27 November 2015
Published 21 January 2016 Volume 2016:9 Pages 21—28
DOI https://doi.org/10.2147/JMDH.S98784
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Randi Andenæs,1 Carolyn E Schwartz1–3
1Department of Nursing and Health Promotion, Faculty of Health Science, Oslo and Akershus University College of Applied Sciences, Oslo, Norway; 2DeltaQuest Foundation, Inc., Concord, 3Departments of Medicine and Orthopaedic Surgery, Tufts University Medical School, Boston, MA, USA
Objective: The aim of this study was to investigate factors related to insomnia in a cohort of people with asthma.
Design: This secondary analysis utilized cross-sectional data from the Norwegian Nord-Trøndelag Health Study, a population-based health survey (n=50,807).
Participants: We used self-reported data from 1,342 men and women with a physician-confirmed asthma diagnosis ranging in age from 19.5 to 91 years.
Measurements: Data on sleep, lifestyle variables (smoking and exercise), anxiety, and depression were included. An insomnia scale and asthma impact scale were constructed using factor analysis. Hierarchical series of multiple regression models were used to investigate direct and mediational relationships between the study variables and insomnia.
Results: The hierarchical models revealed significant independent contributions of female sex, higher age, not exercising, asthma impact, anxiety, and depression on insomnia (R2=25.2%). Further, these models suggested that the impact of smoking on insomnia was mediated by anxiety, and that the beneficial impact of exercise was mitigated by depression symptoms.
Conclusion: Smokers with asthma have more insomnia, and this relationship may be mediated by anxiety. Further, people with asthma who experience depression symptoms are less likely to benefit from physical exercise as a method to enhance sleep quality. Our findings would suggest that helping smokers to manage their anxiety and depression through behavioral methods may reduce their insomnia symptoms, and enable them to engage in other health-enhancing pursuits, such as physical exercise.
Keywords: insomnia, asthma, anxiety, depression, smoking, mediation
Introduction
Sleep problems are widespread both in general population and in clinical practice. Insomnia is the most common sleep disorder, and is characterized by subjective complaints about dissatisfaction with sleep quality or duration, as well as difficulty with initiating sleep, maintaining sleep at bedtime, waking up in the middle of the night or too early in the morning, or nonrestorative, poor quality of sleep.1 Population studies show that up to 48% of adults report “some insomnia problems over the past year”, and 6%–15% report chronic insomnia.2
Sleep patterns change throughout life. Sleep problems are common in adolescents,3 possibly because of the puberty-related circadian shift toward being a “night owl”.4 Menopausal transition status is also related to severe self-reported sleep difficulty,5,6 which may be due to hormonal changes.7 Later in life, people note that they have more difficulty staying asleep at night and, perhaps consequently, more difficulty staying awake during the day.8 The cause of sleep problems in older people is more complex than hormones, and can be related to circadian rhythm changes, medication, medical and psychiatric illness, and other primary sleep disorders.9 Thus, the biological reasons underlying sleep difficulties may change over the life trajectory.
Although primarily defined as a sleep disorder, consequences of insomnia extend beyond the sleep period. Daytime symptoms, such as tiredness, irritability, and decreased cognitive and psychomotor alertness, are typical complaints that motivate people to seek treatment.10 These consequences of insomnia carry a heavy burden for both individuals and the society, as evidenced by their association with fatal accidents, human errors,11 increased sick leave, reduced work capacity, increased health care utilization, and heightened risk of anxiety or depression.11,12
In addition to the social costs of insomnia, there are long-term effects of sleep problems on health. For example, sleep has profound effects on airway function. Indeed, asthma is reported to be the fifth most frequent physical condition related to insomnia, after headache, hypertension, obesity, and arthrosis.13 Many people with asthma experience worsening of symptoms at night, and a greater proportion of people with asthma die at night as compared with general population.14,15 Factors contributing to the pathophysiology of nocturnal asthma are increased airway resistance and bronchial hyperreactivity, and diminished flow rates.16 As one of the most common chronic medical conditions, currently affecting an estimated 235 million people worldwide,17 asthma has reached historically high levels in the US,18 and is associated with high health care utilization. Caused by allergies and respiratory sensitization,19 asthma is exacerbated by active and passive smoking,20,21 viral infections, and poor indoor and outdoor air quality.22
In addition to the above environmental factors, lifestyle factors also contribute to asthma symptom burden. For example, chronic heavy smoking in adulthood is a significant risk factor for insomnia.20,21,23 In contrast, habitual physical activity is protective, reducing the prevalence of insomnia24 and improving sleep quality.25 Other relevant correlates of insomnia are affective disorders, such as depression and anxiety.26–31 Compared with people with no sleep difficulties, people with insomnia have an almost tenfold risk of clinical depression and more than a 17-fold risk of clinical anxiety.27,32
In order to improve interventions, it is important to understand social and mental health determinants of insomnia. Determinants of insomnia in asthma are not well understood. Therefore, the aim of this study was to investigate factors related to insomnia in a cohort of people with asthma.
