Back to Journals » OncoTargets and Therapy » Volume 13
Antitumorigenic Effect of Hsp90 Inhibitor SNX-2112 on Tongue Squamous Cell Carcinoma is Enhanced by Low-Intensity Ultrasound
Authors Nan C, Zheng Y, Fan H, Sun H, Huang S, Li N
Received 27 May 2020
Accepted for publication 24 July 2020
Published 12 August 2020 Volume 2020:13 Pages 7907—7919
DOI https://doi.org/10.2147/OTT.S262174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
Chuanchuan Nan,1 Yuyan Zheng,2 Haidong Fan,2 Haipeng Sun,2 Shengxing Huang,2 Nan Li2
1Department of Intensive Care Unit, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 518020, Guangdong, People’s Republic of China; 2Department of Stomatology, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University; The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, Guangdong, 518020, People’s Republic of China
Correspondence: Nan Li
Department of Stomatology Center, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University; The First Affiliated Hospital, Southern University of Science and Technology), No. 1017, Dongmen North Road, Shenzhen 518020, Guangdong, People’s Republic of China
Tel +86 18845156569
Email [email protected]
Purpose: The novel Hsp90 inhibitor SNX-2112 showed broad antitumor activity. However, it was still necessary to optimize the therapeutic dosage of SNX-2112 applied on tumors to obtain effective therapy with minimal dose to reduce toxicity. We investigated the role of low-intensity US in promoting antitumorigenic effect of low doses of SNX-2112 on tongue squamous cell carcinoma.
Methods: Cell viability was measured using CCK-8 assay or staining with Calcein AM/PI. Relative cumulative levels of SNX-2112 in cells were detected using high-performance liquid chromatography. The production of ROS was analyzed using fluorescence microscope and flow cytometer. Cellular apoptosis was detected using flow cytometer. The expression levels of proteins of the ERS-associated apoptosis signaling pathway were detected using Western blotting analysis. The efficacy and biosafety of SNX-2112 were also investigated in a mouse xenograft model.
Results: Low-intensity US combined with SNX-2112 exhibited significant antitumor effect, increased the absorption of SNX-2112 by cells even with a low dose, enhanced ROS generation and apoptosis. The combination regimen also inhibited the protein expression of Hsp90 and triggered apoptosis through endoplasmic reticulum stress (ERS) by enhancing PERK, CHOP and Bax protein levels, while downregulating the level of Bcl-2. Additionally, N-acetyl-L-cysteine (NAC), ROS scavenger, was able to reverse these results. Low-intensity US combined with SNX-2112 significantly inhibited tumor growth, prolonged survival of mice, decreased proliferation and promoted apoptosis with no visible damage or abnormalities in major organs in the mouse xenograft model with tongue squamous cell carcinoma.
Conclusion: The antitumor effects of SNX-2112 were enhanced by low-intensity US. The most probable mechanism was that US sonoporation induced more SNX-2112 delivery to the cells and enhanced ROS production, triggering the ERS-associated apoptosis signaling pathway. Therefore, low-intensity US may increase the efficiency of conventional chemotherapy and reduce the dosage of SNX-2112 required and its side effects.
