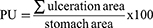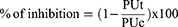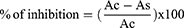Back to Journals » Journal of Experimental Pharmacology » Volume 15
Antiulcer Effect of Aqueous Ethanolic Extracts of Pseudocedrela kotschyi (Schweinf) Harms (Meliaceae) and Ximenia americana L. (Olacaceae)
Authors Delma ET, Ouédraogo M , Ouédraogo AS, Nikiema AW , Abdoulaye Gambo M, Ramde N, Youl EN, Sanou-Lamien A , Lompo OM, Guissou PI
Received 29 December 2022
Accepted for publication 6 May 2023
Published 29 May 2023 Volume 2023:15 Pages 231—240
DOI https://doi.org/10.2147/JEP.S393168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Edwige T Delma,1,2 Moussa Ouédraogo,1,2 Aimé S Ouédraogo,2,3 Arsène W Nikiema,1,2 Moustapha Abdoulaye Gambo,1,2 Norbert Ramde,2,3 Estelle NH Youl,1,2 Assita Sanou-Lamien,2,3 Olga Mélanie Lompo,2,3 Pierre I Guissou1,2
1Laboratoire de Développement du Médicament, Ecole Doctorale Sciences et Santé (ED2S), Université Joseph KI-ZERBO, Ouagadougou, Burkina Faso; 2Faculté des Sciences de la Santé, Université Joseph KI-ZERBO, Ouagadougou, Burkina Faso; 3Laboratoire d’Anatomie Pathologique, Centre Hospitalier Universitaire Yalgado Ouédraogo, Ouagadougou, Burkina Faso
Correspondence: Moussa Ouédraogo, Laboratoire de Développement du Médicament, Université Joseph KI-ZERBO, BP: 7021 Ouagadougou 03, Tel +226 25 30 70 64 /65, Email [email protected]
Purpose: This study aimed to provide pharmacological evidence of Pseudocedrela kotschyi and Ximenia americana in preventing or healing peptic ulcers claimed by traditional healers in Burkina Faso.
Methods: The trunk bark of Pseudocedrela kotschyi and the roots bark of Ximenia americana (Olacaceae) were macerated in mixed ethanol/water (80:20), respectively, to obtain dried extracts. Two models of hydrochloric acid (HCl, 0.3 M/ethanol, 60%) and hypothermic stress-induced peptic ulcer were used. The cytoprotective effect of individual or combined plant extracts was assessed at 1; 10; 30mg/kg. bw. Then, the healing effect of the extracts at 10mg/kg.bw was evaluated within 21 days of treatment on the hydrochloric acid-induced ulcer model. The extracts’ antioxidant activity and phenolic content were assessed to support the plant extracts’ efficiency.
Results: The extracts of P. kotschyi and X. americana at 10 mg/kg.bw reduced ulceration index in hydrochloric acid- and hypothermic stress-ulcer models by more than 83% and 65%, respectively. The extract from X. americana at 10mg/kg.bw allowed complete ulcer healing but not the association of the two plant extracts. The plant extracts had IC50of inhibition of DPPH radical lower than 5μg/mL and total ferric reducing antioxidant power of more than 77 mg EQAA/100mg. The total polyphenolic content was 64.82 ± 0.99 and 53.75 ± 1.39 mg EGA/g of dried extract of P. kotschyi and X. americana, respectively.
Conclusion: X. americana extract is better than the combined two plant extracts in gastric cytoprotection and ulcer healing. Further investigations are needed to highlight mechanism-based effects.
Keywords: Pseudocedrela kotschyi, Ximenia americana, aqueous ethanolic extract, antiulcer, antioxidant
Plain Language Summary
This work aimed to provide a scientific base of traditional medicine use of Pseudocedrela kotschyi and Ximenia americana in peptic ulcer treatment in Burkina Faso. We found that Ximenia americana, alone is sufficient to obtain prevention and healing of the peptic ulcer.