Materials and methods
Data source
We analyzed data from the third wave of the Nord-Trøndelag Health Study (HUNT study), which is a large population database for medical and health-related research. The present study uses data from the most recent survey, HUNT3, which was carried out between October 2006 and June 2008. Nord-Trøndelag is located in central Norway. The area is fairly representative of Norway in terms of geography, economy, industry, and sources of income, as well as in terms of the age distribution, morbidity, and mortality rates of its inhabitants.33,34 The present study was approved by the Regional Committee for Ethics in Medical Research (2015/660) and by the HUNT Publication Review Board.
Study sample
All inhabitants in the county were invited to participate. From among 93,860 who were eligible for participation, 50,807 (54.1%) responded to the first questionnaire (Q1) that was enclosed with the invitation letter. The data were collected in each of the 24 municipalities in the county by using temporarily located health examination sites staffed by certified fieldwork teams. A second questionnaire (Q2) focusing more on symptoms and diseases was handed out to everyone to be completed at home, and returned by mail in a prepaid envelope. Respondents with lung diseases also participated in a substudy (n=12,128). Our data came from this substudy. Eligibility criteria for inclusion in our study sample were: 1) having a physician-confirmed diagnosis of asthma and 2) endorsing having had asthma symptoms in the year prior to the interview. Those who reported having a diagnosis of chronic obstructive pulmonary disease, chronic bronchitis, or emphysema were excluded from the study sample.
Measures
Insomnia
Participants were asked four questions on insomnia symptoms, which included how often during the last 3 months they had 1) experienced difficulties with falling asleep at night; 2) woke up repeatedly during the night; 3) woke up too early without being able to get back to sleep; and 4) felt sleepy during the day. Response options were: 1 (never/seldom), 2 (occasionally), or 3 (several times a week). A factor analysis yielded one component with an eigenvalue greater than 1.0 (Table 1). We computed a total sum score representing insomnia from these four items. This score ranged from 4 to 12, with higher scores reflecting more severe insomnia. The summary score had acceptable internal consistency reliability (Cronbach’s α=0.68). Thus, the HUNT3 questions assessing insomnia symptoms generated a unidimensional score with high internal consistency, and were a sound psychometric composite for analysis.
  | Table 1 Items and factor structure for insomnia and asthma impact measures |
Asthma impact
Questions on the impact of asthma included how much they had experienced asthma symptoms in the last 7 days, and use of asthma medication. Specific questions were: 1) How many of the last 7 days have you noticed symptoms from your respiratory disease? 2) How many of the last 7 days have breathlessness restricted your normal activities (school, sport, work, housekeeping, etc)? and 3) How many of the last seven nights did you wake up/were affected by breathlessness (including cough)? A factor analysis generated one component (eigenvalue =1.78; Cronbach’s α=0.61; Table 1). We thus created a subscale by adding the three asthma items into an asthma impact score, ranging from 0 to 21. Higher scores reflect more asthma symptoms. Thus, the HUNT3 questions assessing asthma impact generated a unidimensional score with high internal consistency, and were a sound psychometric composite for analysis.