Keywords: low-intensity ultrasound, SNX-2112, tongue squamous cell carcinoma, reactive oxygen species, endoplasmic reticulum stress, apoptosis
Introduction
Tongue squamous cell carcinoma (TSCC) is one of the most aggressive and lethal forms of oral carcinoma primarily due to rich blood supply and lymphatic drainage.1 Recently, there is an increasing prevalence of TSCC among younger patients (as early as 45 years of age) worldwide, due to an increase in tobacco and alcohol abuse.2 Surgery has been used as the conventional method of treatment for the elimination of tumors, but it also leads to tongue tissue defection and oral dysfunction. High doses of chemotherapy may result in serious clinical complications that often lead to drug resistance. There is an increasing need for alternative non-invasive and effective methodologies that can improve the treatment regimen for TSCC patients. Sonodynamic therapy (SDT) has been developed as a new non-invasive alternative from photodynamic therapy (PDT). SDT can kill tumor cells specifically and selectively using ultrasound (US), which penetrates deep into tissues and activates sensitizers that are concentrated in tumor tissue.3,4
Some studies have combined ultrasound with chemotherapeutics to treat tumors.5,6 Heat shock protein 90 (Hsp90) has been found to be significantly over expressed in different types of cancer cells, including TSCC tumors, compared with non-cancerous tissue.7–9 Hsp90 has been studied as a potential anticancer target10 because it acts as a molecular chaperone involved in cancer cell proliferation, mostly through its anti-apoptotic function.11,12 SNX-2112 inhibits Hsp90, which degrades proteins by binding to the N-terminal of ATP, thus causing the arrest of cell cycle t and finally apoptosis of cancer cells. SNX-2112 is a potent molecule against diverse cancers, including multiple myeloma, human chronic leukemia, lung cancer and human hepatocellular carcinoma.12,13 However, it is still necessary to optimize the therapeutic dosage of SNX-2112 applied on tumors to exert effective therapy using a minimal dose to reduce toxicity. Therefore, the combination of SNX-2112 and low-intensity US may be a promising alternative.14 Studies have validated that low-intensity US enhanced the efficacy of chemotherapeutic drugs, including doxorubicin,5 carboplatin,15 and cisplatin.16 The ultrasonic cavitation effect of the ultrasound leads to the formation of micro jets, which direct the agent into the cell or disrupt cell membranes, permitting the inflow of external agents.17
Numerous experiments have suggested that endoplasmic reticulum stress (ERS) may be an important regulator of cell apoptosis.18,19 Previous investigations have shown that the antitumor activity of SNX-2112 is partially mediated by the ERS pathway.13 Low-intensity US along with chemotherapeutic agents or a sensitizer can generate significant levels of reactive oxygen species (ROS) to induce cell death through the apoptotic pathway.3,20 Accumulating reports have shown that increased ROS generation could cause protein misfolding in the endoplasmic reticulum, resulting in activation of the ERS pathway.21 Thus, we hypothesized that low-intensity US may also increase ROS production via SNX-2112 and trigger the ERS pathway, ultimately resulting in cell apoptosis.
The purpose of this study was to investigate whether low-intensity US could enhance the antitumor effect of SNX-2112 on TSCC cells, as well as on TSCC xenograft in vivo. We assessed SNX-2112 uptake by TSCC cells after low-intensity US treatment. We also probed the effect of US on ROS generation induced by SNX-2112 and explored whether SNX-2112 combined with US-induced apoptosis through the ERS pathway via ROS generation.
Materials and Methods
Materials and Reagents
SNX-2112 was purchased from Selleck chemicals (USA). Apoptosis Detection kit was purchased from Biosea Biotechnology (Beijing, China). Calcein acetoxymethyl ester (Calcein-AM) and propidium iodide (PI) Double Stain Kit were purchased from Santa Cruz Biotechnology Inc. (USA). 2.7-dichlorofluorescein diacetate (DCFH-DA) ROS assay kit was purchased from Applygen (Beijing, China). Cell Counting kit (CCK)-8 was bought from Beyotime Institute of Biotechnology (Nantong, China). N-acetylcysteine (NAC) was procured from Sigma-Aldrich (USA). Antibodies against the proteins studied were sourced as follows: PERK, CHOP, Bax, Bcl-2, and β-actin from Cell Signaling Technology (Beverly, USA); HRP-conjugated secondary antibody from Santa Cruz Biotechnology (Santa Cruz, USA); and Anti-PCNA from Proteintech (Chicago, USA). Terminal deoxyribonucleotide transferase-mediated nick-end labeling (TUNEL) assay In Situ Cell Death Detection Kit was obtained from Roche (Mannheim, Germany).
Ultrasonic Treatment Protocol
The system used for ultrasound treatment in this study (Figure 1) was created by Harbin Institute of Technology (Harbin, China). For the in vitro study, cells were kept at the center of the transducer along with an aluminum transmission medium. Degassed water was used above the transmission medium and cells were subjected to ultrasound at specifications of 1.0 MHz; 1 W/cm2; 20% duty cycle for a duration of 3 min in the US and SNX-2112+US groups in the dark. For the in vivo experiments, we used the ultrasonic transducer with a tapered 5 mm diameter aluminum buffer head. The transducer was positioned directly in touch with the tumor cells using acoustic couplant grease at specifications of 1.0 MHz; 1 W/cm2; 20% duty cycle for 5 min. The temperature increase was maintained at less than 2°C in all experiments.