Introduction
A peptic ulcer is a gastroduodenal ulcerative disease. It develops in chronic multifactorial conditions spontaneously and by flare-ups. A peptic ulcer consists of a crater evolving from asymptomatic to complicated hemorrhage, stenosis, or perforation lesions.1 Helicobacter pylori, a Gram-negative bacteria, is associated with the genesis and recurrence of peptic ulcer.2 The imbalance between the defense and repair mechanisms of the gastric lining and aggressive factors such as pepsin and hydrochloric acid is critical.3 The prevalence of gastric ulcers is estimated at 2% against 8% for duodenal ulcers.4 In comparison, study reports in Australia and Great Britain showed the prevalence of peptic ulcers between 5.2% and 9.9% in the general population.5
In Africa, peptic ulcer prevalence varies according to the area. Thus, Diarra in Mali, Lawson in Togo, and Ibara in Congo have reported 10.80%, 15.53%, and 30.42%, respectively.6–8 In Burkina Faso, peptic ulcer was reported in the past decade to represent the third most common of all gastric diseases9 with an intra-hospital prevalence rate of 6.59%.10
The treatment of peptic ulcers consists of anti-secretory, antiulcer medicines and two associated antibiotics curing in the presence of H. pylori. The treatment is expensive and takes a more extended period to get healing. In developing countries, traditional healers propose using herbal medicines to treat peptic ulcers. In Burkina Faso, traditional healers use the trunk bark of Pseudocedrela kotschyi (Schweinf) Harms (Meliaceae) and Ximenia americana (Olacaceae) roots in peptic ulcers. Few data are available to support the use of these plants in the treatment of peptic ulcers. This work aimed to elucidate the pharmacological actions on the manifestations of peptic ulcers.
Materials and Methods
Material
Plants Material
It consisted of the trunk bark of Pseudocedrela kotschyi (Schweinf.) Harms (Meliaceae) and the roots bark of Ximenia americana L. (Olacaceae) were harvested in Boromo 100 km west of Ouagadougou. Plants specimens were identified by Dr BELEM Maimounata, a botanist searcher at the National Center for Scientific and Technical Research (CNRST), and confirmed by Prof. OUEDRAOGO Amadé. Vouchers of Pseudocedrela kotschyi (Schweinf.) Harms (Meliaceae) and Ximenia americana L. (Olacaceae) were deposited at the University Joseph KI-ZERBO herbarium under numbers 18024/6984 and 18025/6985, respectively.
Pharmacological Materials
The reagents and the substances used were all analytical grade: lansoprazole (Genpharma, Maroc), isotonic saline solution (Fresenius Kabi, India), and ethanol (Prolabo, France).
Animals
Healthy female mice of the Naval Medical Research Institute (NMRI) strain, three (3) months aged and weighting 30–35 g, were used. They were provided by the International Centre for Research Development on Subhumid Livestock (CIRDES) of Bobo Dioulasso, in the west of Burkina Faso. Mice have raised under 22±1°C temperature and subjected to a cycle of 12 hours of darkness/light. All experiments were conducted by international animal welfare standards as recommended by the European Union on Animal Care (EEC 86/609, UE 2010/63). The protocols were approved by the institutional animal ethical committee of Joseph KI-ZERBO University (CE-UJKZ/2014-04).
Preparation of Extracts
Two hundred grams of powder of each plant were allowed to macerate in an ethanol/water mixture (80:20, v/v) in a proportion of one powder for five parts of solvent at 25°C under magnetic stirring. The extracts were respectively concentrated to dryness in a partial vacuum rotative evaporator (Buchi water bath B-480) at 45°C and left to dry in the oven (Jouan; France). That operation was repeated three times for each plant.
Methods
Total Phenolic and Flavonoid Content
Polyphenols and flavonoid contents in each plant extract using a UV-visible microplate reader spectrophotometer (Epoch 251465, Biotek Instruments, USA).
Total phenolic content determination used the Folin–Ciocalteu reagent method described by Singleton et al.11 A mixture of extracted samples at different concentrations, Folin–Ciocalteu reagent (1N), and solution of Na2CO3 (20%) were incubated for 40 min. Then, the absorbance was read at 760 nm against a control.
Gallic acid was used as a reference compound to produce the standard curve, and the results were expressed as mg of gallic acid equivalents (GAE)/100 g of extract weight.
Total flavonoid content was assessed using the method of Dowd, adapted by Arvouet-Grand et al.12 In the presence of flavonoids; the aluminum trichloride formed a yellow–green complex with a maximal pick of absorbance at 415 nm. A calibration curve of quercetin allowed the determination of the flavonoid content of extracts.