Anxiety and depression
The Hospital Anxiety and Depression Scale35 was used to assess self-reported symptoms of anxiety and depression. The questionnaire consisted of 14 questions: seven for anxiety and seven for depression. Response options were on a 4-point scale ranging from 0 (not at all) to 3 (very often). Recall time was the last week. Responses were summed to provide separate scores for anxiety and depression symptoms, with possible scores ranging from 0 to 21 for each scale. Higher scores indicate greater likelihood of depression or anxiety.35,36 The psychometric properties of the scale have been validated previously.36,37 Cronbach’s α was 0.82 for the anxiety score and 0.76 for the depression score. A cutoff score of 8 on both subscales has been shown to give an optimal balance between sensitivity and specificity at ~0.80 for depression, according to the Diagnostic and Statistical Manual of Mental Disorders (III and IV) and the International Classification of Disorders (ICD-8 and 9).38 The anxiety and depression scores were dichotomized according to this cutoff score for the present analyses.36,37
Demographic and lifestyle variables
The following variables were included to characterize the sample. Age was categorized in 6 decades, from 19 to 79 years, and one additional group with those 80 years and older. Partnership was dichotomized into those living with a partner and those living alone. Data on education were obtained from the National Population Registry of Norway, and highest completed education was classified as: 0, primary and lower secondary school – up to 10 years; 1, high school – 11–13 years; 2, university and high school, short; and 3, university and high school, long. Physical activity was assessed by the single item “How often do you perform exercise?” with response options as: 0, never; 1, less than once a week; 2, once a week; 3, two to three times a week; and 4, almost every day. Smoking was characterized as either “never smoking”, “quit smoking”, “now and then”, or “daily”, with yes/no response options. For daily smokers, number of cigarettes per day was also recorded. Questions on medications included use of prophylactic- and symptom-reducing medication.
Statistical analysis
Hierarchical series of multiple regression analyses were performed with insomnia as dependent variable. Mediation was tested by a multistage regression approach outlined by Baron and Kenny,39 in which the three regression equations were estimated: first, regressing the mediator (M) on the independent variable (X); second, regressing the dependent variable (Y) on the independent variable (X); and third, regressing the dependent variable (Y) on both the independent variable (X) and on the mediator (M).39 The mediator has been called an intervening or process variable. Complete mediation is the case in which variable X no longer affects Y after M has been controlled and so path c′ is zero. Partial mediation is the case in which the path from X to Y is reduced in absolute size but is still different from zero when the mediator is introduced.40 The type I error rate was set to P<0.05, and all tests were two-tailed. The statistical analyses were carried out using SPSS 22.0 for Windows (IBM Corporation, Armonk, NY, USA).41
Results
From the 3,348 participants who reported that they had, or ever had asthma, only those with a current asthma diagnosis, and with no missing data on sleep questions were included. The final asthma sample included 1,342 persons. The age range was 19–91 years (mean 53.0, standard deviation 1.8). The majority was female (60.3%), and there was a high prevalence of current tobacco smokers (33% smoking daily, and now and then). Approximately 11% reported a high level of depression and 18% had a high level of anxiety (Table 2). The asthma sample did not differ from the HUNT3 in age, partnership, or smoking. However, the asthma sample had more women (P≤0.001), a higher prevalence of insomnia (P≤0.001), and fewer people with more than 20 years of education compared with the HUNT3 (P≤0.035). Further, those with asthma had more anxiety (P≤0.001) and depression (P≤0.001), which is consistent with the research literature.12
  | Table 2 Description of study population |
Univariate regressions suggested that reporting insomnia was associated with being female, older, less educated, and/or a smoker. It was also associated with never exercising, having worse reported asthma symptom impact, and reporting anxiety or depression (Table 3). Of study participants, more than 40% used asthma prophylactic medication, and nearly 28% reported that they had used a medication to reduce asthma symptoms in the past 3 days. We evaluated the medication variables in the univariate models, but they showed no significant association with insomnia, and were not kept for later models.
Table 3 shows the results of the hierarchical multiple regression analyses modeling the prediction of insomnia. Because all of the bivariate correlations between variables used in the analysis were r<0.7, we assumed no multicollinearity of variables (Table 3). Inspection of residuals indicated no violation of regression assumptions.