Cell Lines and Cell Culture
Cell lines derived from human tongue cancer, SAS, was sourced from Human Science Research Resources Bank (Osaka, Japan). Human TSCC cell lines SCC-9, and SCC-25 and normal HT293 cells were obtained from Cell Bank of Type Culture Collection, Chinese Academy of Sciences (Shanghai, China). SAS Cells were grown in (RPMI)-1640 medium containing 10% fetal bovine serum (FBS) and 1% antibiotic (penicillin-streptomycin). SCC-9, SCC-25 and normal HT293 were incubated in Dulbecco’s modified Eagle’s medium (DMEM) enhanced with 10% FBS and 1% penicillin-streptomycin. All cells were grown at 37°C in a 5% CO2 atmosphere.
Cellular Cytotoxicity and Apoptosis
Cellular viability was assayed using a Cell Counting kit (CCK)-8, following the manufacturer’s instructions. Exponentially growing TSCC cells (SAS, SCC-9 and SCC-25) were grown overnight in 96-well culture plates (1 × 104 cells/mL). To screen preferable treatment parameters, the TSCC cells were treated with a range of concentrations (0–2μM) of SNX-2112 for 48 h and detected using CCK-8 assay. The cells were sonicated at different ultrasonic intensities (0–4 W/cm2) for a period of 3 min and measured using CCK-8 assay after 48 h of incubation in the dark. The IC50 values were enumerated using the Prism Pad program 6. We chose a suitable dosage of SNX-2112 and ultrasonic intensities to visualize the therapeutic effect of SNX-2112+US treatment on TSCC cells. The 4 groups used in this study are (1) Untreated (C); (2) SNX-2112 (SNX); (3) US (US); and (4) SNX-2112 + US (SNX+US). After 4 h of incubation with SNX-2112, the cells were immediately subjected to US at a frequency of 1.1 MHz, 1 W/cm2, 20% duty cycle for a duration of 3 min. The cells were incubated for 48 h and were subjected to viability measurement using CCK-8 assay and apoptosis analysis. To further confirm these results, each group of cells was stained with Calcein AM (10 μL) and PI (15 μL) at 37°C for a duration of 15 min and the live v/s dead cell fraction was determined. The samples were visualized using a Dmi8 fluorescence inverted microscope (Leica, Buffalo Grove, USA). Calcein AM was used to stain the live cells (which emitted green fluorescence), while a red fluorescence was obtained from dead cells due to PI staining.
Cellular apoptosis was detected using an Annexin V-FITC Apoptosis Detection Kit, as instructions. Cells in all treatment groups were harvested and re-suspended in a binding buffer with a mixture of Annexin V and PI. After a post-incubation period of 15 minutes in the dark, the samples were analyzed using a DxFLEX flow cytometer (Beckman Coulter, California, USA). In addition, to visualize the effect of ROS on cellular apoptosis induced by SNX-2112+US treatment, a group of cells were incubated with 5 mM NAC for 1 h prior to the addition of SNX-2112. Each group included 6 wells of cells and each experiment was performed in triplicate.
Detection of Intracellular ROS Content
The following five groups of cells were used in this experiment: (1) Untreated (C); (2) SNX-2112 (SNX); (3) US (US); (4) SNX-2112 + US (SNX+US); and (5) SNX-2112 + US +NAC (SNX+US+NAC). The production of ROS was measured using an oxidation-sensitive probe DCFH-DA ROS assay kit, following the manufacturer’s instructions. Cells were exposed to 5 μM DCFH-DA in the cell culture medium without serum for 10 min of incubation at 37°C in the dark. ROS generation was then ascertained using an inverted fluorescence microscope at 488 nm (excitation) and 525 nm (emission). In addition, ROS generation was also detected using a DxFLEX flow cytometer (Beckman Coulter, California, USA) and computed using Flowjo.10 software. Each group contained 6 wells and each experiment was performed in triplicate.