Assessment of Plant Extracts’ Anti-Peptic Ulcer Effect
Two mice models of induced ulcers were used to highlight the cytoprotective effect of plant extracts.
HCl/Ethanol-Induced Peptic Ulcer
A mixture of HCl (0.3 M)/ethanol (60%)-induced peptic ulcer model described by Mizui and Doteuchi13 was used. Fifty-four female mice were divided into nine groups. Three groups received by an oral route different doses (1, 10, 30 mg/kg. bw) of each aqueous ethanolic extract of X. americana or P. kotschyi. The reference group received lansoprazole (20 mg/kg.bw). The negative control and the positive control groups received distilled water. Fifty minutes later, except for the negative control group, mice in the other groups received orally 0.2 mL of the mixture of HCl (0.3 M)/ethanol (60%). The negative control group received distilled water. Sixty minutes after administration of the ulcerative agent, the mice were sacrificed under anesthesia with ether vapors.
The stomachs of the mice in different groups were excised and then infused with a 0.9% sodium chloride solution. They were incised following the large curvature and then rinsed with physiological saline solution, after which they were stretched on planks and immersed in 10% formaldehyde solution for 10 minutes. Photographic images of the stomachs of animals were then realized using 12-megapixel resolution camera.
Hypothermic Restraint-Stress-Induced Peptic Ulcer
The method of hypothermic stress-induced ulcer described by Levine was used.14 Thirty-six mice were divided into six groups. Three test groups received X. americana, P. kotschyi, and a combination of both extracts at 10 mg/kg.bw. The reference group received lansoprazole (20 mg/kg.bw); the negative and positive control groups received distilled water.
Sixty minutes after the substances were administered, the mice of the test, reference, and positive control groups were immobilized in cylindrical iron cages with ventilation holes and kept at 4 °C for 3 hours. Mice in the negative control group were kept at room temperature. After 3 hours, the mice of the different groups were sacrificed under anesthesia with ether vapors. Then, the stomachs were removed and treated as before.
The images of stomachs from animal groups were analyzed using a computer equipped with ImageJ® software (version 1.43; 2009). The lesions’ cumulative area and the stomach’s total area were determined, respectively.
The percentage of ulceration (PU) was then calculated according to the formula:
The percentage of inhibition of ulceration was calculated according to the formula:
PUt: percentage of ulceration in the treated group
PUc: percentage of ulceration in the positive control group.
Assessment of the Healing Effect of Plants Extracts
The model of ulcer obtained by administering 0.2 mL of an HCl (0.3 M)/ethanol mixture (60%) to mice was used. All groups except the negative control group received the mixture. Then, 60 min after the ulcer induction, the test groups received one or combined plant extracts at 10 mg/kg. bw, respectively. Control and reference groups received distilled water or lansoprazole at 20 mg/kg.bw.
The mice of each group, priorly fasted (12h), received individual treatment every 2 days for 21 days. A preliminary study showed the persistence of the lesions after 7 days of treatment. On the 21st day, mice were sacrificed under anesthesia with ether vapors.
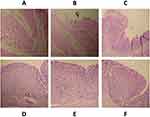
The stomachs of animals were opened, rinsed with a physiological saline solution, and fixed in 10% formaldehyde for histological analysis. Prior, macroscopic examination consisted of weighing and palpating stomach pieces was realized. Then, the area of interest was cut and impregnated in paraffin to allow a thin cross-section. After the paraffin elimination, the pieces were mounted on blades and colored by combining a basic nuclear dye (hematein) with a cytoplasmic dye (eosin). An anatomic pathologist examined and analyzed the blades at magnification 40 on a microscope equipped with a digital camera that allowed the realization of images.
Assessment of Antioxidant Activity
The antiradical activity was evaluated in vitro using the following methods: the 1.1-diphenyl-2-picrylhydrazyl (DPPH) radical reduction test and the Ferric reducing antioxidant power (FRAP) test.
DPPH° Stable Radical Reduction Test
The method described by Blois15 was used. It is based on reducing the radical DPPH° from dark purple to yellow in the presence of a hydrogen atom donor substance.
The reaction mixture contained 200 µL of 0.004% methanolic DPPH° solution and 10 µL of each plant extract at final concentrations of 30, 10, 3, 1, 0.3, and 0.1 μg/mL following a logarithmic scale.