In step 1, controlling for demographic and lifestyle variables, female sex, higher age, current smoking, and never doing physical activity, were all independently associated with insomnia (P<0.001 for all variables). In step 2, asthma impact was entered into the model and was found to have an independent effect on insomnia (β=0.14, P<0.001). In step 3, depression was entered into the model and was found to have an independent effect on insomnia (β=0.36, P<0.001). Further, exercise was no longer a significant predictor of insomnia, suggesting that the beneficial impact of exercise was mitigated by depression symptoms. In step 4, anxiety was entered into the model along with the step 1 and step 2 covariates, and was found to have an independent effect on insomnia (β=0.39, P<0.001). In step 5, depression and anxiety were entered together into the model and were found to have independent effects on insomnia (β=0.20 and 0.28, respectively; P<0.001 in both cases). In step 6, two interaction terms were entered into the model (anxiety x asthma impact, and depression x asthma impact); both were not statistically significant.
Sociodemographic and lifestyle variables (step 1) explained 6.8% of the variance in explaining insomnia, whereas asthma impact (step 2) added only 1.9%. Anxiety and depression accounted for 16.6% variance (step 5), adding most to the explained 25.0% variance in the total model.
This hierarchical approach to modeling insomnia revealed that when anxiety was entered into the model (step 3), the previously significant direct relationship between smoking and insomnia (β=0.06, P<0.01) was no longer significant (β=0.05 in both steps 4 and 5; P=0.06 in step 4 and 0.08 in step 5). This pattern suggests that anxiety mediated the relationship between smoking and insomnia. Figure 1 displays the mediation model.
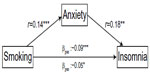  | Figure 1 Anxiety as a mediator of the relationship between smoking and insomnia. |
Discussion
Our findings from this large population-based survey suggest that insomnia in people with asthma is associated with demographic characteristics, lifestyle factors, and asthma impact. By far, the most important explanatory variables were anxiety and depression, accounting for two-thirds of the explained variance in the model. Further, we found that when we adjusted for anxiety, the predictive value of smoking diminished, suggesting that this affective problem mediates the relationship between smoking and insomnia.
Despite the cross-sectional structure of the data used in this secondary analysis, the mediation analysis allows the examination of potentially causal models to generate hypotheses for future longitudinal research.40 As Kenny notes, the mediator is presumed to cause the outcome and not vice versa. One reason for testing mediation is to suggest a mechanism by which the variable affects the outcome. While it is known smoking in people with asthma is associated with anxiety,31 research on the relationship between smoking and insomnia is inconclusive.42,43 In the present sample, more than 31% of the participants reported that they were current smokers. This is a surprising number given that these people have asthma and are likely aware of the harmful effect of cigarette smoking on their lungs. It also puts them at higher risk for the development of chronic obstructive pulmonary disease.44–46 Why would these people with asthma not quit smoking? Perhaps these people continue to smoke despite its harmful effects because nicotine has mood-altering effects that make quitting difficult.47 While it is known that nicotine reduces depression47 and anxiety48 in the short-term (ie, acute effects),49 the relationship is a complex one.48 For example, it is conceivable that over extended periods of time, smoking may actually induce negative affect, through the emergence of a withdrawal syndrome or some other mechanism.50 Our findings suggest that the relationship between smoking and insomnia is related to heightened anxiety, but we are not able to determine whether this anxiety is alleviated or caused by the smoking due to the cross-sectional nature of the data.
It is also possible that other variables in our model interrelate with smoking, anxiety, and insomnia. For example, it is well known that anxiety and panic attacks are associated with asthma,51 and most likely the relationship is bidirectional.52 Persons with asthma having emotional problems may develop a vicious cycle between anxiety and symptoms; they may cope or self-medicate by smoking cigarettes to regulate their affect. This approach to managing their anxiety in the short-term may exacerbate their insomnia in the longer-term, and may lead to more negative affect due to the sleep deprivation (insomnia). This escalation in negative affect may in turn lead to more smoking. Future research might utilize longitudinal data from the HUNT study or another data source to investigate causal models of the relationship between smoking, anxiety, asthma symptoms, and insomnia in people with asthma.
