Back to Journals » Journal of Pain Research » Volume 12
Anxiety-induced hyperalgesia in female rats is mediated by cholecystokinin 2 receptor in rostral ventromedial medulla and spinal 5-hydroxytryptamine 2B receptor
Authors Jiang M, Bo J, Lei Y, Hu F, Xia Z, Liu Y, Lu C, Sun Y, Hou B, Ni K, Ma Z, Gu X
Received 15 September 2018
Accepted for publication 29 May 2019
Published 3 July 2019 Volume 2019:12 Pages 2009—2026
DOI https://doi.org/10.2147/JPR.S187715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Ming Jiang1,*, Jinhua Bo1,*, Yishan Lei,1 Fan Hu,2 Zhengrong Xia,2 Yue Liu,1 Cui’e Lu,1 Yu’e Sun,1 Bailing Hou,1 Kun Ni,1 Zhengliang Ma,1 Xiaoping Gu1
1Department of Anesthesiology, Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing 210008, Jiangsu Province, People’s Republic of China; 2Department of Basic Medicine, Analytical & Testing Center, Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Background: Preoperative anxiety is associated with postoperative hyperalgesia; however, few studies have investigated the mechanism underlying this association in female surgical patients. Research has suggested that ON cells in the rostral ventromedial medulla (RVM) receive nerve impulses via cholecystokinin 2 (CCK2) receptors, facilitating hyperalgesia. Additionally, the downstream serotonergic projection system from the RVM to the spinal cord has a dual regulating effect on pain responses, and the 5-hydoxytryptophan 2B (5-HT2B) receptor in spinal dorsal horn neurons is critically involved in mechanical allodynia.
Methods: Ovariectomized rats were treated with estrogen replacement, single prolonged stress (SPS), and plantar incision. Various receptor agonists and antagonists were then administered into the RVM and spinal cord to study the mechanism underlying postoperative hyperalgesia caused by preoperative anxiety in female rats.
Results: Behavioral testing revealed that preoperative SPS induced postoperative hyperalgesia, as well as the expression of the CCK2 receptor in the RVM and the expression of the 5-HT2B receptor, protein kinase Cγ (PKCγ), and phosphorylation of the N-methyl-d-aspartate receptor1 (p-NR1) in the spinal cord increased confirmed by Western blot. RVM microinjection of the CCK2 receptor agonist CCK-8 and intrathecal injection of the 5-HT2B receptor agonist BW723C86 both produced hyperalgesia in female rats after plantar incision, whereas the CCK2 receptor antagonist YM022, the 5-HT2B receptor antagonist RS127445, and the PKCγ inhibitor C37H65N9O13 decreased the rats’ sensitivity to the same stimulus. Additionally, electrophysiological analysis suggested that activation of the 5-HT2B receptor increased the whole-cell current (IBa) in superficial dorsal horn neurons through the PKCγ pathway.
Conclusion: Our study demonstrated that preoperative anxiety-induced postoperative hyperalgesia in female rats is associated with descending pain pathways. The CCK2 receptor in the RVM and spinal 5-HT2B receptor may play a role in this hyperalgesic effect.
Keywords: cholecystokinin 2 receptor, 5-hydoxytryptophan 2B receptor, preoperative anxiety, postoperative hyperalgesia, rostral ventromedial medulla, spinal cord
Introduction
Preoperative anxiety is a clinically significant problem for patients undergoing surgery, with a reported incidence ranging from 60% to 92%.1,2 Preoperative anxiety is associated with increased pain, nausea, vomiting, and risk of infection in the postoperative period.3–5 Further, studies have shown a positive correlation between preoperative anxiety and postoperative pain intensity.6–8 Enhanced allodynia and pain sensitivity due to stressful stimuli are collectively termed stress-induced hyperalgesia.9–11 Some research have pointed out that gonadal steroid hormones significantly influence pain and anxiety levels differently in male and female. Estrogen, testosterone, and other gonadal hormones have a complex role in anxiety processes and the pain response.12,13 Although animal studies have investigated anxiety-induced hyperalgesia in males,14,15 the underlying mechanism in females remains unclear.
The rostral ventromedial medulla (RVM) is well recognized as a critical relay site for integrating descending modulatory influences on nociceptive input to dorsal horn neurons.16–18 The RVM can facilitate or suppress nociceptive transmission through the actions of two distinct classes of neurons, ON-cells and OFF-cells, which, respectively, exert nociceptive and antinociceptive effects.19 Stress has been shown to produce thermal and mechanical hypersensitivity by activating pain-facilitating ON-cells in the RVM.20,21 Evidence indicates that ON-cells are activated by the peptide cholecystokinin (CCK).22 CCK has been shown to have both pro-nociceptive and anxiogenic properties, which are likely involved in the link between anxiety and hyperalgesia.23 Studies suggest CCK activates ON-cells, which produces hyperalgesia and allodynia via the CCK2 receptor.24 Additionally, treatment with CCK2 antagonists can reduce anxiety-induced hyperalgesia in humans.25 Studies have also shown that the CCK-induced increase in the spinal level of 5-hydroxyindoleacetic acid, a 5-hydoxytryptophan (5-HT) metabolite, can be blocked by pretreatment of the RVM with a CCK2 antagonist.26
The serotonergic system from the RVM to the spinal cord is involved in descending facilitation during the development of persistent pain after tissue and nerve injury.27,28 The family of 5-HT receptors consists of seven subgroups and 15 receptor subtypes,29 including several subtypes associated with selective excitatory and inhibitory functions in neurons.17 However, it is unclear as to which 5-HT receptor subtypes in the spinal dorsal horn are associated with serotonergic modulation of preoperative anxiety-induced postoperative hyperalgesia in female rats. Activation of the 5-hydoxytryptophan 2B (5-HT2B) receptor in spinal dorsal horn neurons has been shown to be critical in the development of thermal and mechanical allodynia.30 Activation of 5-HT2 receptors and downstream protein kinase C (PKC) plays a role of relation to nociception in superficial dorsal horn neuron.31,32 PKCγ is specific to interneurons in the inner part of lamina II33 and widely participates in the processing of pain signals in spinal neuron.34,35 Translocation of PKC to the cell membrane is accompanied by increased phosphorylation of the NR1 subunit Ser-896 of the N-methyl-d-aspartate receptor (NMDAR) in the spinal cord.36 The NR1 subunit is phosphorylated by protein kinase A (PKA) on serine-890 and -897 and by PKC on Ser-896. The phosphorylation of NR1 subunit Ser-896 in spinal cord neurons can enhance pain sensitization. In this study, we investigated whether the CCK2 receptor in the RVM and spinal 5-HT2B receptor contribute to postoperative hyperalgesia after a single prolonged stress (SPS) procedure in female rats and observed the effect of antagonist on relieving postoperative hyperalgesia.