After 30 minutes of incubation at room temperature without light exposure, the optical density of samples in methanol solution was read at 517 nm against a blank (210 µL of methanol). Negative control contains DPPH° (200 µL) and methanol (10µL). The reference was quercetin. The radical scavenging activity was calculated using the formula:
Ac: optical density of negative control (maximal OD).
As the Optical Density of the Test Sample
Ferric Reducing Antioxidant Power
The iron-reducing activity of plant extracts allows for assessing their potential antioxidant activity. It was determined using the method described by Hinneburg.16 It is based on the Fe3+-ion reduction provided by the K3[Fe(CN)6] complex. The absorbance of samples was read at a wavelength of 700 nm.
The results were expressed as mean ± ESM of ascorbic acid equivalent/100mg (AAE/100 mg) of extract in repeated triplicate experiences. We used the Student’s test for comparing two averages and the one-way ANOVA test with the Bonferroni post-test for comparing several means. The data were processed by PRISM 5.03 software and Microsoft Excel 2010. The significance threshold was p<0.05.
Results
Extraction Yield
The maceration procedure allowed a yield of 19.26±0.04% and 22.67±0.02%, respectively, for P. kotschyi and Ximenia americana. The residual humidity was less than 5% for both extracts.
Total Phenolic and Flavonoids
A calibration curve of gallic acid (y = 0.0197x + 0.0264; R² = 0.9948) allowed the polyphenols content to be determined using the Folin–Ciocalteu method (see Table 1).
 |
Table 1 Total Phenolic and Flavonoid Contents in Aqueous Ethanolic Extracts of P. kotschyi and X. americana |
The calibration curve of quercetin for total flavonoid content determination was y = 0.0538x – 0.0002; R² = 0.9972. The results are presented in Table 1.
Antiulcerogenic Activity
Protective Effects
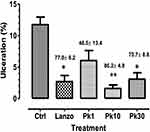
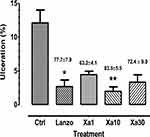
P. kotschyi and X. americana extract at 10 mg/kg.bw dose significantly inhibited ulcer formation at 86.2% (Figure 1) and 83.5%, respectively (Figure 2).
Hypothermic Stress Ulcer
The 10 mg/kg.bw dose of P. kotschyi and X. americana extracts inhibited ulceration by 65.70% and 95.11%, respectively, compared to the negative control group (Table 2).
 |
Table 2 Cytoprotective Effects of P. kotschyi and/or X. americana on Hypothermic Stress-Induced Ulcer in Mice |
The cytoprotective effect of combined extracts at 10 mg/kg.bw dose was better than the individual effect of P. kotschyi extract but less than X. americana extract effect. The inhibition percentages produced by the extracts are higher than those produced by lansoprazole (Figures 1 and 2).
Healing Power
The extract from X. americana, lansoprazole, and mixing of the two extracts at 10 mg/kg.bw dose produced complete regeneration while healing on P. kotschyi after 21 days of treatment has a persistent inflammatory reshuffling. The Lansoprazole group yield partial healing (Figure 3).
Plants Extract Antioxidant Activity
DPPH Radical Reduction Test
The inhibition curves of the radical DPPH° based on the concentrations of aqueous ethanolic extracts of trunk bark of P. kotschyi and roots bark of X. americana allowed the determination of CI50 which are respectively 3.96 and 5.01 versus 1.4 for Quercetin (Figure 4).
Reducing Power Test: FRAP
The reducing power of P. kotschyi and X. americana extracts was determined against a standard curve of ascorbic acid (y = 0.027x-0.114; R2 = 0.973). The plant extracts yielded 77.01±2.72 and 77.69±2.06mg EAA/100 mg of dry extract, respectively, for P. kotschyi and X. americana.
Discussion
This study showed cytoprotective and healing effects of the trunk bark of Pseudocedrela kotschyi (Schweinf.) Harms (Meliaceae) and roots bark of Ximenia americana L. (Olacaceae) on HCl/Ethanol and hypothermic stress-induced peptic ulcer. Each plant extract or both at 10 mg/kg.bw prevented ulcers induced by cold or acidified ethanol. Healing of the ulcer lesion is obtained in 21 days of treatment with plant extracts versus lansoprazole, an inhibitor of the proton pump of gastric borders cells.