Increase in asthma symptoms significantly associated with insomnia
In addition to the intriguing mediation effect discussed earlier, we also found robust independent effects of asthma impact on insomnia. Based on these findings, one might speculate that nocturnal asthma – exacerbations of asthma that occur during the night – may further disrupt sleep, causing difficulty initiating and maintaining sleep as well as poor overall sleep quality.53,54 Future research might examine the effectiveness of proactive or preventative use of asthma medication (eg, inhalers) just before going to bed at night to reduce insomnia.
Prescribing physical activity
Along with identifying health behaviors associated with worse insomnia, our model also identified a robust and independent impact of engaging in physical exercise in reducing insomnia. It is notable that this effect was mediated by depression, suggesting that the salutogenic effect of exercise is explained by (reduced) depression. Thus, engaging in exercise may reduce individuals’ depression symptoms and thus enable a good night’s sleep. When anxiety was entered into the model, this mediation effect was no longer present, suggesting that exercise did not buffer the impact of anxiety on insomnia. Since asthma guidelines55 aim to help people with asthma to remain active and sleep well, future research might investigate whether exercise-based interventions can be tailored to mitigate both depression and anxiety and thereby improve sleep in people with asthma.55
Strengths and limitations of the study
This study has a number of notable strengths, including the sample size, the population-based sampling strategy, and the quality of the data collected. Our analyses built on these strengths to ask a research question with clear clinical significance and implications. The limitations of the present study must, however, be acknowledged. First, the main limitation is that the data were not collected for the purpose of the specific research question for our study. Consequently, the measures might not be the best operationalization of a construct of interest; the sample might not be the most fitting to ask the question (eg, including asthmatics who are using medications that could interfere with sleep). Second, although the HUNT3 has a reasonable compliance rate (54%)56 for such a large study, nonparticipant analyses suggest that the study sample tends to include relatively healthy people (ie, those with a lower mortality rate), and underrepresents lower socioeconomic status.56 Third, the flip side of the power advantages of large samples is that analyses of such sample can yield statistically significant findings that have questionable clinical significance. In our analysis, the difference in beta coefficients between model step 1 and 4 was 0.04, yet the significance changed from P<0.001 to P<0.06. Future research might replicate these analyses in an independent sample to evaluate their robustness. Finally, our mediation analysis is not able to make causal inferences about the relationships among the study variables. It is intended to suggest causal hypotheses that can be tested in future longitudinal investigations.
Conclusion
It is well known that an untreated sleep problem can have a negative effect on an individual’s physical and psychosocial functioning.52,57 We found that while the impact of asthma on insomnia is modest, smokers with asthma have more insomnia, and anxiety seems to mediate this relationship. Further, people with asthma who have symptoms of depression do not get the benefit of exercise on insomnia. Our findings would suggest that helping smokers to manage their anxiety and depression through behavioral methods may reduce their insomnia symptoms, and enable them to engage in other health-enhancing pursuits, such as physical exercise. Future research should investigate the impact on sleep and other health behaviors of behavioral interventions aimed at negative-affect reduction in people with asthma.
Acknowledgments
The Nord-Trøndelag Health Study (the HUNT study) is a collaboration between the HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology), the Nord-Trøndelag County Council, the Central Norway Health Authority, and the Norwegian Institute of Public Health. The authors thank Carolina Campos, RN, MNSc for helpful discussions earlier in the course of this project. This study was performed at the Department of Nursing and Health Promotion, Oslo and Akershus University College, Oslo, Norway.
Author contributions
Both authors contributed to the conception and design, analysis, and interpretation of data. Further, RA drafted the article with substantial contributions from CES, and both authors revised it critically for important intellectual content, and approved the final version. The authors agree that they are accountable for all aspects of the work, confirming that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no other conflicts of interest in this work.
