Back to Journals » Journal of Experimental Pharmacology » Volume 12
Antiviral Effects of Oleandrin
Authors Newman RA , Sastry KJ, Arav-Boger R, Cai H , Matos R , Harrod R
Received 4 September 2020
Accepted for publication 25 October 2020
Published 16 November 2020 Volume 2020:12 Pages 503—515
DOI https://doi.org/10.2147/JEP.S273120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Arthur E. Frankel
Robert A Newman, 1, 2 K Jagannadha Sastry, 3 Ravit Arav-Boger, 4 Hongyi Cai, 5 Rick Matos, 6 Robert Harrod 7
1Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77054, USA; 2Phoenix Biotechnology, Inc, San Antonio, TX 78217, USA; 3Departments of Thoracic, Head and Neck Medical Oncology and Veterinary Sciences, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; 4Division of Infectious Diseases, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI 53226, USA; 5National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA; 6Innovar, LLC, Plano, TX 75025, USA; 7Department of Biological Sciences, the Dedman College Center for Drug Discovery, Design & Delivery, Southern Methodist University, Dallas, TX 75275, USA
Correspondence: Robert A Newman Tel +1-7138578921
Email [email protected]
Abstract: Over the past 15 years, investigators have reported on the utility and safety of cardiac glycosides for numerous health benefits including those as treatments for malignant disease, stroke-mediated ischemic injury and certain neurodegenerative diseases. In addition to those, there is a growing body of evidence for novel antiviral effects of selected cardiac glycoside molecules. One unique cardiac glycoside, oleandrin derived from Nerium oleander, has been reported to have antiviral activity specifically against ‘enveloped’ viruses including HIV and HTLV-1. Importantly, a recent publication has presented in vitro evidence for oleandrin’s ability to inhibit production of infectious virus particles when used for treatment prior to, as well as after infection by SARS-CoV-2/COVID-19. This review will highlight the known in vitro antiviral effects of oleandrin as well as present previously unpublished effects of this novel cardiac glycoside against Ebola virus, Cytomegalovirus, and Herpes simplex viruses.
Keywords: oleandrin, Nerium oleander, virus, Na, K-ATPase, antiviral therapy
Background
The class of compounds referred to as cardiac glycosides suffer from an unfortunate moniker. While the benefits of compounds such as digitalis to treat congestive heart failure and cardiac arrhythmias have been well established, the pharmacologic effects of cardiac glycosides are now known to extend well beyond the ability to augment cardiac function via inhibition of Na,K-ATPase activity. In fact, it is only recently that scientists have learned that there are a series of endogenous cardiac glycoside compounds within mammals whose varied functions are only now becoming clear.1,2 In contrast, exogenously administered cardiac glycosides have been shown to be beneficial as targeted therapies against malignant disease,3–6 stroke and neurodegenerative diseases,7,8 and certain bacterial and fungal diseases.9 Less well appreciated is a growing list of antiviral activities of cardiac glycosides against both RNA and DNA enveloped viruses. Recent reviews of the antiviral effects of compounds that cause Na,K-ATPase inhibition, for example, cite activities of various cardiac glycosides against Cytomegalovirus, Herpes simplex virus I and II, adenovirus, Chikungunya virus, coronaviruses, respiratory syncytial virus, Ebola virus, Influenza virus, the human T-cell leukemia virus type-1 (HTLV-1) and the human immunodeficiency virus type-1 (HIV-1).10–12 Oleandrin, a unique lipid-soluble molecule derived exclusively from Nerium oleander (aka Nerium indicum, Common oleander, Adelfa, Kaner, Nerium odorum),13 has been shown to be the active principle ingredient in the botanical extract drug PBI-05204 which has been through both Phase I and Phase II trials in patients with malignant disease.14,15 These clinical studies demonstrated that oral administration of defined doses of oleandrin, as part of the extract, can be administered without serious adverse effects, suggesting its use for other diseases beyond cancer may be appropriate to consider.
Because of the recent SARS-CoV-2 (COVID-19) world-wide pandemic, attention has been drawn to the antiviral effects of cardiac glycosides and oleandrin as extracts containing this molecule may represent ‘re-purposing’ of a novel molecule. Published reports from in vitro cell culture studies have shown that oleandrin and extracts containing this molecule are effective against HIV, Ebola virus as well as the HTLV-1 and other viruses important to human health and wellbeing;16–19 however, it is the unique antiviral mechanisms of oleandrin that merits additional attention. The ability of oleandrin to inhibit the relative infectivity of progeny virus particles may offer a unique approach to treating certain viral diseases. The antiviral efficacy of oleandrin has now been shown against SARS-CoV-2,20 and, if proven safe and effective in appropriate animal models and then in human studies, oleandrin and extracts containing this molecule may offer a unique therapeutic approach to treat COVID-19-related disease. The reported specific antiviral effects and suggested mechanisms of antiviral activity of oleandrin, as well as extracts containing this singular molecule, will be presented.
The list of viruses reported being inhibited by oleandrin or extracts that contain this molecule continues to expand – with activities observed against both DNA as well as RNA viruses (Table 1). While these viruses may differ in the composition and organization of their nucleic acid genomes as well as in their mechanisms of replication, they are all considered to be ‘enveloped’ viruses with the exception of poliovirus. The viral envelope is a viral glycoprotein (usually arranged as spikes or peplomers) as well as a phospholipid bilayer which comes from the infected host cell during the egress of newly produced virus particles through a “budding” process. Through the release and exit of mature virus particles from an infected host cell during the budding process, newly formed virus particles become “enveloped” or wrapped in an outer coat that is made from components of the cell’s plasma membrane which also includes host proteins that were embedded in the lipid bilayer. As discussed in this review, the effect of oleandrin on the formation and perhaps subsequent functioning of the envelope appears to be essential, at least in part, to the activities of this molecule.
 |
Table 1 Antiviral Activity of Oleandrin and Extracts of N. oleander |
Antiviral Effects of Oleandrin and N. oleander Extracts Against RNA Viruses
HIV-1
In 2005 an article appeared in The Lancet by Jan Balzarini that offered a prescient hypothesis suggesting that targeting the glycans of envelope glycoproteins such as gp120 with carbohydrate-binding agents (CBA) might present a novel therapeutic approach against HIV-1.21 He reasoned that damaging the glycans of the HIV-1 viral envelope may provide an opportunity for enhanced immune response against the virus. While no CBA molecules have yet been developed for use as antiviral agents, the concept that alteration of the viral envelope making progeny virus particles less infective was considered novel.