Immunoblot Analysis
The cells were lysed using a RIPA buffer (Pierce, USA) and the tumor tissues were homogenized. The total protein concentration was estimated using a BCA protein assay kit (Pierce, USA). Protein samples were resolved using SDS polyacrylamide gel and transferred onto a nitrocellulose membrane (Pall Corporation, USA). Upon blocking with a buffer (composed of 5% non-fat milk dissolved in TBST), the membranes were layered with primary antibodies against PERK, CHOP, Bax, Bcl-2, and β-actin overnight at 4°C. The following day, membranes were treated with secondary antibodies. Chemiluminescence was detected using a detection system (LI-COR Biosciences, USA) and protein band densities were calculated using Odyssey 3.0 software. Finally, the blots were subjected to densitometry analysis with GADPH used as the standard. Each group contained 6 wells of cells and each experiment was performed in triplicate.
TSCC Xenograft Experiments
4–6-week-old male BALB/c nude mice were purchased from Shanghai Laboratory Animal Center (SLAC, Shanghai, China). The mice were maintained and treated according to the ARRIVE guidelines and the experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act. 1 × 106 SAS cells suspended in 200 μL RPMI-1640 (without FBS) were injected through the subcutaneous route to generate TSCC tumors. When tumors reached a volume of 100 mm3, animals with tumor burden were segregated into 8 groups (n= 6 animals/group). Four treatment categories were used to estimate the antitumor efficacy of the treatment regime. The treatment groups used were as follows: control, SNX-2112, US, and SNX-2112+US. SNX-2112 solution was intraperitoneally introduced into the tumor-bearing mice of the SNX-2112 and SNX-2112+US groups at a dosage of 5 mg/kg. In the absence of SNX-2112, 0.9% normal saline solution (used as the placebo control) was injected into tumor-bearing mice of the control and only US groups. The tumor-bearing mice in the US group were exposed to ultrasound (1.1 MHz, 1 W/cm2, 20% duty cycle) for a duration of 5 minutes in the absence of light. Mice in the SNX-2112+US group were treated with US after 1 hour of SNX-2112 administration. The groups were exposed to repeated treatment 3 times a week for 2 weeks. Tumor parameters were measured to estimate its volume for 15 days using the formula: V = π/6 × L × S2, where L and S are the long diameter and short diameter, respectively. At day 15 post treatment, the mice were sacrificed and various tissues, such as tumor, heart, liver, kidney, spleen, and lung tissue, were obtained to be used for histopathological examination. We calculated survival by dividing the mice into four groups in the same manner as above. We monitored the mice daily and euthanized them when their weight decreased to 10% of their initial weight.
Absorption of SNX-2112 in Cells
The absorption of SNX-2112 in cells was studied using an Ultimate 3000 HPLC system (Thermo, MA, USA). 1 × 106 cells were suspended in 5 μg/mL SNX-2112 (for 4 h) were sonicated for 3 min and continued incubation for 24 h, while another group of cells were only incubated with SNA-2112 for 24 h. We washed the cellular suspension twice in PBS prior to finally suspending the cells in 1 mL of PBS. The cell suspension was lysed using an ultrasonic cell crusher (work 6 s, intermittent 8 s, 200 W ultrasound 25 times) followed by centrifugation at 10,000 rpm for 15 minutes at a temperature of 4°C. The sample was purified following a previously described method.1 We used a ZORBAX Eclipse plus C18 column (4.6 × 250 mm, 5 μm), which in the mobile phase was eluted with acetonitrile–water (40:60, v/v). The flow rate was maintained at 1.0 mL/min and the column temperature was 30°C. The sample was detected at an absorption wavelength of 251 nm and the sample injection volume was kept steady at 10 μL. Each group contained 6 wells of cells and each experiment was performed in triplicate.
Tissue Staining with Hematoxylin and Eosin (H&E)
Tissues were fixed using 4% formaldehyde prior to embedding in paraffin wax. Samples embedded in paraffin wax were sectioned and subjected to H&E staining and studied under a light microscope (Axio Scope A1 FL; Carl Zeiss, Germany).
TUNEL Assay
Tumor sections embedded in paraffin were used for TUNEL assay, following the manufacturer’s instructions. Cells stained brown under a fluorescence microscope were considered to be TUNEL-positive. The apoptosis index (AI) was calculated using the number of cells that stained positive in 10 randomly selected fields at a magnification of × 200.