Materials and methods
Animals and estrogen replacement
Adult female Sprague–Dawley rats, weighing from 250 to 300 g, were provided by the Laboratory Animal Centre of Drum Tower Hospital. The rats were housed in standard laboratory conditions with free access to food and tap water. Every effort was made to minimize suffering, and only the necessary number of rats was used to obtain valid results. This study was carried out in accordance with the principles of the Basel Declaration and recommendations of animal management regulations, State Scientific and Technological Commission of People’s Republic of China. The protocol was approved by the Experimental Animals Welfare and Ethics Committee of Nanjing Drum Tower Hospital.
All of the female rats were anesthetized with sevoflurane and underwent bilateral ovariectomy. A small incision was made on bilateral sides of the back to expose the ovaries retroperitoneally. The ovaries were clamped and removed; the uterine tubes were ligated; then the skin was sutured.37 Prophylactic gentamicin 8 mg/mL, 1mL/kg was delivered i.p. following surgery. For estrogen replacement therapy, the ovariectomized rats were subcutaneously injected with 0.1 mg/kg/day of 17b-estradiol-3-benzoate (E2; Sigma, St. Louis, MO, USA), which has been previously shown to maintain an average concentration of plasma E2 between the nadir and peak levels of a normal estrous cycle.38
SPS experiment and incisional surgery
In the SPS experiment, female rats were restrained for 2 hrs inside a plastic animal holder, and then they were placed in a plastic cylinder (30 cm in diameter and 50 cm tall), which was filled with water (25°C) to two-thirds of its height. The rats were then forced to swim individually for 20 mins on day 7 after estrogen replacement.39 Afterward, the rats were allowed to rest for 15 mins and then exposed to ether until they lost consciousness.40 A sham group of SPS rats was placed in another room without any further treatment.
On day 8 after estrogen replacement, incisional surgery was performed under sterile conditions following the procedure described by Brennan et al.41 Briefly, ovariectomized rats were first anesthetized with sevoflurane by a nose mask. After disinfection of the skin, a 1-cm longitudinal incision was made through the skin and fascia on the right hind paw, starting 0.5 cm from the edge of the heel and extending toward the toes. The plantaris muscle was elevated by forceps, leaving the muscle origin and insertion intact. Finally, the skin was closed with two mattress sutures using 5–0 nylon and the wound site was treated with aureomycin ointment.
Nociceptive behavioral tests
The paw withdrawal mechanical threshold (PWMT) was measured on day 6 (1day prior to SPS, baseline), as well as on days 8, 9, and 10 (2, 6, 24, and 48 hrs after incisional surgery, respectively). The rats were placed in plastic boxes with a wire mesh bottom and allowed to acclimate for 30 mins. PWMT was conducted by the “up-and-down” method using a set of von Frey filaments. Each von Frey filament was applied vertically with sufficient force to the plantar surface of the right hind paw, adjacent to the wound, for 6–8 s. Positive responses were defined by paw flinching or briskly withdrawing. The data were recorded and analyzed using the method described by Chaplan.42
RVM microinjection procedure and intrathecal injection
In order to investigate the effects of drugs injected into RVM in animal, a microinjection system was constructed by the method described by Mitchell et al.43 Female rats were mounted on a stereotaxic frame under anesthesia with 10% chloral hydrate. After locating the bregma, a stainless-steel guide cannula was inserted unilaterally into the RVM and fixed to the skull using dental zinc cement. The stereotaxic coordinates for the RVM were −11.0 mm posterior to the bregma, ±0.6 mm lateral to the midline, and −7.5 mm ventral to the skull surface, according to the atlas of Paxinos and Watson (1986). A stainless steel dummy cannula was inserted into the guide cannula and remained in place when the guide cannula was not in use. After removal from the stereotaxic apparatus, the rats were placed in a thermally controlled cage to avoid hypothermia until they completely recovered from anesthesia. The animals were allowed to recover from the implantation surgery for 5 days prior to undergoing microinjection, and they were monitored for signs of motor impairment. Rats with any neurological dysfunction caused by the implantation procedure were excluded from subsequent experiments. The injection cannula was connected to a 5 µL Hamilton microsyringe. A total volume of 0.5 µL was injected over 2 mins. To ensure complete drug diffusion, the injector was left in place for an additional 2 mins before it was slowly removed.
Intrathecal injections were administered once a day from day 6 to day 8 after estrogen replacement. The procedures of intrathecal administrations were described as previously described.44 Following an optimal flexion of the rat lumbar spine under prone position, a 27-gauge needle attached to a 50 μL syringe (Hamilton, Reno, Nevada) was inserted into the midline of the lumbar 4–5 (L4-L5) intervertebral space of the rat.45 Neurobehavioral assessments were conducted after intrathecal administrations.
Drug preparation and experimental design
For estrogen replacement, we used E2 diluted in sesame seed oil. Experimental drugs administered via RVM microinjection included a CCK2 receptor agonist (CCK-8, Sigma) and a CCK2 receptor antagonist (YM022; Tocris, Bristol, UK), both of which were dissolved in saline. Experimental drugs administered intrathecally included a 5-HT2B receptor agonist (BW723C86; Tocris), a 5-HT2B receptor antagonist (RS127445; Tocris), and a PKCγ translocation inhibitor peptide (C37H65N9O13; Sigma). BW723C86 and C37H65N9O13 were dissolved in artificial cerebrospinal fluid (aCSF; in mmol/L: 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 1.2 CaCl2, 1.2 MgSO4, 10 D-(±) glucose; pH 7.4) and RS127445 was dissolved in 1% dimethyl sulfoxide (DMSO). The dosages of E2 and other drugs were adopted according to our preliminary experiments and previous studies.30,46 There is no influence of 1% DMSO on the function of neurons in the superficial spinal dorsal horn.47 The schematic representation of the experimental design and time schedule of the protocol are shown in Figure 1.