Research with cardiac glycoside compounds such as digoxin demonstrated anti-HIV activity through multiple mechanisms – including the suppression of HIV-1 replication through an alteration of viral RNA processing.16 This was followed by a report of a cell-based high-throughput screen for inhibitors of HIV-1 gene expression and budding that identified the potential of cardiac glycoside compounds that prevent the production and release of HIV-1 RNA-containing virions.22 A subsequent article by Agostini et al,23 then suggested cardiac glycosides such as ouabain mediate reduced infectivity of HIV-1 through a viral Tat protein interaction with Na, K-ATPase – again suggesting that cardiac glycoside treatment of HIV-1 infected cells results in the reduced infectivity of progeny virions. By 2018 it had become clear that the TAT transactivator protein is an essential component of the viral particle and that loss of or defective formation of the envelope protein on released particles would correspond with a loss of infectivity.24
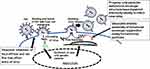
Singh et al advanced this research on the utility of cardiac glycosides as novel agents against HIV-1 by demonstrating the effectiveness of oleandrin and a Nerium oleander plant extract’s ability to inhibit HIV-1 infection.17 Unlike current widely used anti-HIV reagents such as AZT that limit virus production by inhibition of viral replication, treatment with oleandrin resulted in the production of virus progeny with a significantly impaired ability to infect target cells. This novel anti-HIV effect of oleandrin was observed against both T-cell-tropic and macrophage/monocyte- tropic strains of HIV-1. Studies to identify the active components in an N. oleander extract revealed that the CG-enriched fraction containing oleandrin was important for the observed anti-HIV effects. This study was the first to reveal the novel anti-HIV activity of oleandrin. Investigation into the underlying mechanism of reduced infectivity of the progeny virus without changes in the total amount of virus produced following treatment with oleandrin revealed a significant reduction in the concentration of the viral envelope protein glycoprotein gp120 -an essential event during the infection involving sequential binding of the viral envelope surface protein gp120 with host cell receptor CD4 and the chemokine co-receptor CXCR4 or CCR5.25 Studies have demonstrated that the reduced expression of gp120 – in the viral envelope surface component compromises HIV-1’s ability to infect target cells.26,27 The effectiveness in significantly reducing infectivity and involving reduced gp120 concentration of the progeny virus demonstrated in this study was specific to oleandrin because ouabain was found to be ineffective. The lack of efficacy of ouabain is particularly interesting because it shares substantial structural similarity with oleandrin; however, it is known that minor differences in the structures of cardiac glycoside compounds correlate with major effects on their pharmacologic activities.2 The current understanding of the antiviral activity of oleandrin against HIV as demonstrated in the study by Singh et al,17 and others are shown in Figure 1.
HTLV-1
The HTLV-1 is an enveloped delta oncoretrovirus, similar to HIV-1, and bloodborne pathogen that is endemic to tropical equatorial regions, particularly, Southeast Asia, Central and South America, certain islands of the Caribbean, Northern Africa, as well as the Middle East.28–31 It is estimated there are approximately 10–15 million HTLV-1-infected individuals worldwide and this virus is considered to be an emerging health threat.31–34 The HTLV-1 infects CD4+ T-lymphocytes, monocytes, and dendritic cells and causes adult T-cell leukemia/lymphoma (ATLL) –a rare, yet aggressive hematological malignancy with high rates of therapy-resistance correlated with generally poor clinical outcomes.35–39 The HTLV-1 also causes a progressive demyelinating neuroinflammatory disease, known as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP)-associated with an autoimmune response against virus replication and viral antigens in the central nervous system (CNS), which results in the demyelination of the lower spinal cord and possible paralysis or coma.40–47 HTLV-1-infections are also etiologically linked with other auto-inflammatory diseases, including infectious dermatitis, rheumatoid arthritis, uveitis, keratoconjunctivitis, sicca syndrome, and Sjögren’s syndrome.48–54 Based upon the demonstrated ability of oleandrin to inhibit the replication and infectivity of HIV-1 –a related human retrovirus,17 investigators examined whether oleandrin or a phytoextract from N. oleander could inhibit the infectivity and transmission of HTLV-1.
The findings reported in Hutchinson et al19 demonstrated that oleandrin or an N. oleander extract did not significantly inhibit the production and release of extracellular HTLV-1 particles into the culture supernatants of treated HTLV-1+ SLB1 cells, as determined by performing anti-HTLV-1 p19-Gag ELISAs. These studies further showed that low concentrations of oleandrin or the N. oleander phytoextract were not significantly cytotoxic and exhibited cellular apoptosis levels similar to the vehicle negative control in HTLV-1+ SLB1 T-cells. Importantly, however, the newly-synthesized virus particles collected from the supernatants of oleandrin (or N. oleander extract)-treated HTLV-1+ SLB1 cells were defective, and impaired in their ability to infect primary human peripheral blood mononuclear cells (huPBMCs).19 The lower concentrations of oleandrin were found to be minimally cytotoxic in huPBMCs as compared to the vehicle control, although the N. oleander extract resulted in higher cytotoxicity and cellular apoptosis likely attributable to other cytopathic chemical products present in the crude phytoextract.
As the transmission of HTLV-1 typically requires direct cell-to-cell contact and occurs across an intercellular junction, known as the virological synapse (VS), which forms between an HTLV-1-infected cell and an uninfected target cell19,55,56(Figure 2), oleandrin was examined for its ability to inhibit the direct intercellular transmission of HTLV-1. These studies demonstrated that both oleandrin and the N. oleander extract markedly inhibited the envelope-dependent intercellular transmission of HTLV-1 particles across the VS between mitomycin C-treated HTLV-1+ SLB1 T-lymphocytes co-cultured with primary huPBMCs. Moreover, to directly visualize and quantify the inhibitory effects of oleandrin and the N. oleander extract upon VS-formation and the infection of target huPBMCs, mitomycin C-treated HTLV-1+ SLB1 T-cells that were stably transduced with a lentiviral-green fluorescent protein (GFP) expression vector, were co-cultured with primary huPBMCs in vitro. The formation of VSs and the infection of huPBMCs were observed and quantified by performing immunofluorescence-confocal microscopy using an Anti-HTLV-1 gp21-Env primary antibody, with the visualization of GFP-positive/gp21-positive HTLV-1+ SLB1 T-cells and GFP-negative/gp21-positive huPBMCs. These data further confirmed the Anti-HTLV-1 p19-Gag ELISA results from previous co-culture experiments and demonstrated that oleandrin and the N. oleander phytoextract inhibited HTLV-1 infectivity and Env-dependent VS-formation.19
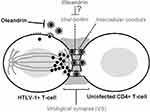 |
Figure 2 Oleandrin inhibits HTLV-1 infectivity and virological synapse formation. The figure illustrates the intercellular transmission of infectious HTLV-1 particles across the virological synapse (VS). The polarization of HTLV-1 p19-Gag core particles along cellular microtubules toward the intercellular junction is indicated. The intercellular conduits/nanotubules which facilitate the trafficking of virus particles between an HTLV-1-infected cell and an uninfected target CD4+ T-cell are shown. The viral glycan-rich biofilm surrounding the VS is also depicted. Oleandrin inhibits the incorporation of the viral glycoprotein into mature extracellular HTLV-1 particles budding from an infected cell and could also inhibit the synthesis of the viral biofilm to prevent VS-formation.19 |
As others have reported that oleandrin can readily cross the blood-brain barrier and penetrate the CNS in mouse xenograft models of malignant glioma and exhibited neuroprotective effects in vivo models of ischemic stroke,7,57,58 this molecule could potentially be used to inhibit HTLV-1 replication and viral gene expression in the CNS and may have therapeutic implications for the treatment of HAM/TSP as well as other HTLV-1-associated inflammatory diseases. Intriguingly, the formation of the VS between an HTLV-1-infected cell and an uninfected target cell involves the formation of a glycan-rich extracellular biofilm19,59 which likely protects the trafficking of infectious particles across the intercellular conduits of the VS.60,61 It is mechanistically possible, therefore, that oleandrin could inhibit the synthesis of the viral biofilm –in addition to blocking the incorporation of the HTLV-1 envelope glycoprotein into newly synthesized, mature virus particles (Figure 2). The aggregate findings of Hutchison et al,19 together with the observations of Singh et al,17 suggest that the botanical glycoside oleandrin has broad antiviral activity against enveloped viruses and inhibits viral maturation at the cell membrane by blocking the incorporation of the envelope glycoprotein into newly-synthesized particles. This represents a stage of the infection cycle that is not currently targeted by existing highly active antiretroviral therapy (HAART).