References
Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379:1129−1141. | |
Lichstein KLT, Daniel J, McCrae, Christina S. Insomnia: epidemiology and risk factors. In: Kryger MH, Roth T, Dement, WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier Saunders; 2011:827−838. | |
Reiter J, Rosen D. The diagnosis and management of common sleep disorders in adolescents. Curr Opin Pediatr. 2014;26:407−412. | |
Kronholm E, Puusniekka R, Jokela J, et al. Trends in self-reported sleep problems, tiredness and related school performance among Finnish adolescents from 1984 to 2011. J Sleep Res. 2015;24:3−10. | |
Tom SE, Kuh D, Guralnik JM, Mishra GD. Self-reported sleep difficulty during the menopausal transition: results from a prospective cohort study. Menopause. 2010;17:1128−1135. | |
Partinen M, Hublin C. Epidemiology of sleep disorders. In: Kryger MH, Roth T, Dement, William C, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier Saunders; 2011:695−715. | |
Lee KA, Moe K. Menopause. In: Kryger MH, Roth T, Dement, William C, editors. Principles and Practice of Sleep Medicine. St. Louis, MO.: Elsevier Saunders; 2011:1592−1601. | |
Feinsilver SH. Sleep in the elderly. What is normal? Clin Geriatr Med. 2003;19:177–188. | |
Ancoli-Israel S, Shochat T. Insomnia in older adults. In: Kryger MH, Roth T, Dement, WC, editors. Principles and Practice of Sleep Medicine. St. Louis, MO: Elsevier Saunders; 2011:1544–1550. | |
Leger D, Poursain B. An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin. 2005;21:1785–1792. | |
Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35:1382–1393. | |
Morin CM, Benca RM. Insomnia: nature, diagnosis, and treatment. In: Montagna P, Chokroverty S, editors. Handbook of Clinical Neurology, Sleep Disorders, Part II. Vol 99. Amsterdam: Elsevier B.V; 2011. | |
Uhlig BL, Sand T, Ødegård SS, Hagen K. Prevalence and associated factors of DSM-V insomnia in Norway: the Nord-Trøndelag Health Study (HUNT 3). Sleep Med. 2014;15:708–713. | |
Douglas NJ. Asthma at night. Clin Chest Med. 1985;6:663–674. | |
Douglas NJ. Nocturnal asthma. Q J Med. 1989;71:279–289. | |
Khan WH, Mohsenin V, D’Ambrosio CM. Sleep in asthma. Clin Chest Med. 2014;35:483–493. | |
WHO. Asthma. Fact sheet No. 307. 2013. Geneva: World Health Organization. Available from: http://www.who.int/mediacentre/factsheets/fs307/en/. Accessed October 19, 2015. | |
Zhang X, Morrison-Carpenter T, Holt JB, Callahan DB. Trends in adult current asthma prevalence and contributing risk factors in the United States by state: 2000–2009. BMC Public Health. 2013;13:1156. | |
Pallasaho P, Juusela M, Lindqvist A, Sovijarvi A, Lundback B, Ronmark E. Allergic rhinoconjunctivitis doubles the risk for incident asthma – results from a population study in Helsinki, Finland. Respir Med. 2011;105:1449–1456. | |
Coogan PF, Castro-Webb N, Yu J, O’Connor GT, Palmer JR, Rosenberg L. Active and passive smoking and the incidence of asthma in the Black Women’s Health Study. Am J Respir Crit Care Med. 2015;191:168–176. | |
Magnus MC, Håberg SE, Karlstad Ø, Nafstad P, London SJ, Nystad W. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: the Norwegian Mother and Child Cohort Study. Thorax. 2015;70:237–243. | |
Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. 2009;181:E181–E190. | |
Brook DW, Rubenstone E, Zhang C, Brook JS. Trajectories of cigarette smoking in adulthood predict insomnia among women in late mid-life. Sleep Med. 2012;13:1130–1137. | |
Inoue S, Yorifuji T, Sugiyama M, Ohta T, Ishikawa-Takata K, Doi H. Does habitual physical activity prevent insomnia? A cross-sectional and longitudinal study of elderly Japanese. J Aging Phys Act. 2013;21:119–139. | |
Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58:157–163. | |
Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. | |
Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–1464. | |