Experiment 1
The rats were randomly divided into four groups (n=6 per group): Group C underwent sham SPS and sham incisional surgery; Group I underwent incisional surgery, but not SPS; Group S underwent SPS, but not incisional surgery; and Group S+I underwent SPS on day 7 and incisional surgery on day 8 after estrogen replacement. The PWMT was measured on days 6, 8, 9, and 10. After the last behavioral test, the rats were sacrificed, and specimens of the RVM and spinal dorsal horn were collected for either immunofluorescence or Western blot analysis.
Experiment 2
The rats were randomly divided into seven groups (n=6 per group). All RVM microinjections were administered once a day from day 6 to day 8 after estrogen replacement. Group C underwent sham SPS and sham incisional surgery; Group I underwent incisional surgery, but not SPS; Group CCK-8 received an RVM microinjection of CCK-8 (20 pmol, 0.5 μL) and Group YM022 received an RVM microinjection of YM022 (2 pmol, 0.5 μL), but neither group underwent SPS or incisional surgery; Group CCK-8+I received microinjection of CCK-8 (20 pmol, 0.5 μL) and underwent incisional surgery, but not SPS; Group YM022+I received microinjection of YM022 (2 pmol, 0.5 μL) and underwent both SPS and incisional surgery; Group CCK-8+S received an RVM microinjection of CCK-8 (20 pmol, 0.5 μL) and underwent SPS without incisional surgery; Group Vehicle received an RVM microinjection of saline (0.5 μL) without SPS and incisional surgery. The PWMT was measured on days 6, 8, 9, and 10. After the last behavioral test, the rats were sacrificed, and specimens of the RVM were collected for either immunofluorescence or Western blot analysis.
Experiment 3
The rats were randomly divided into 10 groups (n=6 per group). All intrathecal injections were administered once a day from day 6 to day 8 after estrogen replacement, Group C underwent sham SPS and sham incisional surgery; Group I underwent incisional surgery, but not SPS; Group BW723C86 received intrathecal injections of BW723C86 (4 μM, 20 μL), Group RS127445 received intrathecal injections of RS127445 (10 μM, 20 μL), and Group C37H65N9O13 received intrathecal injections of C37H65N9O13 (50 μM, 20 μL); however, none of these 3 groups underwent SPS or incisional surgery; Group BW723C86+I received intrathecal injections of BW723C86 (4 μM, 20 μL) and underwent incisional surgery, but not SPS; Group RS127445+I received intrathecal injections of RS127445 (10 μM, 20 μL) and Group C37H65N9O13+I received intrathecal injections of C37H65N9O13 (50 μM, 20 μL), and both groups underwent SPS and incisional surgery; Group Vehicle1 received intrathecal injections of a CSF (20 μL) and Group Vehicle2 received intrathecal injections of 1% DMSO (20 μL), and both groups did not undergo SPS and incisional surgery. The PWMT was measured on days 6, 8, 9, and 10. After the last behavioral test, the rats were sacrificed, and specimens of the spinal dorsal horn were collected for either immunofluorescence or Western blot analysis.
Immunofluorescence
On day 10 after estrogen replacement, the rats were deeply anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg) and perfused with 200 mL normal saline followed by 500 mL 0.1 M phosphate buffer containing 4% paraformaldehyde (pH =7.4). The brain and lumbosacral section of the spinal cord were quickly resected and placed in a fixative solution overnight. The RVM and spinal cord specimens were cut into sections. After washing with PBS, the sections were blocked with 10% FBS at room temperature for 1 hr. The sections were then incubated at 4°C for 48 hrs with the following primary antibodies: goat polyclonal antibodies against the CCK2 receptor (Abcam, Cambridge, UK, 1:200), goat polyclonal antibodies against the 5-HT2B receptor (Y-15; Santa Cruz Biotechnology, Dallas, TX, USA, 1:250), rabbit polyclonal antibodies against the PKCγ receptor (C-19; Santa Cruz Biotechnology, 1:250), and mouse monoclonal antibody against the NMDA receptor subunit NR1 (Millipore, Billerica, MA, USA, 1:1,000). After washing in PBS, sections were incubated with secondary fluorescent antibodies at room temperature for 1 hr. The RVM samples were incubated with NL 557-conjugated donkey anti-goat antibodies (R&D Systems, Minneapolis, MN, USA, 1:1,500). The spinal cord samples were incubated with secondary antibodies, including NL 557-conjugated donkey anti-goat, NL 493-conjugated donkey anti-rabbit (R&D Systems, 1:1,500), and Alexa Fluor 405-conjugated donkey anti-mouse (Abcam, 1:2,000) antibodies. Tissue sections were then washed, mounted on glass slides, and covered with coverslips. The specimens were examined by use of NA0.45×20 and NA0.75×20 objective with a confocal microscope (LSM710, ZEISS, Stuttgart, Germany). Thickness of obtained confocal sections was 10×5μm and 20×2μm. The images were captured and postprocessing was measured by ZEN2.3 software (ZEISS).
Western blot analysis
After being deeply anesthetized with sevoflurane, the rats were euthanized. We took the brain tissue and put it in liquid nitrogen. After that, the brain was placed into the brain matrices and sliced with a 0.5 mm thickness, and then the RVM region of the brain tissue was taken out according to the atlas. The spinal dorsal horn segments (right dorsal part of L4–L5) were removed and stored in liquid nitrogen. The collected tissue samples were homogenized in a lysis buffer, the homogenate was centrifuged at 13,000 rpm for 10 mins at 4°C, and the supernatant was removed. Protein samples were separated by SDS-PAGE and then transferred onto a polyvinylidene difluoride membrane (Millipore). The filter membranes were blocked with 5% skim milk for 1 hr at room temperature and incubated overnight at 4°C with primary antibodies against the following antigens: CCK2 receptor (Abcam, 1:500), 5-HT2B receptor (Santa Cruz Biotechnology, 1:500), PKCγ (Santa Cruz Biotechnology, 1:1,000), and the antibody against p-NR1 subunit Ser-896, anti-NMDAR1 (phospho S896), purchased from Abcam (ab75680, 1:2,000). The membranes were then washed and incubated with secondary antibody for 1 hr at room temperature. β-actin was used as a loading control for total protein. Protein band visualization was achieved using the enhanced chemiluminescence method, and protein quantification was performed with Image-Pro Plus software.