Filoviruses (Ebola and Marburg)
The members of this virus family, including the Ebola and Marburg viruses, are highly pathogenic, may be transmissible through aerosols, and cause severe hemorrhagic fevers; and are classified as Biosafety level-4 (BSL-4) infectious select agents. These filamentous viruses, of the order Mononegavirales, contain a single copy of a negative single-stranded RNA (-ssRNA) genome and the helical ribonucleoprotein core is surrounded by an envelope which is comprised of peplomers, or spikes, of the viral glycoprotein and the lipid bilayer form an infected cell. Filoviruses are endotheliotropic and infect vascular endothelial cells (ie, associated with the blood leakage and visceral liquefication characteristic of disease) as well as monocytes and macrophages which allow the virus infection to become systemically disseminated throughout the body. The indirect inhibition of calcium regulation by cardiac glycosides suggests that these drugs may also be effective against filoviruses, as calcium channel blockers have demonstrated efficacy against this family of viruses.62 Interestingly, calcium channel blockers have been demonstrated to mediate their antiviral effect by inhibiting new virus particles from budding.63 In studies from USAMRIID conducted in 2017 and presented at an international meeting, a defined extract (PBI-05204) derived from Nerium oleander as well as purified oleandrin were used to pretreat Vero cells prior to and post-infection with MARV and EBOV.18 An immunofluorescence-based assay was used to determine antiviral efficacy 48hr post-infection. For passaging experiments, Vero cells were infected in the presence of PBI-05204 or oleandrin and supernatants were collected 24hr or 48hr later. The supernatants were then assayed for the presence of infectious particles. An EBOV mini-genome was used to assess viral transcription in the presence of PBI-05204 or oleandrin.
The broad-spectrum efficacy observed against EBOV, MARV and certain alpha viruses may be especially critical as certain therapeutic elements within the PBI-05204 botanical drug can be found to accumulate in the CNS,18 which is essential for viruses that have demonstrated neuropathic effects. PBI-05204 and oleandrin fully inhibited MARV and EBOV infection in Vero cells. No infectious progeny virus was recovered from the supernatants of cells infected with EBOV or MARV when treated with PBI-05204 or oleandrin. Virus transcription was not inhibited by treatment with PBI-05204 or oleandrin, indicating the inhibition does appear to be linked to viral polymerase functions. Preliminary results also indicated that PBI-05204 and oleandrin also have antiviral efficacy against other enveloped viruses such as Western equine encephalitis virus (data not shown), demonstrating a broad antiviral profile.
Influenza Virus
There are four types of influenza viruses: A, B, C and D. Human influenza A and B viruses cause seasonal epidemics of disease (often referred to as the flu season). All of these are RNA viruses and make up four of the seven genera of the family Orthomyxoviridae. Influenza viruses are similar in overall structure; all contain an envelope wrapped around a central core of genetic material comprised of 8–9 segments of negative single-stranded RNA (-ssRNA). The envelope of the influenza virus is made up of trimers of the viral hemagglutinin (HA) protein and the viral neuraminidase (N) protein together with the lipid bilayer from an infected cell. Influenza viruses primarily infect respiratory epithelial cells and include some of the most well-known and pathogenic viruses known to cause serious illness and even death in humans. For example, influenza viruses include H1N1, which caused the Spanish flu pandemic in 1918 and the Swine Flu pandemic in 2009; H2N2, which caused Asian Flu in 1957; H3N2, which caused the Hong Kong Flu in 1968; and H5N1, which caused what was called Bird Flu in 2004.
In a study of plants from Saudi Arabia, Kiohara et al found that a methanolic extract contained an active compound identified as oleandrigenin-B-D-glucosyl (1-4)-B-D-digitalose which inhibited viral titer by 69.3% at a concentration of 1 ug/mL.64 Given the similarity of this compound to oleandrin and the fact that influenza viruses are enveloped it would be of interest to test the antiviral activity of oleandrin and extracts containing this molecule against these common influenza strains. More recently, several investigators observed that cardiac glycosides decrease influenza virus replication by inhibiting cell protein translation machinery.65,66
Poliovirus
The causative agent of polio (a neuromuscular disease also known as poliomyelitis), is a serotype of the species Enterovirus C, in the family of Picornaviridae. Poliovirus is composed of an RNA genome and a protein capsid. The genome is composed of positive single-stranded RNA (+ssRNA) genome and an icosahedral protein capsid and these viruses characteristically lack an envelope. Because of widespread vaccination efforts by the WHO, the poliovirus was eliminated from the Western Hemisphere in 1994.67 However, it continues to be present in developing countries like Afghanistan, Pakistan, and Nigeria, with occasional migratory spread to neighboring countries. While vaccination against polio continues to be an effective medical control strategy, a report by Sanna et al present data showing that both hot as well as cold extracts of N. oleander inhibited proliferation of poliovirus type-1 (Sb-1) in vitro. They observed that inhibition of the poliovirus was not due to direct inactivation of virions, but rather could be attributed to an interference by the extracts within a step along the viral life cycle.68
Chikungunya Virus
Chikungunya virus (CHIKV), is a member of the genus Alphavirus, and family Togaviridae that is transmitted by mosquitoes and is responsible for vector-borne epidemics around the world, particularly, in tropical equatorial regions including certain islands of the Caribbean. It was first isolated in 1953 in Tanzania has a positive single-stranded RNA (+ssRNA) genome of about 11.6kb. Chikungunya virus infections can cause debilitating muscular-joint diseases and it is considered an arthralgia-inducing pathogen. In the United States, it is classified as a category B priority pathogen, and work with this agent requires Biosafety level-3 (BSL-3) precautions. As of 2016, there was no vaccine or specific treatment available for the sometimes-devastating effects of this viral infection.69 Like the other viruses highlighted in this review, Chikungunya virus particles contain structural proteins that include the capsid and two envelope glycoproteins, E1 and E2, which form heterodimeric spikes on the virion surface.