Immunohistochemical Staining
1% BSA solution was used to incubate deparaffinized tumor sections for 30 min followed by treatment with an anti-PCNA primary antibody at a dilution of 1:200. The number of PCNA stained nuclei were counted. The proliferation index was scored as the fraction of PCNA-positive cells over the total number of cells counted in 10 randomly selected high-power fields (magnification of × 200).
Analysis of Statistical Significance
All data are expressed as mean ±standard deviation (SD). We used ANOVA followed by Tukey’s multiple comparison test to analyze the deviation between the different treatment groups. Survival analyses were evaluated using the Kaplan–Meier method and compared using the Log rank test. Prism 6.0 software was used to determine statistical differences among the groups. We considered a P value of <0.05 to be a statistically significant difference among groups.
Results
Antitumor Effect of SNX-2112 on TSCC Cells Increased Upon the Application of Low-Intensity US
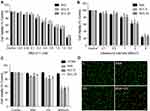
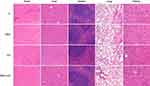
To investigate the effects of combinatorial therapy using SNX-2112 and US on TSCC cell viability in vitro, we determined the suitable dosage of SNX-2112 and ultrasonic intensity. We observed TSCC cell viability (SAS, SCC-9, and SCC-2) using CCK-8 assay, post treatment with various dosages of SNX-2112 for 48 h. SNX-2112 exhibited dose-dependent inhibition of cell viability and the IC50 values for SAS, SCC-9 and SCC-25 obtained were 1.152 μM, 0.982 μM, and 0.856 μM, respectively (Figure 2A). US also suppressed cell viability with an IC50 of 2.125 W/cm2, 2.233 W/cm2 and 2.524 W/cm2 for SAS, SCC-9 and SCC-26 cells, respectively (Figure 2B). A SNX-2112 dose of 0.2 μM and the US intensity of 1.0 W/cm2 were recommended for use on normal liver HT293 cells with no decrease in cell viability. These treatment parameters were chosen for further experiments because they can safely be applied on normal cells. As shown in Figure 2C, cell viability was much lower in the SNX-2112+US treatment group, compared with the SNX-2112 and US treatment groups alone (P <0.05). Moreover, SNX-2112 and US combination treatment did not result in significant levels of cell death of non-cancerous HT293 cells, suggesting the specificity of its effect. We also stained each group of cells with Calcein AM/PI to observe the ratio of live/dead cells using fluorescence microscopy, as shown in Figure 2D. SNX-2112 combined with US could lead to the death of SAS cells at a significant rate, compared with the single treatment regimen groups. These results illustrate that low-intensity US could enhance the inhibition of TSCC cell growth induced by SNX-2112.
Low-Intensity US Enhanced the Uptake of SNX-2112 in Cells Facilitating ROS Generation
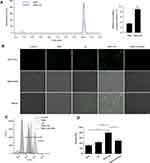
To investigate the causal mechanism by which low-intensity US enhanced the SNX-2112-induced anti-cancer effects, we monitored uptake of intracellular SNX-2112 in SAS cells in vitro. As shown in Figure 3A, compared to SNX-2112 alone, after the application of ultrasound, the levels of SNX-2112 in SAS cells increased about 3.1-fold (where P <0.05). As shown in Figure 3B, the green fluorescence represented ROS positive cells, and we observed no or little green fluorescence from control, SNX alone and US alone groups of cells, and in contrast, high fluorescence intensity was observed after SNX-2112 combined with low-intensity US treatment. This increase in the fluorescence of ROS created in the SNX-2112+US group can be weakened by the ROS scavenger, NAC. Subsequently, we validated ROS generation in vitro using flow cytometry and the results showed a similar trend to that observed with fluorescence microscopy (Figure 3C and D). Relative mean fluorescence intensities (MFI) of ROS in the SN-2112+US treatment showed significant increase as much as 2.53-fold (P< 0.05 vs SNX-2112 group), and fluorescence intensities decreased significantly upon the use of the ROS scavenger, NAC (Figure 3D).