Primary spinal cord dorsal horn neurons culture
Primary cultures of spinal cord superficial dorsal horn neurons from 14-day-old pregnant fetal rats were prepared following a previously described method.48 This results in cultures composed primarily of neurons from laminae II, although the presence of lamina III neurons cannot be entirely ruled out. The tissue fragments were incubated at 37°C for 30 mins in a medium containing Hank’s balanced salt solution (HBSS) and papain (15 U/mL; Worthington Biochemical, Lakewood, NJ, USA), followed by a series of rinses, first with HBSS and then with a culture medium containing Neurobasal medium (Gibco, Grand Island, NE, USA), fetal calf serum (5%), heat-inactivated horse serum (5%), l-glutamax-1 (2 mM), B-27 (2%, Gibco), and glucose (20 mM). The resulting cell suspension was plated onto 12 mm poly-d-lysine- and collagen-coated coverslips and was cultured for 3–5 days in humidified air with 5% CO2 at 37°C.
Electrophysiological analysis
The electrophysiological recordings were performed on cultured cells of the spinal cord.33,34 In our study, spinal cord superficial dorsal horn was dissected with a surgical blade cut in approximately lamina II. Although we isolated only the most superficial portion of the dorsal horn, the cultures may contain some cells from deeper laminae. In superficial dorsal horn neurons, the whole-cell Ca2+ channel currents (IBa) were elicited by a 100-ms stepping voltage from a holding potential of −60 to 0 mV. Whole-cell patch-clamp electrophysiology was applied to measure the voltage-gated Ca2+ currents at room temperature (22–24°C) with a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA). The Pclamp 10.2 program was used to extract and analyze all collected data. Coverslips were placed in a small laminar flow perfusion chamber and were continuously perfused with an extracellular solution. Signals were filtered at 1 kHz by a low-pass Bessel filter and digitized at 5 kHz. Series resistance and capacitance readings were collected directly from the amplifier after electronically subtracting the effects of capacitive transients. Series resistance was compensated to the maximal extent (at least 75%). Current traces were corrected using online P/6 trace subtraction. Currents were measured at their peak amplitude within the 200-ms test pulse and were used to determine the percentage of current decrease.
Statistical analysis
Data were expressed as mean ± SD. Pain behaviors were analyzed by two-way repeated measures ANOVA. Bonferroni post-hoc tests were conducted to determine the source(s) of differences when significant main effects were observed. Kruskal–Wallis test was used for comparisons of the nonparametric data regarding Western blot. Treatment effects reflected by electric currents from the electrophysiological analysis were statistically analyzed by a paired or independent Student’s t-test. All statistical analyses were performed using SPSS 17.0 software (SPSS Inc, Chicago, IL, USA), and P≤0.05 was set as the level of statistical significance.
Results
Preoperative SPS-induced allodynia following incisional surgery in female rats
Group S, Group I, and Group S+I showed a decrease in the PWMT from 2 hrs to 48 hrs when compared with Group C (Figure 2). Further, PWMT nociceptive threshold levels decreased more in Group S+I than in Group S and Group I from 2 hrs to 48 hrs after incisional surgery. The same group did not induce significant changes in the PWMT among different times.
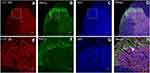
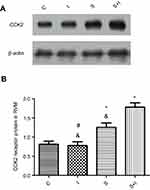
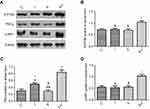
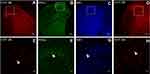
Neuronal receptors were observed under the confocal microscope. The CCK2 receptor was observed in the RVM neurons in Group S+I (Figure 3), and the triple immunofluorescence stained images of Group S+I showed the co-expression of the 5-HT2B receptor, NR1, and PKCγ in the superficial laminae of the spinal dorsal horn (Figure 4). Western blot analysis demonstrated that the expression of the CCK2 receptor in the RVM, as well as the 5-HT2B receptor, PKCγ, and p-NR1 in the spinal dorsal horn were significantly higher in Group S+I than in other groups (Figure 5 and Figure 6).
Upregulation of the CCK2 receptor in the RVM is associated with SPS-enhanced postoperative hyperalgesia in female rats
To explore whether the upregulation of the CCK2 receptor in RVM neurons contributed to SPS-enhanced hyperalgesia in female rats, a selective CCK2 receptor agonist (CCK-8) and antagonist (YM022) were administered into the RVM (Figure 7). A decreased tolerance to nociceptive stimulus was observed in Group CCK-8 compared with Group C from 2 hrs to 48 hrs. Furthermore, Group CCK-8+I rats were relatively more sensitive to the same stimulus. No significant changes were found in the PWMT between Group YM022+I and Group I, and the nociceptive threshold of Group YM022+I was relatively lower than that of Group C from 2 hrs to 48 hrs. No significant differences were observed in the PWMT between Group Vehicle and Group C. The treatment of the same group did not induce significant changes in the PWMT among different times from 2 hrs to 48 hrs.
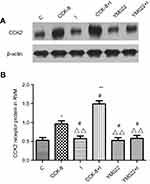
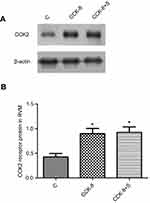
Western blot analysis showed that microinjection of CCK-8 into the RVM significantly upregulated the expression of the CCK2 receptor in Group CCK-8 and Group CCK-8+I relative to Group C. Further, Group CCK-8+I showed greater upregulation of the expression of the CCK2 receptor than Group CCK-8. Conversely, RVM microinjection of YM022 markedly decreased the expression of the CCK2 receptor in the RVM (Figure 8). Microinjection of CCK-8 combined with SPS showed a higher intensity of CCK2 expression than Group C, and no significant changes were found in the expression of CCK2 compared with Group CCK-8 (Figure 9). Additionally, the CCK2 receptor was observed in immunofluorescence stained images of the RVM in Group CCK-8+I and Group YM022+I (Figure 3). These findings suggest that expression of the CCK2 receptor in the RVM is important for preoperative anxiety to mediate postoperative nociceptive stimulation in female rats.