A carefully performed series of experiments by Ashbrook et al found digoxin to be effective at inhibiting CHIKV although they state that this cardiac glycoside also inhibits infection of a number of plus-strand, minus-strand and double-stranded RNA viruses as well.70 Infection is inhibited by digoxin at post-viral entry steps and the authors determined that inhibition of the Na,K-ATPase was an essential part of viral inhibition. Further investigation of the relative antiviral activities of other cardiac glycosides including oleandrin was determined to be important.
Coronavirus
Human coronaviruses were discovered in the 1960s and consist of a group of related glycoprotein-enveloped RNA viruses that cause diseases in mammals and birds. In humans, these viruses cause respiratory tract infections that can range from mild to lethal. Mild illnesses include some cases of the common cold (which is also caused by other viruses, predominantly rhinoviruses), while more lethal varieties can cause SARS (severe acute respiratory syndrome), MERS (human Middle East respiratory syndrome), and COVID-19 (coronavirus disease 2019).71 While multiple therapeutic strategies exist to either prevent or treat coronaviral diseases development and use of specific vaccines appear to be the most effective. These vaccines, however, must be developed against specific strains of coronaviruses and this may not happen until well after the virus has caused serious incidences of infection and disease.
Many small molecules have been reported to exert anti-coronavirus activity via targeting either viral entry or the intracellular viral life cycle.72,73 Among these are cardiac glycosides that have been reported to be effective therapeutic agents against coronaviruses such as the porcine transmissible gastroenteritis virus (TGEV). Yang et al,74 identified the membrane-associated Na,K-ATPase as an anti-viral novel target and presented evidence that antagonists of this enzyme, the cardenolides, may serve as effective therapeutic agents. In a list of cardiac glycoside molecules tested oleandrin was shown to be one of the most potent against TGEV. Treatment with oleandrin significantly blocked viral replication and diminished viral yields. They suggest further exploration of the anti-viral activity of cardenolides such as oleandrin against other coronaviruses is warranted.
With the continued expansion of the COVID-19 pandemic, antiviral drugs are desperately needed to treat patients at a high risk of life-threatening disease and even to limit the spread of viral infection. A recent report has shown that oleandrin has a powerful effect against SARS-CoV-2.20 Prophylactic treatment of Vero cells with as little as 50 ng/mL concentration resulted in a significant 800-fold reduction in virus production, and a 100 ng/mL oleandrin concentration resulted in a greater than 3000-fold reduction in the infectious titer as well as a similar reduction in viral RNA. Given recent reports of the long, lingering deleterious health effects of COVID-19 infection, such as cognitive injury and decline as well as heart and lung tissue damage, it is important to note that oleandrin has been shown to readily traverse the blood-brain barrier and induce brain-derived neurotrophic factor (BDNF) which may assist with the recovery from virus-associated neurological disease.58 In addition, oleandrin and extracts containing this cardiac glycoside have been shown to produce a strong anti-inflammatory response through the activation of Nrf-2 antioxidant genes, which may be of benefit in preventing hyper-inflammatory responses to infection with SARS-CoV-2.8
Antiviral Effects of Oleandrin and N. oleander Extracts Against DNA Viruses
HSV-1 and HSV-2
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are alpha-herpesviruses with double-stranded DNA (dsDNA) packed within an icosahedral core that is surrounded by a lipid envelope containing the viral glycoprotein. These enveloped viruses infect epithelial cells as well as neurons and cause chronically recurrent blistering lesions, associated with viral latency and reactivation which can last for the lifetime of the infected person. Most currently licensed drugs for the systemic and/or topical treatment of herpesvirus infections target the viral DNA polymerase or the viral thymidine kinase enzyme associated with HSV replication and reactivation in non-dividing (postmitotic) neuronal cells. In 2001 Rajbhandari et al reported that methanolic extracts of N. indicum (aka N. oleander) showed considerable in vitro antiviral activity against HSV-1 thereby indicating a new class of molecules with anti-HSV activity.75 Six years later investigators used a chemical screening approach that identified ouabain as a cardiac glycoside capable of decreasing viral yield by 100-fold without affecting cellular metabolic activity.76 The antiviral potencies of other cardiac glycosides correlated with their potencies against the known target of these compounds, cellular Na, K-ATPase. Interestingly, the cardiac glycosides studied did not inhibit viral attachment or entry but did reduce the expression of viral immediate early and early genes by at least 5-fold. Of special interest was the finding that the authors were not able to generate resistant viral mutants to ouabain.76
Su et al have further advanced our understanding of the efficacy of cardiac glycosides against HSV-1 and HSV-2.77 Their data showed that the antiviral effects of digitoxin, largely similar in aglycone structure to oleandrin, was likely to involve an early stage of HSV-1 replication and the viral release stage. They also presented data demonstrating that this cardiac glycoside was effective against acyclovir-resistant viruses. Recently, Boff et al78 published their experience with several semisynthetic cardenolides, C10 (3β-[(N-(2-hydroxyethyl) aminoacetyl]amino-3-deoxydigitoxigenin)) and C11 (3β-(hydroxyacetyl) amino-3-deoxydigitoxigenin), both of which inhibited HSV-1 and HSV-2 at nM concentrations. Both compounds inhibited the intermediate and final steps of HSV replication, but not the earlier stages, based on the complete reduction in the expression of UL42 (β) and gD (γ) proteins and partial reduction of ICP27 (α).
Unpublished studies of oleandrin have shown that this cardiac glycoside was also active at nontoxic cellular concentrations against both HSV-1 and HSV-2 in infected cultured human foreskin fibroblasts (personal communication from Ravit Arav-Boger, MD). Human foreskin fibroblasts were infected with HSV-1 luciferase or a clinical isolate of HSV-2 and the antiviral activities of selected cardiac glycosides were compared. The results showed similar efficacy of cardiac glycosides against HSV-1 and HSV-2, suggesting a shared mechanism of action against both herpesviruses. Oleandrin and digitoxin were the most effective against HSV-1 and HSV-2.
CMV
This member of the herpesvirus family is also enveloped and causes opportunistic infections, as well as infectious mononucleosis. Kapoor et al reported that digoxin, digitoxin, and ouabain all inhibit human Cytomegalovirus (CMV) at nM concentrations in vitro. The compounds did not inhibit virus entry but acted at a time before DNA replication.79 Using a high-content screen, Cohen et al identified the cardiac glycoside convallatoxin as an effective inhibitor of human CMV infection.80 The proposed mechanism of CMV inhibition was reduction of methionine import, leading to decreased immediate-early gene expression without significant toxicity. Convallatoxin dramatically reduced replication of clinical CMV strains. In unpublished data, oleandrin also inhibited human CMV replication in vitro (EC50 of 0.007 µM (7 nM) and CC50 0.67 µM, selectivity index 95±2; Personal communication from R. Arav-Boger).