The phytochemical contents of plant extracts are based on biological properties. Previous studies have determined the phytochemical contents of the two plants. Phytochemical screening of the stem bark of P. kotschyi retrieved tannins, alkaloids, anthraquinones, saponins, glycosides, flavonoids, and triterpenoids. Three limonoids, highly oxygenated triterpenes with furanyl steroid core structure, and pseudorelone A-B have also been isolated and widely revised.17 X. americana roots bark is reported to contain phenolic, tannins, flavonoids, triterpenes, and fatty acids, the major plant derivatives and the main active ingredients.18
Hypothermic stress in mice is reported to activate the vagus nerve19 and direct vasoconstriction, leading to a decrease in blood flow, mucus production20, and an increase in gastrin release.
Flavonoids have good stomach protection by stimulating protective factors such as vasodilation21 and mucus secretion.22 X. americana23,24 and P. kotschyi25 are reported to contain flavonoids and polyphenols. At 10 mg/kg.bw, X. americana prevented better P. kotschyi gastric ulceration (see Table 2). Beyond this qualitative chemical contents of the two plants, other chemical compounds could participate in peptic ulcer prevention. X. americana has fewer flavonoids and polyphenol contents but yields a better preventive antiulcer effect than P. kotschyi. The anticholinergic activity of plant extracts can reduce chlorohydric acid secretion or increase mucus production and release.
In acidified ethanol-induced lesions, potential mechanisms are solubilizing mucus components, modifying ion movements, and releasing histamine, pepsin, and hydrochloric acid, accelerating the process.15,24,26 Ethanol also promotes leukocyte recruitment that stimulates inflammatory responses by increasing levels of proinflammatory cytokines such as TNF-α and IL-1β26,27 and reactive oxygen species (ROS) production.28 Anti-inflammatory properties have been reported in X. americana29 and P. kotschyi.17 If stopping the inflammatory process is beneficial, at higher doses of plant extracts, a decrease in prostaglandins synthesis is damaging for the gastric mucosa. Parallelly, the blockage of prostaglandins synthesis can promote the synthesis of leukotrienes and other mediators, increasing permeability to ions H+ and Na+ and reversing membrane potential.30
On the other hand, plant extracts effectively inhibited the radical DPPH° with IC50 below 5 μg/mL. The reduction of the radical DPPH° is a widely used test for evaluating the antioxidant activity of active compounds. This test is based on the ability of an active substance to transfer hydrogen or electron atoms to the radical DPPH°.31 These results show a robust antiradical activity related to polyphenol content.
Reactive oxygen species contribute to the pathogenesis of oxidative stress-related diseases such as cancer, aging, heart failure, and peptic ulcer.32 In general, plant extracts can mediate anti-peptic ulcer activity by increasing the gastric levels of enzymatic and non-enzymatic antioxidants, namely catalase, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and GSH reductase and reduction of malondialdehyde (MDA) level.33 Polyphenols, including flavonoids, reported as antioxidants, can participle in the antiulcer activity of the two studied plants. The Ferric Reducing Antioxidant Power (FRAP) test assesses the ability of plant extracts to reduce Fe3+ ions in Fe2+ ions through electron transfer. The aqueous ethanolic extracts of P. kotschyi and X. americana showed a high concentration of reducing compounds. There is no difference between the reductive power of the two extracts—the contents of the polyphenolic compounds are almost identical in both plants. Plant polyphenols are predominantly antioxidant substances.34–36 In addition to polyphenols, several studies reported the antioxidant activity of saponin.37
Chronic gastric ulcer healing is favored by gastroprotective, anti-secretory, antioxidant agents, epithelial cell proliferation, and release of prostaglandins. Also, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and their receptors are essential in tissue repair and wounding ulcers. The secondary metabolites like saponins and flavonoids are evoked to be gastroprotective to prevent gastric ulcer complication or healing.38 The critical content of reducing compounds in both extracts could justify the extracts’ protective and healing effect on the induced gastric ulcer. However, there is no synergic effect as the association of the two extracts is not better than that of X. americana alone in improving gastroprotective or healing effect.
Conclusion
This study demonstrated the protective and healing effect of the hydroethanolic extract of P. kotschyi (Schweinf.) Harms (Meliaceae) and X. americana L. (Olacaceae) on induced gastric ulcers. These results partially justify plants’ traditional use based on active phytochemical compounds and do not yield synergistic effects between plant extracts.