SPS-enhanced postoperative allodynia triggered 5-HT2B receptor-mediated PKCγ upregulation and NR1 phosphorylation in dorsal horn neurons
To examine the role of the signaling pathway in the spinal cord dorsal horn in SPS-enhanced postoperative hyperalgesia, a selective 5-HT2B receptor agonist and antagonist, and a PKCγ translocation inhibitor peptide were administered by intrathecal injection (Figure 10). No significant difference in the PWMT was found between the sham and experimental groups prior to incisional surgery. The nociceptive threshold in Group BW723C86+I was lower than in Group RS127445+I and Group C37H65N9O13+I from 2 hrs to 48 hrs. No significant changes were found in the PWMT between Group RS127445+I and Group C37H65N9O13+I, and the nociceptive thresholds of these two groups were relatively lower than that of Group C from 2 hrs to 48 hrs. In addition, intrathecal injection without incisional surgery did not induce significant allodynia in Group BW723C86, Group RS127445, or Group C37H65N9O13 compared with the control group from 2 hrs to 48 hrs. No significant differences in the PWMT were observed in Group Vehicle1 or Group Vehicle2 compared with Group C. The treatment of the same group did not induce significant changes in the PWMT among different times from 2 hrs to 48 hrs.
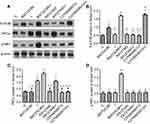
Results from the Western blot analysis showed that intrathecal injection of BW723C86 significantly upregulated the expression of the 5-HT2B receptor, PKCγ, and p-NR1 in the dorsal horn after incisional surgery. Group BW723C86 and Group C37H65N9O13+I showed a higher intensity of 5-HT2B receptor expression than did the control group. Additionally, the expression level of PKCγ in Group I and Group RS127445+I was both distinctly higher than in Group C (Figure 11). The specificity of the primary antibodies was tested in the Western blot studies. For instance, the control group samples showed that the antibody of PKCγ was positive only in the molecular weight range corresponding to PKCγ, which proved the specificity of the antibody (Figure 12). Immunofluorescence stained image of Group BW723C86+I showed the 5-HT2B receptor in dorsal horn neurons. Furthermore, the expression of PKCγ, 5-HT2B receptor, and NR1 in dorsal horn was observed in Group RS127445+I (Figure 13).
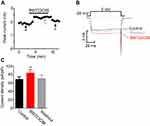
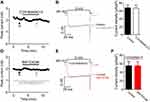
The electrophysiological recordings showed that adding BW723C86 to the bath significantly increased the peak IBa level by 18.65% over the basal level (n=7; Figure 14A, B). After removing BW723C86, the IBa amplitude partially recovered within 5 mins (Figure 14B, C). However, C37H65N9O13 did not have any significant effect on the IBa in superficial dorsal horn neurons (n=9; Figure 15A-C). Pretreatment with C37H65N9O13 completely abolished the BW723C86-induced IBa response (n=6; Figure 15D-F). This phenomenon suggests that the activation of the 5-HT2B receptor increases the IBa through the PKCγ pathway in superficial dorsal horn neurons.
Discussion
The present study showed that preoperative anxiety may induce postoperative hyperalgesia in female rats. Female rats that received an RVM microinjection of CCK-8 and underwent incisional surgery were relatively more sensitive to pain, and microinjection of YM022 into RVM relieved postoperative hyperalgesia. Furthermore, female rats treated with the intrathecal BW723C86 exhibited hyperalgesia until 48 hrs after incisional surgery, whereas the hyperalgesia quickly resolved after incisional surgery in rats that received an intrathecal injection of RS127445 or C37H65N9O13. These findings suggest that activation of CCK2 receptor in the RVM and spinal 5-HT2B receptor contributes to the hyperalgesia in female rats.
Preoperative anxiety among surgical patients is associated with acute postoperative pain.49 Although laboratory evidences have demonstrated the correlation between preoperative anxiety and postoperative pain in males,14,15 few studies were conducted to investigate this association and the underlying mechanism in females. In the present study, ovariectomized rats treated with estrogen replacement that underwent preoperative SPS conditioning and incisional surgery exhibited postoperative allodynia. The mechanical allodynia developed from 2 hrs to 48 hrs after the incision. Recently, several studies found that rats exposed to SPS exhibited long-lasting allodynia and hyperalgesia, and this phenomenon is considered to be associated with the activation of related receptor within the central nervous system neurons.50
The RVM can facilitate nociception by activating pronociceptive ON-cells. Furthermore, blocking the activation of ON-cells reverses hyperalgesia following inflammation and nerve injury and prevents behavioral hyperalgesia after acute injury.51 The RVM has been shown to be critical for some forms of stress-induced hyperalgesia by activating pain-facilitating ON-cells.21 As the pro-nociceptive and anxiogenic properties, CCK is found in pain-related regions of the central nervous system, including the RVM.52 Local application of CCK into the RVM can activate the firing of ON-cells, which in turn produces behavioral allodynia and hyperalgesia via the CCK2 receptor;24 this effect can be attenuated by administering CCK2 antagonists.53 It is well known that anxiety can lead to the exacerbation of existing pain and reduce the efficacy of analgesic drugs in humans.54 Similarly, non-nociceptive stressful stimulation can induce hyperalgesia in animals.55 Previous studies have demonstrated the involvement of central cholecystokininergic systems and the CCK2 receptor in these phenomena. Moreover, intravenous administration of CCK receptor agonists produces panic-like anxiety in humans, which can be prevented by pretreatment with a CCK2 receptor antagonist.56 Additionally, the administration of CCK2 agonists that cross the blood–brain barrier causes anxiogenic behavior in rats.57 Thus, it appears that CCK is highly relevant in the mechanism linking anxiety with hyperalgesia, and CCK receptor antagonists are effective in reducing this hyperalgesia.25 However, most of these findings regarding anxiety-induced hyperalgesia have come from experiments in male animals.