Given their activities against both alpha and beta herpesviruses at low nM concentrations, timing prior to initiation of viral DNA, and high slope of the dose-response curve, it is anticipated that cardiac glycosides target a cellular pathway that is linked with additional targets. Kapoor et al have shown that the antiviral activity of cardiac glycosides in infected human fibroblasts correlated with the expression of the potassium channel gene, hERG. CMV infections upregulated hERG, whereas digitoxin significantly downregulated its expression.79 These findings suggest that cardiac glycosides may inhibit human CMV by modulating cellular targets associated with hERG. Analysis of the human CMV host cell transcriptome revealed induction of potassium channels at all times tested.79–81 Ten potassium transporter RNAs were up-regulated at 72 and 120 h post infection, including three subunits of the Na/K/ATPase pump – ATP1A1, ATP1A3, and ATP1B3.
To link the binding of cardiac glycosides to the Na,K-ATPase and subsequent cellular pathways, Mukhopadhyay et al reported on autophagy induction as a novel mechanism used by cardiac glycosides to inhibit human CMV.82 Treatment with digitoxin induced autophagy flux through the Na, K-ATPase α1 subunit. Both CMV infection and digitoxin induced AMPK phosphorylation, but ULK1 was differentially phosphorylated at unique sites leading to opposing effects upon autophagy. The suppression of autophagy during infection occurred via ULK1 phosphorylation at Ser757. Digitoxin continuously phosphorylated AMPK, leading to ULK1 phosphorylation at Ser317, and suppressed mTOR, resulting in increased autophagy flux and CMV suppression. The α1 subunit of the Na,K-ATPase pump was required for autophagy induction by digitoxin, since in α1 deficient cells, neither AMPK nor autophagy was activated and CMV was not inhibited by digitoxin.
Discussion
Any meaningful discussion of the potential therapeutic benefits of cardiac glycosides must include commentary of their relative safety/toxicity as well.4,83–85 Recently obtained knowledge of natural as well as endogenous cardiac glycosides point out the variability in pharmacologic activities and potential toxicities of these individual compounds.4 In addition, an increased understanding of binding of these molecules to different combinations of alpha subunits of Na, K-ATPase is known to influence the specific ability of cardiac glycosides to inhibit tissue ion pump activity.4 Beyond this is knowledge that individual tissues are now known to vary in relative expression of the importance of the four known alpha subunits thus making generalizations of cardiac glycoside toxicity complex. This review refers specifically to oleandrin derived from Nerium oleander. Although sometimes referred to as “oleander or yellow oleander” oleandrin is not found in the highly poisonous plant Thevetia peruviana which is often used for attempted suicide.83 Indeed, no published attempts have been reported using extracts of Thevetia peruviana for pharmaceutical purposes.
While high doses of oleandrin or extracts of Nerium oleander can indeed be considered as toxic, the same is true of many approved pharmaceutical products as well. The human mortality associated with oleander ingestion is generally very low, even in cases of intentional consumption (ie, suicide attempts).86 PBI-05204, a carefully defined extract of N. oleander containing a controlled concentration of oleandrin was evaluated in both Phase I and Phase II clinical trials for treatment of advanced cancer patients.14,15 Those studies showed that oleandrin, the active principle ingredient, and other compounds in the extract could be safely administered to humans without serious adverse effects. The Swiss physician Paracelsus has been quoted as saying that “The dose makes the poison”. This is true of oleandrin as well as other cardiac glycosides such as digoxin that have been and continue to be used for medicinal purposes. It must be stressed that no attempt to use unapproved extracts of N. oleander should ever be made for medicinal purposes without FDA approval, strict knowledge of oleandrin content and proper pharmaceutical formulation.
Cardenolides such as oleandrin have many reported therapeutic applications. Recent in vitro and in vivo toxicological results, as well as epidemiologic data support new roles for this class of molecules in the treatment of several diseases, including cancer, neurological diseases as well as viral infections. Few cardenolides, however, have been proposed as effective antiviral agents. One exception is oleandrin – either as a single molecule or contained within simple plant extracts. Clinical trials of oleander extracts with carefully defined concentrations of oleandrin have shown promise that this cardenolide, at least when included in the PBI-05204 extract, can be safely administered to patients with therapeutic intent.14,15
At the present time, the specific mechanism(s) by which oleandrin, or extracts containing this molecule, provide antiviral activity against specific viruses is not known; however, multiple mechanisms of the antiviral activity of oleandrin have been suggested and deserve further inquiry. For example, in addition to targeting HIV-1,17 the findings by Hutchison et al,19 that oleandrin and a N. oleander phytoextract can inhibit the infectivity and Env-dependent intercellular transmission of HTLV-1 across the VS allude to the therapeutic potential of oleandrin as a broad-spectrum antiviral to combat infections by enveloped viruses. While these studies demonstrated that oleandrin could block the incorporation of the viral envelope glycoprotein into newly-synthesized progeny particles, it is also possible this molecule could have inhibited the synthesis of the extracellular glycan-rich biofilm associated with the formation of the VS between an HTLV-1-infected cell and an uninfected T-lymphocyte during viral pathogenesis (Figure 2), and more studies are needed in this area. Although the hematological malignancy, ATLL, is associated with latent viral infections and the clonal proliferation of an HTLV-1-transformed subpopulation of T-cells,34–38,87,88 the HTLV-1-associated inflammatory diseases, such HAM/TSP, infectious dermatitis, rheumatoid arthritis, uveitis, and others, are caused by autoreactive immune-responses to persistent virus replication and the chronic production of viral antigens.39–53 It is thus possible that oleandrin and its ability to enter the CNS7,57,58 could provide a plausible therapeutic strategy for the future clinical treatment and management of HTLV-1-induced inflammatory diseases.
The therapeutic potential of oleandrin and extracts containing this unique molecule against a wide variety of ‘enveloped’ viruses is evident from the preclinical studies reviewed in this article. Clearly, additional safety and efficacy studies involving appropriately designed in vivo experiments using vertebrate animal models must precede actual clinical studies. However, the speed of development and international spread of viruses such as SARS-CoV-2 necessitates consideration of a new strategy against these viruses that can so quickly lead to epidemics and even global pandemics. While the development of targeted vaccines is always an appropriate approach, the fact is that this type of therapeutic option requires a significant amount of time to rigorously evaluate its therapeutic efficacy and safety and often, by necessity, follows the heels of the spread of viral disease. Moreover, coronaviruses are known to exhibit a relatively high rate of mutation (REF), thereby making development of a broad-spectrum vaccine highly problematic. Indeed, a vaccine that has been demonstrated to be both safe and highly effective against SARS or MERS-CoV has still not been developed. Having a compound or plant extract with significant demonstrated potential to prevent as well as treat a wide variety of viruses deserves serious consideration. This is not to suggest that proper safety and efficacy studies against a given disease or virus should not be performed. Indeed, the FDA mandated development of ‘botanical’ drugs is similar in terms of regulatory requirements as are ‘pharmaceutical’ drugs. Given the fact that oleandrin affects viral infectivity in a unique manner highlights the possibility that it represents a novel strategy to block a virus’s ability to infect new cells and could serve as a viable therapeutic option against diseases such as COVID-19. In addition, having the ability to cross the blood–brain barrier and therein activate Nrf-2 and antioxidant genes may also present a new strategy against the prolonged secondary effects of COVID-19 since it has only recently been recognized that SARS-CoV-2 adversely affects neuronal and brain tissues2,89. The preventative as well as therapeutic potential of a new method to broadly target enveloped viruses should be given serious consideration in the era of the current coronavirus pandemic.