Acknowledgments
We want to thank all the faculty of Health Sciences, those of the Biochemistry and Applied Chemistry Laboratory, and all the Anatomy and Physiopathology Department staff of the University Hospital Center – Yalgado Ouédraogo (CHU/YO), Burkina Faso.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bouvenot G, Devulder B, Guillevin L, Queneau PSA. L’ulcère gastroduodénal. Abrégé de Pathologie Médicale Masson. 1995;5:32–34.
2. Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7(11):629–641. doi:10.1038/nrgastro.2010.154
3. Yeomans ND. The ulcer sleuths: the search for the cause of peptic ulcers. J Gastroenterol Hepatol. 2011;26:35–41. doi:10.1111/j.1440-1746.2010.06537.x
4. Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors, and mortality. Digestion. 2011;84:102–113. doi:10.1159/000323958
5. Groenen M, Kuipers E, Hansen B, Ouwendijk RT. L’incidence d’ulcères duodénaux et d’ulcères gastriques au sein d’une population occidentale: de retour à la case de départ. J Can Gastro Enterol. 2009;23:604–608. doi:10.1155/2009/181059
6. Diarra M, Konaté A, Traoré C, Soukao A, Kanate B, Diallo A. Ulcères gastroduodénaux en milieu rural au Mali. Mali Med. 2009;24:1–3.
7. Lawson-Ananissoh LM, Bouglouga O, Bagny A, El-Hadj Yakoubou R, Kaaga L, Redah D. Profil épidémiologique des ulcères gastroduodénaux au centre hospitalier et universitaire Campus de Lomé (Togo). J Afr Hépatol Gastroenterol. 2015;9(3):88–103.
8. Ibara JR, Ikourou A, Itoua Ngaporo A. Les ulcères gastriques et duodénaux à Brazzaville. à propos de 728 cas. Med Afr Noire. 1993;40:459–465.
9. Koura M, Passolguewindé D, Napon Z, et al. Upper Gastrointestinal Endoscopy at University Hospital Sourou Sanou Bobo-Dioulasso (Burkina Faso), about 1022 Cases. Signs Lesions Observed. 2017;1:287–296.
10. Coulibaly A, Sermé AK, Godonou H, et al. Peptic ulcer disease in CHUYO. J Gastroenterol. 2016;2:353–361.
11. Singleton LV, Orthofer R, Lamuela-Raventos RM. Analysis of total phenol and other oxidation substrates and antioxidants using Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178.
12. Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardisation d’un extrait de propolis et identification des principaux constituants. J Pharm Belg. 1994;49:462–468.
13. Mizui T, Doteuchi M. Effect of polyamines on the acidified ethanol-induced gastric lesion in rats. Japan J Pharmacol. 1983;33:939–945. doi:10.1016/S0021-5198(19)52438-6
14. Levine RJ. A method for rapid production of stress ulcers in rats. In: Pfeiffer CJ, editor. Peptic Ulcer. Copenhagen: Munksgaard; 1971:92–97.
15. Blois M. Antioxidant determinations by the use of a stable free radical. Nature. 1958;29:1199–1200. doi:10.1038/1811199a0
16. Hinneburg I, Dorman HJD, Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–129. doi:10.1016/j.foodchem.2005.03.028
17. Alhassan AM, Ahmed QU, Malami I, Zakaria ZA. Pseudocedrela kotschyi: a review of ethnomedicinal uses, pharmacology and phytochemistry. Pharm Biol. 2021;59:955–963. doi:10.1080/13880209.2021.1950776
18. Denou A, Togola A, Diakite K, et al. Investigation phytochimique et activité antiradicalaire de quatre plantes utilisées dans la prise en charge traditionnelle du cancer au Mali. Bamako Technol Med Tradit. 2021;20:65–71.