Katon WJ, Richardson L, Lozano P, McCauley E. The relationship of asthma and anxiety disorders. Psychosom Med. 2004;66:349–355. | |
de Miguel Díez J, Hernández Barrera V, Puente Maestu L, Carrasco Garrido P, Gómez García T, Jiménez García R. Psychiatric comorbidity in asthma patients. Associated factors. J Asthma. 2011;48:253–258. | |
Urrutia I, Aguirre U, Pascual S, et al. Impact of anxiety and depression on disease control and quality of life in asthma patients. J Asthma. 2012;49:201–208. | |
Gada E, Khan DA, DeFina LF, Brown ES. The relationship between asthma and self-reported anxiety in a predominantly healthy adult population. Ann Allergy Asthma Immunol. 2014;112:329–332. | |
Baglioni C, Spiegelhalder K, Nissen C, Riemann D. Clinical implications of the causal relationship between insomnia and depression: how individually tailored treatment of sleeping difficulties could prevent the onset of depression. EPMA J. 2011;2:287–293. | |
Holmen J, Midthjell K, Øystein Krüger, et al. The Nord-Trøndelag Health Study 1995-97 (HUNT 2): objectives, contents, methods and participation. Norsk Epidemiol. 2003;13:19–32. | |
Langhammer A, Krokstad S, Romundstad P, Heggland J, Holmen J. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol. 2012;12:143. | |
Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. | |
Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. | |
Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–544. | |
Sivertsen B, Lallukka T, Salo P, et al. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT Study in Norway. J Sleep Res. 2014;23:124–132. | |
Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. | |
Kenny DA. Mediation. 2014. Available from: http://davidakenny.net/cm/mediate.htm. Accessed October 19, 2015. | |
IBM Corporation. SPSS. Version 22. Armonk, NY: IBM Corporation. | |
Mak K-K, Ho S-Y, Thomas GN, et al. Smoking and sleep disorders in Chinese adolescents. Sleep Med. 2010;11:268–273. | |
Sutton DA, Moldofsky H, Badley EM. Insomnia and health problems in Canadians. Sleep. 2001;24:665–670. | |
Harmsen L, Gottlieb V, Makowska Rasmussen L, Backer V. Asthma patients who smoke have signs of chronic airflow limitation before age 45. J Asthma. 2010;47:362–366. | |
Vignoud L, Pin I, Boudier A, et al. Smoking and asthma: disentangling their mutual influences using a longitudinal approach. Respir Med. 2011;105:1805–1814. | |
Perret JL, Walters EH, Abramson MJ, McDonald CF, Dharmage SC. The independent and combined effects of lifetime smoke exposures and asthma as they relate to COPD. Expert Rev Respir Med. 2014;8:503–514. | |
Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031. | |
Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. | |
Kassel JD, Unrod M. Smoking, anxiety, and attention: support for the role of nicotine in attentionally mediated anxiolysis. J Abnorm Psychol. 2000;109:161–166. | |
Parrott AC. Does cigarette smoking cause stress? Am Psychol. 1999;54:817–820. | |
Goodwin RD, Eaton WW. Asthma and the risk of panic attacks among adults in the community. Psychol Med. 2003;33:879–885. | |
Hasler G, Buysse DJ, Gamma A, et al. Excessive daytime sleepiness in young adults: a 20-year prospective community study. J Clin Psychiatry. 2005;66:521–529. | |
Klink ME, Dodge R, Quan SF. The relation of sleep complaints to respiratory symptoms in a general population. Chest. 1994;105:151–154. | |
Mastronarde JG, Wise RA, Shade DM, Olopade CO, Scharf SM. Sleep quality in asthma: results of a large prospective clinical trial. J Asthma. 2008;45:183–189. | |
Institute NHLaB. EPR 3 Guidelines on Asthma 2012. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/full-report. Accessed October 19, 2015. | |
Krokstad S, Langhammer A, Hveem K, et al. Cohort profile: the HUNT study, Norway. Int J Epidemiol. 2013;42:968–977. | |
Luyster FS, Strollo PJ Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–734. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.