Disclosure
Robert A. Newman is the Chief Science Officer and a Director of Phoenix Biotechnology, Inc., reports personal fees from Phoenix Biotechnology, Inc., during the conduct of the study, and has a patent issued: 10,729,735. K Jagannadha Sastry reports personal fees from Phoenix Biotechnology Inc., during the conduct of the study and outside the submitted work. Ravit Arav-Boger reports grants from Nerium Biotechnology, during the conduct of the study and has a patent, Inhibition of Human Cytomegalovirus Replication by Novel Digitoxin Analogs Depends on Specific Alpha Isoforms of the Na-K-ATPase Pump, issued to US patent application US20160143934.
Rick Matos is a Director and consultant for Phoenix Biotechnology. Robert Harrod reports personal fees from Phoenix Biotechnology, Inc., outside the submitted work.
The other authors report no other potential conflicts of interest for this work.
References
1. Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology. And novel therapeutic targets. Pharmacol Rev. 2009;61(1):9–38. doi:10.1124/pr.108.000711
2. Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7(3):173–189.
3. Kanwal N, Rasul A, Hussain G, et al. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem Toxicol.2020;143:111570. doi:10.1016/j.fct.2020.111570
4. Botelho AFM, Pierezan F, Soto-Blanco B, Melo MM. A review of cardiac glycosides: structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon. 2019;158:63–68.
5. Babula P, Masarik M, Adam V, Provaznik I, Kizek R. From Na+/K+-ATPase and cardiac glycosides to cytotoxicity and cancer treatment. Anticancer Agents Med Chem. 2013;13(7):1069–1087. doi:10.2174/18715206113139990304
6. Slingerland M, Corella C, Guchelaar HJ, Diederich M, Geiderblum H. Cardiac glycosides in cancer therapy: from preclinical investigations towards clinical trials. Invest New Drugs. 2013;31(4):1087–1094.
7. Van Kanegan MJ, He DN, Dunn DE, et al. BDNF mediates neuroprotection against oxygen-glucose deprivation by the cardiac glycoside oleandrin. J Neurosci. 2014;34(3):963–968. doi:10.1523/JNEUROSCI.2700-13.2014
8. Van Kanegan MJ, Dunn DE, Kaltenbach LS, et al. Dual activities of the anti-cancer drug candidate PBI-05204 provide neuroprotection in brain slice models for neurodegenerative diseases and stroke. Sci Rep. 2016;6:25626. doi:10.1038/srep25626
9. Gupta V, Mittal P. Phytochemical and pharmacological potential of Nerium oleander: A Review. International Journal of Pharmaceutical Sciences and Research. 2010;1(3):21–27.
10. Amarelle L, Leucona E. The antiviral effects of Na,K-ATPase inhibition: A Minireview. Int J Mol Sci. 2018;19(8):2154. doi:10.3390/ijms19082154
11. Dey P, Chaudhuri TK. Pharmacological aspects of Nerium indicum Mill: A comprehensive review. Pharmacogn Rev. 2014;8(16):156–162. doi:10.4103/0973-7847.134250
12. Kalita D, Saikia J. Ethnomedicinal, antibacterial and antifungal potentiality of Centella asiatica, Nerium indicum and Cuscuta reflexa - widely used in Tiwa tribe of Morigaon district of Assam, India. Int J Phytomed. 2012;4:380–385.
13. Farooqui S, Tyagi T. Nerium oleander: its application in basic and applied science: A review. Int J Pharm and Pharm Sci. 2018;10(3):1–4. doi:10.22159/ijpps.2018v10i3.22505
14. Hong DS, Henary H, Falchook GS, et al. First-in-human study of PBI-05204, an oleander-derived inhibitor of Akt, FGF-2, Nf-kB and p70s6k, in patients with solid tumors. Invest New Drugs. 2014;32(6):1204–1212. doi:10.1007/s10637-014-0127-0
15. Roth MT, Cardin DB, Borazanci EH, et al. A phase II single-arm open-label bayesian adaptive efficacy and safety study of PBI 05204 in patients with stage IV metastatic pancreatic adenocarcinoma. Oncologist. 2020. 25. doi:10.1634/theoncologist.2020-0440
16. Wong RW, Lingwood CA, Ostrowski MA, Cabral MA, Cochrane A. Cardiac glycosides/aglycones inhibit HIV-1 gene expression by a mechanism requiring MEK ½-ERK ½ signaling. Sci Rep. 2018;8(1):850. doi:10.1038/s41598-018-19298-x
17. Singh S, Shenoy S, Nehete PN, et al. Nerium oleander derived derived cardiac glycoside oleandrin is a novel inhibitor of HIV infectivity. Fitoterapia. 2013;84:32–39. doi:10.1016/j.fitote.2012.10.017
18. James RM, Dorosky DE, Stonier SW, Newman RA, Dye JM Antiviral potency of an extract from Nerium oleander. 2017. 9th International Symposium on Filoviruses. Marburg, Germany. Poster presentation.
19. Hutchinson T, Yapindi L, Malu A, Newman RA, Sastry KJ, Harrod R. The botanical glycoside oleandrin inhibits human T-cell leukemia virus Type-1 infectivity and Env-dependent virological synapse formation. J Antivir and Antiretrovir. 2019;11(3):184.
20. Plante KS, Plante JA, Fernandez D, et al. Prophylactic and therapeutic inhibition of in vitro SARS-CoV-2 replication by oleandrin. bioRxiv. 2020.
21. Balzarini J. Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect Dis. 2005;5:726–731. doi:10.1016/S1473-3099(05)70271-1
22. Laird GM, Eisele EE, Rabi SA, Nikolaeva D, Siliciano RF. A novel cell-based high-throughput screen for inhibitors of HIV-1 gene expression and budding identities the cardiac glycosides. J Antimicrob Chemother. 2014;69(4):988–994. doi:10.1093/jac/dkt471
23. Agostini S, Ali H, Vardabasso C, et al. Inhibition of non-canonical HIV-1 Tat secretion through the cellular Na+, K+-ATPase Blocks HIV-1 infection. E BIO Medicine. 2017;21:170–181.
24. Spector C, Mele AR, Wigdahl B, Nonnemacher MR. Genetic variation and function of the HIV-1 Tat protein. Med Microbiol Immunol. 2019;208(2):131–169.
25. Ugolini S, Moulard M, Mondor I, et al. HIV-1 gp120 induces association between CD4 and the chemokine receptor CXCR4. J Immunol. 1994;159(6):3000–3008.