SNX-2112 Combined with US Triggered Apoptosis Through the ERS Pathway
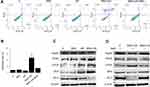
Differentially treated SAS cells (including the group with the ROS scavenger, NAC) were subjected to flow cytometry to detect apoptotic changes. As shown in Figure 4A and B, the SNX-2112+US group exhibited an apoptosis index of 23.7%, which was significantly higher compared with the other three groups. Interestingly, apoptosis significantly decreased to 5.0% in the SNX-2112 + US group in the presence of NAC. Compared with the control, SNX-2112 and US groups, the protein expression of Hsp90 decreased in the SNX-2112 +US group. SNX-2112+US could increase PERK, CHOP and BAX protein levels, whereas it downregulated the expression of Bcl-2 in SAS cells (Figure 4C). The NAC (ROS scavenger) reversed the expression trend of Hsp90, PERK, CHOP, BAX and Bcl-2 in the SNX-2112+US group (Figure 4D).
Effects of SNX-2112 Combined with US on in vivo Studies
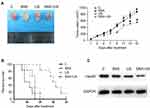
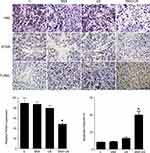
The in vivo effect of SNX-2112+US was determined in a xenograft model of BALB/c mice with SAS tumor. Compared with the control, SNX-2112+US treatment mediated approximately 38% of tumor volume inhibition after 14 days, while SNX-2112 alone and US alone failed to show obvious tumor growth suppression (Figure 5A). We also observed significantly prolonged survival of mice after SNX-2112+US treatment, compared with mice treated with SNX-2112 or US alone (P < 0.001; Figure 5B). Accordingly, we investigated whether treatment with SNX-2112 combined with low-Intensity US changed Hsp90 levels in the xenograft tumors. The results showed that the expression of Hsp90 decreased in the SNX-2112+US group in vivo, compared with the other three groups. In addition, histopathological analysis further validated that after treatment with SNX-2112+US, most of tumor tissue turned necrotic and died, while cells exhibited regular morphology (Figure 6). As shown in Figure 6, the PCNA staining intensities were significantly lower after SNX-2112+US treatment (48.22 ± 4.8%; P < 0.05 vs other three groups), while the TUNEL score increased significantly, compared to the other 3 groups (P < 0.05). As shown in Figure 7, no visible damage or abnormalities were observed in major organs in any of the groups.
Discussion
The main treatment of TSCC involves chemotherapy. Hsp90 affects the expression and function of more than 100 client proteins, such as Akt, Raf-1, Cdk4, MMP2, and VEGFR2, which regulate cancer cell proliferation, invasion and angiogenesis.22 The Hsp90 inhibitor, SNX-2112, has been shown to exert antitumor effects on a variety of tumors. However, therapeutic doses of SNX-2112 can also exert significant off-target toxicity. If the dose is limited to reduce toxicity, the therapeutic effect will be poor.23 Combination anticancer treatments are necessary to deliver a suitable low dose that can target cancerous tissues. In this study, we determined that low-intensity ultrasound can enhance the antitumor effect of low-dose SNX-2112 and its potential mechanism in TSCC.
First, through the cell cytotoxicity experiment, we determined that when combined, 1.0 W/cm2 intensity of US and 0.2 μM dose of SNX-2112 could result in significant TSCC cell death and tumor inhibition. But when used alone, it is safe for both normal and tumor cells (Figure 2). Meanwhile, the results indicated that low-intensity US effectively increased the absorption of SNX-2112 into the cells (Figure 3). Therefore, we hypothesized that SNX-2112 combined with US exerted excellent anticancer activity, which may be due to US increasing the level of SNX-2112 delivery to the cell. Some studies have shown that US improved cellular uptake of adriamycin and promoted its antitumor effect.24,25 Escoffre et al inferred that sonoporation significantly increased the delivery of nucleic acid to tumors, skeletal muscle, and kidney.26,27 Low-intensity US could affect the penetrability of the cell membrane, which can promote the delivery of chemotherapeutic drugs into tumor cells.27,28 Ultrasound cavitation is considered to be a major cause of US-induced porosity of cell membranes.29 Oscillating ultrasonic cavitation microbubbles can result in sonoporation at a fixed intensity and frequency close to the cell membrane. This mechanical oscillation creates transient microporous membranes that have a half-life of 10 minutes and a girth of up to 150 nanometers.30 Therefore, the low-intensity US can effectively make the cell membrane more permeable and results in higher uptake of SNX-2112 at a nontoxic, low dose.