In women, the luteal phase of the ovarian cycle is associated with changes in mood and anxiety levels, which raises the possibility that CCK may be involved in anxiety-induced hyperalgesia in females.23 In this study, rats in Group CCK-8+I were relatively more sensitive to pain than rats in Group C. The results of Western blot analysis revealed that the preoperative stress without the incisional surgery upregulated the expression of the CCK2 receptor protein within the RVM, and the expression further increased when the preoperative stress was followed by the incisional surgery. The reason for this phenomenon might be the sole preoperative stress-activated CCK2 receptor in female rats, and we speculate that plantar incision after preoperative stress might act as a positive feedback further activated CCK2 receptor within the RVM in this hyperalgesic effect. Additionally, the expression of the CCK2 receptor was significantly increased in Group CCK-8 and Group CCK-8+I as well as markedly decreased in Group YM022+I. Previous studies suggest that CCK facilitates hyperalgesia and allodynia via the CCK2 receptor, and this effect can be reduced by administering CCK2 antagonists.53 We found CCK-8 microinjection into the RVM elevated the CCK2 receptor protein, and the expression of the CCK2 receptor further increased when CCK-8 microinjection combined with incisional surgery. Of course, further studies are still needed to confirm this hypothesis. No significant difference in the intensity of CCK2 expression was found between Group CCK-8+S and Group CCK-8. Based on this finding, there is the possibility that the CCK2 receptor of RVM neurons increased to a certain extent after CCK-8 microinjection and did not increase further when CCK-8 microinjection combined with preoperative stress, which may be related to the different intensities of stimulation. Together, these results suggest that the CCK2 receptor in the RVM neurons is important for preoperative anxiety to mediate postoperative hyperalgesia in female rats. These findings support the likelihood that CCK increases the excitability of ON-cells that mediate descending pain facilitation through the CCK2 receptor in the RVM.
As a key brainstem region for controlling the response to noxious stimuli, the RVM is a major source of serotonergic input to the spinal cord.58 Exposure to noxious stimuli has been shown to activate serotonergic neurons in the RVM and to accelerate the turnover of 5-HT in the spinal cord.59 Furthermore, the CCK-induced increase in the spinal level of 5-hydroxyindoleacetic acid can be blocked by pretreatment of the RVM with a CCK2 antagonist.26 The downstream serotonergic projection system from the RVM to the spinal cord has a dual regulating effect on pain responses. The role of 5-HT in the inhibition or facilitation of pain responses is associated with the site (peripheral or central) and the 5-HT receptor subtype.60 Several subtypes of 5-HT receptors are associated with selective excitatory or inhibitory functions in neurons.17 Thus, it is likely that a certain subtype of the 5-HT receptor in the spinal cord facilitates preoperative anxiety-induced postoperative hyperalgesia in female rats.
A previous study reported that the 5-HT2B receptor in dorsal horn neurons is critically involved in thermal and mechanical allodynia.30 The 5-HT2B receptor utilizes a Gq-mediated signaling cascade, leading to activation of a signal transduction pathway that utilizes phospholipase C and diacyl glycerol/inositol trisphosphate.61 Diacyl glycerol acts as a second messenger that activates PKC, and inositol trisphosphate helps in the phosphorylation of some proteins. Upon stimulation, the bioactive PKC translocates from the cytoplasm to the cell membrane and phosphorylates corresponding receptors or ion channels, further regulating the permeability of the membrane.62,63 PKCγ is specific to interneurons in the inner part of lamina II33 and is widely involved in pain signal processing, as well as the formation, development, and maintenance of spinal neuron sensitization.34,35 Translocation of PKC to the cell membrane is accompanied by increased phosphorylation of the NR1 subunit Ser-896 of the NMDA receptor in the dorsal horn, which can enhance NMDA receptor ion channel function.64 The phosphorylation of NR1 in spinal cord neurons can increase neurons’ sensitivity to pain stimulation and promote pain sensitization.36 The Ca2+ influx via calcium channels further regulates pacemaking activity, action potential burst firing, and pain transmission. Activated PKCγ, adhered onto or inserted into the cell membrane, promotes phosphorylation of NR1, which allows ion channels to remove the Mg2+ blockade and cause an influx of Ca2+.30 This influx of Ca2+ further activates intracellular calcium-dependent PKCγ. After the newly activated PKCγ translocates from the cytoplasm to the cell membrane, an increased number of phosphorylated NR1 receptors are activated and the influx of Ca2+ increases. This positive feedback loop ultimately leads to dorsal horn neuron hyperexcitability to noxious input and pain. The rats which received an intrathecal injection of RS127445 or C37H65N9O13 quickly resolved hyperalgesia after incisional surgery. We provided direct evidence that adding BW723C86 caused a significant increase in voltage-gated whole-cell Ca2+currents in superficial dorsal horn neurons. However, pretreatment with C37H65N9O13 completely abolished the BW723C86-induced IBa response. These observations suggest that preoperative anxiety-induced postoperative hyperalgesia in female rats occurs through the 5-HT2B receptor signaling pathway in spinal cord neurons.
Conclusion
Together, the results of this study support the hypothesis that preoperative anxiety may induce postoperative hyperalgesia in female rats. During this process, increased expression of the CCK2 receptor likely enhances the excitability of neurons in the RVM, and 5-HT2B receptor signaling pathway in spinal cord neurons enhances the neurons’ sensitivity to noxious stimuli.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grand number 81500955, 81771142, 81371207, 81471129, 81400914, 81671087), Jiangsu Planned Projects for Postdoctoral Research Funds, China (grand number 1701022B), Nanjing Medical Science and Technique Development Foundation (grand number QRX17053, QRX17138) and the Fundamental Research Funds for the Central Universities (grand number 021414380074).
Author contributions
MJ, ZM, and XG designed the studies. JB and YL performed the animal behavioral tests. FH and ZX performed the molecular biological test. YL and CL performed data collection and statistical analysis. YS, BH, and KN interpreted the results and drafted the manuscript. All the authors discussed the results and revised the manuscript critically for important intellectual content. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Perks A, Chakravarti S, Manninen P. Preoperative anxiety in neurosurgical patients. J Neurosurg Anesthesiol. 2009;21(2):127–130. doi:10.1097/ANA.0b013e31819a6ca3
2. Frazier SK, Moser DK, Daley LK, et al. Critical care nurses’ beliefs about and reported management of anxiety. Am J Crit Care. 2003;12(1):19–27.