19. Sun S, Yamaha H. Effects of a polysaccharide fraction from the roots of Bupleurum falcatum L. on experimental gastric ulcer models in rats and mice. J Pharm Pharmacol. 1991;43:699–704 p. doi:10.1111/j.2042-7158.1991.tb03461.x
20. Kitagawa H, Fujiwara M, Osumi Y. Effects of water-immersion stress on gastric secretion and mucosal blood flow in rats. Gastroenterology. 1979;77:298–302. doi:10.1016/0016-5085(79)90281-6
21. Serafim C, Araruna ME, Diniz M. A review of the role of flavonoids in peptic ulcer (2010–2020). Molecules. 2020;25(22):5431. doi:10.3390/molecules25225431
22. Atmani D, Chaher N, Atmani D, Berboucha M, Debbache N, Boudaoud H. Flavonoids in human health: from structure to biological activity. Curr Nutr Food Sci. 2009;5:225–237. doi:10.2174/157340109790218049
23. Ballo M, Youl ENH, Haidara M, et al. Etude des constituants chimiques et activités antiradicalaires des extraits de huit plantes médicinales récoltées au mali. Revue RAMReS. Série Pharm Méd Trad Afr. 2021;20(2):72–79.
24. Soro TY, Traore F, Datte JY, Nene-Bi AS. Pharmacognosie antipyrétique de l’extrait aqueux de Ximenia americana. Phytothérapie. 2009;7:297–303. doi:10.1007/s10298-009-0507-3
25. Ayo RG, Audu OT, Ndukwe GI, Ogunshola AM. Antimicrobial activity of extracts of leaves of Pseudocedrela kotschyi (Schweinf.) Harms. Afr J Biotechnol. 2010;9:7733–7773.
26. Ahmed OAA, Fahmy UA, Bakhaidar R, et al. Omega-3 self-nanoemulsion role in gastroprotection against indomethacin-induced gastric injury in rats. Pharmaceutics. 2020;12(2):140. doi:10.3390/pharmaceutics12020140
27. Song SH, Kim JE, Sung JE, et al. Antiulcer effect of Gallarhois extract with antioxidant activity in an ICR model of ethanol/hydrochloride acid-induced gastric injury. J Tradit Complement Med. 2019;9(4):372–382. doi:10.1016/j.jtcme.2017.07.001
28. Abdelfattah MS, Elmallah MIY, Ebrahim HY, Almeer RS, Eltanany RMA, Abdel Moneim AE. Prodigiosins from a marine sponge associated actinomycete attenuate HCl/ethanol-induced gastric lesion via antioxidant and anti-inflammatory mechanisms. PLoS One. 2019;14(6):e0216737. doi:10.1371/journal.pone.0216737
29. Shettar AK, Kotresha K, Kaliwal BB, Vedamurthy AB. Evaluation of in vitro antioxidant and anti-inflammatory activities of Ximenia americana extracts. Asian Pacific J Trop Dis. 2015;5:918–923. doi:10.1016/S2222-1808(15)60957-4
30. Takeuchi K, Amagase K. Roles of Cyclooxygenase, Prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr Pharm Des. 2018;24:2002–2011. doi:10.2174/1381612824666180629111227
31. Molyneux P. The use of the stable free radical diphenylpicryl hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;2(26):211–219.
32. Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49(3):345–361. doi:10.2165/00003495-199549030-00003
33. Mei X, Xu D, Xu S, Zheng Y, Xu S. Novel role of Zn(II)-curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated* oxidative damage of ethanol-induced acute gastric ulcers. Chem Biol Interact. 2012;197:31–39. doi:10.1016/j.cbi.2012.03.006
34. Dharmesh S, Srikanta B. Anti- Helicobacter pylori, proton pump inhibitory and antioxidant properties of selected dietary medicinal plants. Int J Pharmacol. 2012;4:573–581.
35. Meziti A, Meziti H, Boudiaf K, Mustapha B, Bouriche H. Polyphenolic profile and antioxidant activities of Nigella sativa seed extracts in vitro and in vivo. Int J Biotechnol Bio Eng. 2012;6:109–117.
36. Pereira DM, Valentão P, Pereira JA, Andrade PB. Phenolics: from chemistry to biology. Molecules. 2009;14:2202–2211. doi:10.3390/molecules14062202
37. Velásquez BOJ, Murillo PE, Méndez JJ, Murillo AW, Alexandra ND. Quantification, chemical and biological characterization of the saponosides material from Sida cordifolia L. (escobilla). Rev Cuba Plant Med. 2013;18:298–314.
38. Sisay ZW, Jemere AT. Evaluation of the antiulcer activity of hydromethanolic crude extract and solvent fractions of the root of Rumex nepalensis in rats. J Exp Pharmacol. 2020;12:325–337. doi:10.2147/JEP.S258586
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.