Furthermore, our results indicated that a combination regimen could effectively increase ROS production (Figure 3B and C). This increase in ROS generation may be due to increased SNX-2112 uptake allowed by low-intensity US. ROS are the main products of oxygen metabolism that occur under SDT treatment or US-enhanced chemotherapy.3,27,31 The imbalance of ROS levels in cells can lead to organelle injury and cell apoptosis.32 In vitro and in vivo studies have shown that the combination of low-intensity US and SNX-2112 increased apoptosis (Figures 4A, B and 6), and that the ROS scavenger, NAC, could weaken the enhanced level of apoptosis (Figure 4A and B). Therefore, we hypothesized that apoptosis induced by ROS may be an additional mechanism by which the anti-cancer effect of SNX-2112+US is induced.
The endoplasmic reticulum is considered as an important cell organelle for protein folding and gathered.33 When cell processes are abnormal, ERS is activated and triggers the unfolded protein response (UPR) to avoid cell damage.34 Excessive ER stress can induce cell death through apoptosis induced by UPR.35 The UPR is initiated by three regulating pathways that proceed through three ERS sensors, PKR like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol requiring kinase 1 (IRE1).33 These three signaling pathways can activate the downstream apoptosis of signaling molecules, such as CAAT/enhancer binding protein homologous protein (CHOP), BAX and Bcl-2.36 Among them, the PERK signaling pathway is necessary for the expression of CHOP.37 Saha et al reported that combination therapy using 3.0 W/cm2 US and the Hsp90 inhibitor, 17AAG, modulated unfold protein response and sensitized prostate cancer to 17AAG.38 In our study, SNX-2112+US could gently decrease Hsp90 expression (Figures 4C and 5C), and increased the expression of PERK, CHOP and BAX, compared with the SNX-2112 or US alone groups, whereas it downregulated the level of Bcl-2 in SAS cells (Figure 4C). These results illustrated that SNX-2112 combined with US-induced apoptosis of TSCC may be partially mediated by ERS by increasing the level of CHOP via activating the PERK signaling pathway. Excessive production of ROS can result in apoptosis through mitochondria-mediated or death receptor-mediated signaling pathways.39 Previous literature has concluded that increase of ROS production could induce oxidative stress and change protein expressions in ERS-mediated apoptosis.19 It has been shown that ROS activated the GRP78/PERK signaling pathway, which induced apoptosis via ERS in cervical cancer cells. Emerging evidence has shown that SNX-2112 may trigger tumor cell death through ERS-mediated pathways.12 To explore the mechanism by which ROS induced apoptosis in SNX-2112+US, we examined the effects of NAC on the expressions of proteins involved in ERS-associated apoptosis of SAS cells. More importantly, the ROS scavenger, NAC, significantly decreased the inhibition effect of SNX-2112+US on Hsp90 and ERS and the expressions of PERK, CHOP, BAX and Bcl-2 recovered, indicating that combined SNX-2112+US treatment definitively regulated the expression of Hsp90 and ERS-related proteins through ROS production.
In summary, our study provided a new strategy for the use of combination therapy of non-invasive low-intensity US and a non-toxic, low dose of SNX-2112 that induced apoptosis through the ERS pathway via ROS generation. The advantage of this combination strategy is that locational application of low-intensity US transiently permeabilized the cell membrane and consequently enhanced the intracellular delivery of SNX-2112 to TSCC. As a result, this regimen showed enhanced antitumor efficacy, and avoided side effects caused by a high-dose of a chemotherapy drug. Our in vivo study determined that no visible damage or abnormalities were observed in major organs (Figure 7), indicating that SNX-2112+US treatment may be relatively safe from long-term damage.
Conclusions
Our in vitro and in vivo experiments confirmed that a combinatorial regimen of SNX-2112 and low-intensity US significantly enhanced antitumor efficacy, compared with groups treated with SNX-2112 or US alone. This may be due to the sonoporation effect ultrasound waves of low intensity can bring about, which induces permeability of cell membrane, resulting in significantly increased rate of absorption and transfer of SNX-2112. In addition, increased ROS production after combination treatment triggered apoptosis induced by SNX-2112 via the ERS-associated apoptosis pathway. In conclusion, our results suggested the possibility of combination therapy with low-intensity US along with chemotherapy. As a non-invasive, targeted treatment the combination therapy may be advantageous over conventional chemotherapy on cancer by reducing the side effects caused by drug dosage.