3. Foggitt PS. Anxiety in cataract surgery: pilot study. J Cataract Refract Surg. 2001;27(10):1651–1655. doi:10.1016/S0886-3350(01)00859-8
4. Bailey L. Strategies for decreasing patient anxiety in the perioperative setting. Aorn J. 2010;92(4):445–457. doi:10.1016/j.aorn.2010.04.017
5. Pokharel K, Bhattarai B, Tripathi M, Khatiwada S, Subedi A. Nepalese patients’ anxiety and concerns before surgery. J Clin Anesth. 2011;23(5):372–378. doi:10.1016/j.jclinane.2010.12.011
6. Ali A, Altun D, Oguz BH, Ilhan M, Demircan F, Koltka K. The effect of preoperative anxiety on postoperative analgesia and anesthesia recovery in patients undergoing laparascopic cholecystectomy. J Anesth. 2014;28(2):222–227. doi:10.1007/s00540-013-1712-7
7. Raichle KA, Osborne TL, Jensen MP, Ehde DM, Smith DG, Robinson LR. Preoperative state anxiety, acute postoperative pain, and analgesic use in persons undergoing lower limb amputation. Clin J Pain. 2015;31(8):699–706. doi:10.1097/AJP.0000000000000150
8. Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111(3):657–677. doi:10.1097/ALN.0b013e3181aae87a
9. Wood PB. Stress and dopamine: implications for the pathophysiology of chronic widespread pain. Med Hypotheses. 2004;62(3):420–424. doi:10.1016/j.mehy.2003.10.013
10. Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192.
11. Qi J, Chen C, Lu YC, et al. Activation of extracellular signal-regulated kinase1/2 in the medial prefrontal cortex contributes to stress-induced hyperalgesia. Mol Neurobiol. 2014;50(3):1013–1023. doi:10.1007/s12035-014-8707-8
12. Manson JE. Pain: sex differences and implications for treatment. Metabolism. 2010;59(Suppl 1):S16–S20. doi:10.1016/j.metabol.2010.07.013
13. McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. 2014;35(1):42–57. doi:10.1016/j.yfrne.2013.09.001
14. Andre J, Zeau B, Pohl M, Cesselin F, Benoliel JJ, Becker C. Involvement of cholecystokininergic systems in anxiety-induced hyperalgesia in male rats: behavioral and biochemical studies. J Neurosci. 2005;25:7896–7904. doi:10.1523/JNEUROSCI.0743-05.2005
15. Quintero L, Cardenas R, Suarez-Roca H. Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain. 2011;152:1909–1922. doi:10.1016/j.pain.2011.04.017
16. Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5(12):1319–1326. doi:10.1038/nn966
17. Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474.
18. Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46(3):295–309. doi:10.1016/j.brainresrev.2004.07.004
19. Chen Q, Roeder Z, Li MH, Zhang Y, Ingram SL, Heinricher MM. Optogenetic evidence for a direct circuit linking nociceptive transmission through the parabrachial complex with pain-modulating neurons of the rostral ventromedial medulla (RVM). eNeuro. 2017;4(3):
20. Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142(3):236–244. doi:10.1016/j.pain.2009.01.011
21. Reynolds J, Bilsky EJ, Meng ID. Selective ablation of mu-opioid receptor expressing neurons in the rostral ventromedial medulla attenuates stress-induced mechanical hypersensitivity. Life Sci. 2011;89(9–10):313–319. doi:10.1016/j.lfs.2011.06.024
22. Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92(4):1982–1989. doi:10.1152/jn.00411.2004
23. Lovick TA. Pro-nociceptive action of cholecystokinin in the periaqueductal grey: a role in neuropathic and anxiety-induced hyperalgesic states. Neurosci Biobehav Rev. 2008;32(4):852–862. doi:10.1016/j.neubiorev.2008.01.003
24. Zhang W, Gardell S, Zhang D, et al. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain. 2009;132(Pt 3):778–787. doi:10.1093/brain/awn330
25. Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26(46):12014–12022. doi:10.1523/JNEUROSCI.2947-06.2006
26. Marshall TM, Herman DS, Largent-Milnes TM, et al. Activation of descending pain-facilitatory pathways from the rostral ventromedial medulla by cholecystokinin elicits release of prostaglandin-E₂ in the spinal cord. Pain. 2012;153(1):86–94. doi:10.1016/j.pain.2011.09.021
27. Wei F, Dubner R, Zou S, et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 2010;30(25):8624–8636. doi:10.1523/JNEUROSCI.5389-09.2010
28. Bowker RM, Westlund KN, Sullivan MC, Coulter JD. Organization of descending serotonergic projections to the spinal cord. Prog Brain Res. 1982;57:239–265. doi:10.1016/S0079-6123(08)64132-1
29. Viguier F, Michot B, Hamon M, Bourgoin S. Multiple roles of serotonin in pain control mechanisms–implications of 5-HT₇ and other 5-HT receptor types. Eur J Pharmacol. 2013;716(1–3):8–16. doi:10.1016/j.ejphar.2013.01.074
30. Aira Z, Buesa I, García Del Caño G, et al. Transient, 5-HT2B receptor-mediated facilitation in neuropathic pain: up-regulation of PKCγ and engagement of the NMDA receptor in dorsal horn neurons. Pain. 2013;154(9):1865–1877. doi:10.1016/j.pain.2013.06.009
31. Hori Y, Endo K, Takahashi T. Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. J Physiol. 1996;492(Pt 3):867–876. doi:10.1113/jphysiol.1996.sp021352
32. Li P, Kerchner GA, Sala C, et al. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nat Neurosci. 1999;2(11):972–977. doi:10.1038/14771
33. Hughes AS, Averill S, King VR, Molander C, Shortland PJ. Neurochemical characterization of neuronal populations expressing protein kinase C gamma isoform in the spinal cord and gracile nucleus of the rat. Neuroscience. 2008;153(2):507–517. doi:10.1016/j.neuroscience.2008.01.082
34. Xiaoping G, Xiaofang Z, Yaguo Z, Juan Z, Junhua W, Zhengliang M. Involvement of the spinal NMDA receptor/PKCgamma signaling pathway in the development of bone cancer pain. Brain Res. 2010;1335:83–90. doi:10.1016/j.brainres.2010.03.083
35. Xie HY, Xu F, Li Y, et al. Increases in PKC gamma expression in trigeminal spinal nucleus is associated with orofacial thermal hyperalgesia in streptozotocin-induced diabetic mice. J Chem Neuroanat. 2015;63:13–19. doi:10.1016/j.jchemneu.2014.12.001
36. Meng X, Zhang Y, Lao L, et al. Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain. 2013;154:294–305. doi:10.1016/j.pain.2012.10.022
37. Lam KK, Hu CT, Ou TY, Yen MH, Chen HI. Effects of oestrogen replacement on steady and pulsatile haemodynamics in ovariectomized rats. Br J Pharmacol. 2002;136(6):811–818. doi:10.1038/sj.bjp.0704762
38. Xu HL, Vetri F, Lee HK, et al. Estrogen replacement therapy in diabetic ovariectomized female rats potentiates postischemic leukocyte adhesion in cerebral venules via a RAGE-related process. Am J Physiol Heart Circ Physiol. 2009;297:H2059–H2067. doi:10.1152/ajpheart.00445.2009
39. Wang HN, Peng Y, Tan QR, et al. Quetiapine ameliorates anxiety-like behavior and cognitive impairments in stressed rats: implications for the treatment of posttraumatic stress disorder. Physiol Res. 2010;59:263–271.