26. Cicala C, Nawaz F, Jelicic J, Arthros J, Fauci AS. HIV-1 gp120: A target for therapeutics and vaccine design. Curr Drug Targets. 2016;17(1):122–135. doi:10.2174/1389450116666150825120735
27. Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2(8):a006866. doi:10.1101/cshperspect.a006866
28. Afonso PV, Cassar O, Gessain A. Molecular epidemiology, genetic variability and evolution of HTLV-1 with special emphasis on African genotypes. Retrovirology. 2019;16(1):39. doi:10.1186/s12977-019-0504-z
29. Harrod R. Silencers of HTLV-1 and HTLV-2: the pX-encoded latency-maintenance factors. Retrovirology. 2019;16(1):25. doi:10.1186/s12977-019-0487-9
30. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi:10.3389/fmicb.2012.00388
31. Einsiedel LJ, Pham H, Woodman RJ, Pepperill C, Taylor KA. The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med J Aust. 2016;205(7):305–309. doi:10.5694/mja16.00285
32. Blas MM, Alva IE, Garcia PJ, et al. High prevalence of human T-lymphotropic virus infection in indigenous women from the Peruvian Amazon. PLoS One;. 2013;8(9):e73978. doi:10.1371/journal.pone.0073978
33. Martin F, Tagoya Y, Gallo R. Time to eradicate HTLV-1: an open letter to WHO. Lancet. 2018;391:1893–1894. doi:10.1016/S0140-6736(18)30974-7
34. Watanabe T. Adult T-cell leukemia: molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood. 2017;129(9):1071–1081. doi:10.1182/blood-2016-09-692574
35. Bangham CRM, Ratner L. How does HTLV-1 cause adult T-cell leukaemia/lymphoma (ATL)? Curr Opin Virol. 2015;14:93–100. doi:10.1016/j.coviro.2015.09.004
36. Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscett FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14(4):429–436. doi:10.1038/nm1745
37. de Castro-amarante MF, Pise-Masison CA, McKinnon K, et al. Human T-cell leukemia virus type 1 infection of the three monocyte subsets contributes to viral burden in humans. J Virol. 2015;90(5):2195–2207.
38. Cook LB, Fuji S, Hermine O, et al. Revised adult t-cell leukemia-lymphoma international consensus meeting report. J Clin Oncol. 2019;37(8):677–687. doi:10.1200/JCO.18.00501
39. Barmak K, Harhaj EW, Wigdahl B. Mediators of central nervous system damage during the progression of human T-cell leukemia type-I-associated myelopathy/tropical spastic paraparesis. J Neurovirol. 2003;9:522–529. doi:10.1080/13550280390218689
40. Cavrois M, Gessain A, Gout O, Wain-Hobson S, Wattel E. Common human T-cell leukemia virus type 1 (HTLV-1) integration sites in cerebrospinal fluid and blood lymphocytes of patients with HTLV-1-associated myelopathy/tropical spastic paraparesis indicate that HTLV-1 crosses the blood-brain barrier via clonal HTLV-1-infected cells. J Infect Dis. 2000;182:1044–1050.
41. Yamano Y, Sato T. Clinical pathophysiology of human T-lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis. Front Microbio. 2012;3:389. doi:10.3389/fmicb.2012.00389
42. Izumo S, Umehara F, Kashio N, Kubota R, Sato E, Osame M. Neuropathology of HTLV-1-associated myelopathy (HAM/TSP). Leukemia. 1997;11:82–84.
43. Levin MC, Lee SM, Kalume F, et al. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med. 2002;8:509–513. doi:10.1038/nm0502-509
44. Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–593. doi:10.3109/13550289809114225
45. Anderson MR, Pleet ML, Enose-Akahata Y, et al. Viral antigens detectable in CSF exosomes from patients with retrovirus associated neurologic disease: functional role of exosomes. Clin Transl Med. 2018;7(1):24. doi:10.1186/s40169-018-0204-7
46. Azodi S, Nai G, Enose-Akahata Y, et al. Imaging spinal cord atrophy in progressive myelopathies: HTLV-I-associated neurological disease (HAM/TSP) and multiple sclerosis (MS). Ann Neurol. 2017;82(5):719–728. doi:10.1002/ana.25072
47. Taniguchi A, Mochizuki H, Yamashita A, Shiomi K, Asada Y, Nakazato M. Spinal cord anteroposterior atrophy in HAM/TSP: magnetic resonance imaging and neuropathological analyses. J Neurol Sci. 2017;381:135–140. doi:10.1016/j.jns.2017.08.3243
48. Mahé A, Chollet-Martin S, Gessain A. HTLV-I-associated infective dermatitis. Lancet. 1999;354(9187):1386. doi:10.1016/S0140-6736(05)76239-5
49. Yakova M, Lézin A, Dantin F, et al. Increased proviral load in HTLV-1-infected patients with rheumatoid arthritis or connective tissue disease. Retrovirology. 2005;2:4. doi:10.1186/1742-4690-2-4
50. Pinheiro SR, Martins-Filho OA, Ribas JG, et al. Immunologic markers, uveitis, and keratoconjunctivitis sicca associated with human T-cell lymphotropic virus type. Am J Ophthalmol. 2006;142(1):811–815. doi:10.1016/j.ajo.2006.06.013
51. Lima CM, Santos S, Dourado A, et al. Association of sicca syndrome with proviral load and proinflammatory cytokines in HTLV-1 infection. J Immunol Res. 2016;8402059.
52. Hida A, Imaizumi M, Sera N, et al. Association of human T lymphotropic virus type 1 with Sjogren syndrome. Ann Rheum Dis. 2010;69:2056–2057. doi:10.1136/ard.2010.128736
53. Martin F, Taylor GP, Jacobson S. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev Clin Immunol. 2014;10:1531–1546.
54. Igakura T, Stinchcombe JC, Goon PKC, et al. Spread of HTLV-I between lymph ocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi:10.1126/science.1080115
55. Pais-Correia AM, Sachse M, Guadagnini S, et al. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat Med. 2010;16:83–89. doi:10.1038/nm.2065
56. Majorovits E, Nejmeddine M, Tanaka Y, Taylor GP, Fuller SD, Bangham CRM. Human T-lymphotropic virus-1 visualized at the virological synapse by electron microscopy. PLoS One. 2008;3:e2251. doi:10.1371/journal.pone.0002251
57. Garofalo S, Grimaldi A, Chece G, et al. The glycoside oleandrin reduces glioma growth with direct and indirect effects on tumor cells. J Neurosci. 2017;37:3926–3939. doi:10.1523/JNEUROSCI.2296-16.2017
58. Dunn DE, He DN, Yang P, Johansen M, Newman RA, Lo DC. In vitro and in vivo neuroprotective activity of the cardiac glycoside oleandrin from Nerium oleander in brain slice-based stroke models. J Neurochem. 2011;119:805–814.