40. Yamamoto S, Morinobu S, Takei S, et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26(12):1110–1117. doi:10.1002/da.20629
41. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501.
42. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63.
43. Mitchell JM, Lowe D, Fields HL. The contribution of the rostral ventromedial medulla to the antinociceptive effects of systemic morphine in restrained and unrestrained rats. Neuroscience. 1998;87(1):123–133.
44. Tzeng JI, Wang JN, Wang JJ, Chen YW, Hung CH. Intrathecal rimantadine induces motor, proprioceptive, and nociceptive blockades in rats. Neurosci Lett. 2016;618:94–98. doi:10.1016/j.neulet.2016.02.061
45. Sun Y, Zhang W, Liu Y, Liu X, Ma Z, Gu X. Intrathecal injection of JWH015 attenuates remifentanil-induced postoperative hyperalgesia by inhibiting activation of spinal glia in a rat model. Anesth Analg. 2014;118(4):841–853. doi:10.1213/ANE.0000000000000146
46. Tomim DH, Pontarolla FM, Bertolini JF, et al. The pronociceptive effect of paradoxical sleep deprivation in rats: evidence for a role of descending pain modulation mechanisms. Mol Neurobiol. 2016;53(3):1706–1717. doi:10.1007/s12035-014-9059-0
47. Jiang M, Zhang W, Ma Z, Gu X. Antinociception and prevention of hyperalgesia by intrathecal administration of Ro 25-6981, a highly selective antagonist of the 2B subunit of N-methyl-D-aspartate receptor. Pharmacol Biochem Behav. 2013;112:56–63. doi:10.1016/j.pbb.2013.09.007
48. Hu HJ, Glauner KS, Gereau R. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol. 2003;90:1671–1679. doi:10.1152/jn.00340.2003
49. Harden RN, Bruehl S, Stanos S, et al. Prospective examination of pain-related and psychological predictors of CRPS-like phenomena following total knee arthroplasty: a preliminary study. Pain. 2003;106:393–400.
50. Sun R, Zhang W, Bo J, et al. Spinal activation of alpha7-nicotinic acetylcholine receptor attenuates posttraumatic stress disorder-related chronic pain via suppression of glial activation. Neuroscience. 2017;344:243–254. doi:10.1016/j.neuroscience.2016.12.029
51. Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60(1):214–225. doi:10.1016/j.brainresrev.2008.12.009
52. Ghilardi JR, Allen CJ, Vigna SR, McVey DC, Mantyh PW. Trigeminal and dorsal root ganglion neurons express CCK receptor binding sites in the rat, rabbit, and monkey: possible site of opiate-CCK analgesic interactions. J Neurosci. 1992;12(12):4854–4866.
53. Xie JY, Herman DS, Stiller C-O, et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25(2):409–416. doi:10.1523/JNEUROSCI.4054-04.2005
54. Kain ZN, Sevarino F, Alexander GM, Pincus S, Mayes LC. Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeated-measures design. J Psychosom Res. 2000;49(6):417–422.
55. Jørum E. Analgesia or hyperalgesia following stress correlates with emotional behavior in rats. Pain. 1988;32(3):341–348.
56. Bradwejn J, Koszycki D, Couëtoux du Tertre A, van Megen H, den Boer J, Westenberg H. The panicogenic effects of cholecystokinin-tetrapeptide are antagonized by L-365,260, a central cholecystokinin receptor antagonist, in patients with panic disorder. Arch Gen Psychiatry. 1994;51(6):486–493.
57. Wang H, Wong PT, Spiess J, Zhu YZ. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci Biobehav Rev. 2005;29(8):1361–1373. doi:10.1016/j.neubiorev.2005.05.008
58. Okamoto K, Katagiri A, Rahman M, Thompson R, Bereiter DA. Inhibition of temporomandibular joint input to medullary dorsal horn neurons by 5HT3 receptor antagonist in female rats. Neuroscience. 2015;299:35–44. doi:10.1016/j.neuroscience.2015.04.037
59. Braz JM, Basbaum AI. Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits. J Comp Neurol. 2008;507(6):1990–2003. doi:10.1002/cne.v507:6
60. Lin SY, Chang WJ, Lin CS, Huang CY, Wang HF, Sun WH. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. J Neurosci. 2011;31(4):1410–1418. doi:10.1523/JNEUROSCI.4682-10.2011
61. Niebert M, Vogelgesang S, Koch UR, et al. Expression and function of serotonin 2A and 2B receptors in the mammalian respiratory network. PLoS One. 2011;6(7):e21395. doi:10.1371/journal.pone.0021395
62. Xin W, Chun W, Ling L, Wei W. Role of melatonin in the prevention of morphine-induced hyperalgesia and spinal glial activation in rats: protein kinase C pathway involved. Int J Neurosci. 2012;122:154–163. doi:10.3109/00207454.2011.635828
63. Roh DH, Choi SR, Yoon SY, et al. Spinal neuronal NOS activation mediates sigma-1 receptor-induced mechanical and thermal hypersensitivity in mice: involvement of PKC-dependent GluN1 phosphorylation. Br J Pharmacol. 2011;163:1707–1720. doi:10.1111/j.1476-5381.2011.01316.x
64. Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Res. 2004;1020:95–105. doi:10.1016/j.brainres.2004.06.017
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.