59. Millen S, Gross C, Donhauser N, et al. (COL4A1, COL4A2), a component of the viral biofilm, is induced by the HTLV-1 oncoprotein Tax and impacts virus transmission. Front Microbiol. 2019;10:2439. doi:10.3389/fmicb.2019.02439
60. Van Prooyen N, Gold H, Andresen V, et al. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc Natl Acad Sci, USA. 2010;107:20738–20743.
61. Omsland M, Pise-Masison C, Fujikawa D, et al. Inhibition of tunneling nanotube (TNT) formation and human T-cell leukemia virus type-1 (HTLV-1) transmission by cytarabine. Sci Rep. 2018;8:11118. doi:10.1038/s41598-018-29391-w
62. Garcia-Dorival I, Wu W, Dowall S, et al. Elucidation of the Ebola virus VP24 cellular interactome and disruption of virus biology through targeted inhibition of host-cell protein function. J Proteome Res. 2014;13(11):120–135. doi:10.1021/pr500556d
63. Freedman BD, Harry RN. Calcium and filoviruses: a budding relationship. Future Microbiol. 2016;11:713–715. doi:10.2217/fmb-2016-0057
64. Kiyohara H, Ichino C, Kawamura Y, et al. In vitro anti-influenza virus activity of a cardiotonic glycoside from Adenium obesum (Forssk.). Phytomed. 2012;19(2):111–114. doi:10.1016/j.phymed.2011.07.004
65. Katzen AL, Shigemura M, Welch LC, et al. Cardiac glycosides decrease influenza virus replication by inhibiting cell protein translational machinery. Am J Physiol Lung Cell Mol Physiol. 2019;316(6):L1094–L1106. doi:10.1152/ajplung.00173.2018
66. Mi S, Li Y, Yan J, Gao GF. Na(+)/K(+) ATPase β1 subunit interacts with M2 proteins of influenza A and B viruses and affects the virus replication. Sci China Life Sci. 2010;53(9):1098–1105. doi:10.1007/s11427-010-4048-7
67. Mehndiratta MM, Mehndritta P, Pande R. Poliomyelitis: historical facts, epidemiology, and current challenges in eradication. Neurohospitalist. 2014;4(4):223–229. doi:10.1177/1941874414533352
68. Sanna G, Madeddu S, Serra A, et al. Anti-poliovirus activity of Nerium oleander aqueous extract. Nat Prod Res. 2019;1–4. doi:10.1080/14786419.2019.1582046
69. Burt FJ, Chen W, Miner JJ, et al. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infectious Disease. 2017;17(4):e107–e117.
70. Ashbrook AW, Lentscher AJ, Zamora PF, et al. Antagonism of the sodium-potassium ATPase impairs Chikungunya virus infection. mBio. 2016;7(3):e00693–16.
71. Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660–694.
72. De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Anti Infect Ther. 2006;4(2):291–302. doi:10.1586/14787210.4.2.291
73. Totura AL, Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expert Opin Drug Discov. 2019;14(4):397–412. doi:10.1080/17460441.2019.1581171
74. Yang C-W, Chang H-Y, Hsu H-Y, et al. Identification of antiviral activity of the cardenolides, Na+/K+-ATPase inhibitors, against porcine transmissible gastroenteritis virus. Tox Appl Pharmacol. 2017;332:129–137. doi:10.1016/j.taap.2017.04.017
75. Rajbhandari M, Wegner U, Julich M, Schopke T, Mentel R. Screening of Nepalese medicinal plants for antiviral activity. J Ethnopharm. 2001;74:251–255. doi:10.1016/S0378-8741(00)00374-3
76. Dodson AW, Taylor TJ, Knipe DM, Coen DM. Inhibitors of sodium potassium ATPase that impair herpes simplex virus replication identified via a chemical screening approach. Virology. 2007;366(2):340–348. doi:10.1016/j.virol.2007.05.001
77. Su CT, Hsu JT, Hsieh HP, et al. Anti-HSV activity of digitoxin and its possible mechanisms. Antiviral Res. 2008;79(1):62–70. doi:10.1016/j.antiviral.2008.01.156
78. Boff L, Schneider NFZ, Munkert J, et al. Elucidation of the mechanism of anti-herpes action of two novel semisynthetic cardenolide derivatives. Arch Virol. 2020;165(6):1385–1396. doi:10.1007/s00705-020-04562-1
79. Kapoor A, Cai H, Forman M, He R, Shamay M, Arav-Boger R. Human cytomegalovirus inhibition by cardiac glycosides: evidence for involvement of the hERG gene. Antimicrobial Agents and Chemotherapy. 2012;5(9):4891–4899.
80. Cohen T, Williams JD, Opperman TJ, Sanchez R, Lurain NS, Tortorella D. Convallatoxin-induced reduction of methionine import effectively inhibits human cytomegalovirus infection and replication. J Virol. 2016;90(23):10715–10727. doi:10.1128/JVI.01050-16
81. Oberstein A, Shenk T. Cellular responses to human cytomegalovirus infection: induction of a mesenchymal-to-epithelial transition (MET) phenotype. Proc Nat Acad Sci USA. 2017;114(39):E8244–E8253. doi:10.1073/pnas.1710799114
82. Mukhopadhyay R, Venkatadri R, Katnelson J, Arav-Boger R. Digitoxin suppresses human cytomegalovirus replication via Na+, K+/ATPase α1 subunit-dependent AMP-activated protein kinase and autophagy activation. J Virol. 2018;92(6):e01861–17. doi:10.1128/JVI.01861-17
83. Pandit VR, Kadhiravan T, Prakash RKNJ. Cardiac arrhythmias, electrolyte abnormalities and serum cardiac concentrations in yellow oleander (Cascabela thevetia) poisoning – a prospective study. Clin Toxicol. 2019;57(2):104–111. doi:10.1080/15563650.2018.1499930
84. Kanjo S, MacLean RD. Cardiac glycoside toxicity: more than 200 years and counting. Crit Care Clin. 2012;28(4):527–535. doi:10.1016/j.ccc.2012.07.005
85. Langford SD, Boor PJ. Oleander toxicity: an examination of human and animal toxic exposures. Toxicology 1996. 1996;109(1):1–13.
86. Koralnik IJ, Tyler KL. COVID-19: A global threat to the nervous system. Ann Nerol. 2020;88(1):1–11. doi:10.1002/ana.25807
87. Malpica L, Pimentel A, Reis IM, et al. Epidemiology, clinical features, and outcome of HTLV-1-related ATLL in an area of prevalence in the United States. Blood Adv. 2018;2(6):607–620. doi:10.1182/bloodadvances.2017011106
88. Johnson JM, Harrod R, Franchini G. Molecular biology and pathogenesis of the human T-cell leukaemia/lymphotropic virus type-1 (HTLV-1). Int J Exp Pathol. 2001;82(3):135–147. doi:10.1046/j.1365-2613.2001.00191.x
89. Cuadrado A, Pajares M, Benito C, et al. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol Sci. 2020;41(9):598–610. doi:10.1016/j.tips.2020.07.003
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.