Ethics Approval
This study was approved by ethical committee of Shenzhen People’s Hospital. Medical ethics committee number: LL-KY-2,020,262. This was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank Professor Wenwu Cao of Harbin Institute of Technology for kindly providing the ultrasonic transducer used in this study and Professor Jinhua Zheng from Harbin Medical University for his expert guidance.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest to disclose for this work.
References
1. Shield KD, Ferlay J, Jemal A, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67(1):51–64. doi:10.3322/caac.21384
2. Hussein AA, Helder MN, de Visscher JG, et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: a systematic review. Eur J Cancer. 2017;82:115–127. doi:10.1016/j.ejca.2017.05.026
3. Liu RG, Zhang QY, Lang YH, et al. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn Ther. 2017;19:159–166. doi:10.1016/j.pdpdt.2017.06.003
4. Costley D, Mc Ewan C, Fowley C, et al. Treating cancer with sonodynamic therapy: a review. Int J Hyperthermia. 2015;31(2):107–117. doi:10.3109/02656736.2014.992484
5. Tinkov S, Coester C, Serba S, et al. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Control Release. 2010;148(3):368–372. doi:10.1016/j.jconrel.2010.09.004
6. Nesbitt H, Sheng Y, Kamila S, et al. Gemcitabine loaded microbubbles for targeted chemo-sonodynamic therapy of pancreatic cancer. J Control Release. 2018;279:8–16. doi:10.1016/j.jconrel.2018.04.018
7. Chanthammachat P, Promwikorn W, Pruegsanusak K, et al. Comparative proteomic analysis of oral squamous cell carcinoma and adjacent non-tumour tissue from Thailand. Arch Oral Biol. 2013;58(11):1677–1685. doi:10.1016/j.archoralbio.2013.08.002
8. Thiel UJ, Feltens R, Adryan B, et al. Analysis of differentially expressed proteins in oral squamous cell carcinoma by MALDI-TOF MS. J Oral Pathol Med. 2011;40(5):369–379. doi:10.1111/j.1600-0714.2010.00982.x
9. Khiavi MM, Anvari E, Hamishehkar H, et al. Assessment of the blood parameters, cardiac and liver enzymes in oral squamous cell carcinoma following treated with injectable doxorubicin-loaded nano-particles. Asian Pac J Cancer Prev. 2019;20(7):1973–1977. doi:10.31557/APJCP.2019.20.7.1973
10. Jin L, Huang R, Huang X, et al. Discovery of 18β-glycyrrhetinic acid conjugated aminobenzothiazole derivatives as Hsp90-Cdc37 interaction disruptors that inhibit cell migration and reverse drug resistance. Bioorg Med Chem. 2018;26(8):1759–1775. doi:10.1016/j.bmc.2018.02.021
11. Wang X, Wang SX, Liu YT, et al. The Hsp90 inhibitor SNX-2112 induces apoptosis of human hepatocellular carcinoma cells: the role of ER stress. Biochem Biophys Res Commun. 2014;446(1):160–166. doi:10.1016/j.bbrc.2014.02.081
12. Arya R, Mallik M, Lakhotia SC. Heat shock genes – integrating cell survival and death. J Biosci. 2007;32(3):595–610. doi:10.1007/s12038-007-0059-3
13. Liu X, Cheng X, Wang F, et al. Targeted delivery of SNX-2112 by polysaccharide-modified graphene oxide nanocomposites for treatment of lung cancer. Carbohydr Polym. 2018;185:85–95. doi:10.1016/j.carbpol.2018.01.014
14. Deng XD, Guan W, Qing XC, et al. Ultrafast low-temperature photothermal therapy activates autophagy and recovers immunity for efficient antitumor treatment. ACS Appl Mater Interfaces. 2020;12(4):4265–4275. doi:10.1021/acsami.9b19148
15. Li HX, Zheng JH, Ji L, et al. Effects of low-intensity ultrasound combined with low-dose carboplatin in an orthotopic hamster model of tongue cancer: a preclinical study. Oncol Rep. 2018;39(4):1609–1618. doi:10.3892/or.2018.6262
16. Yu T, Yang Y, Liu S, et al. Ultrasound increases DNA damage attributable to cisplatin in cisplatin-resistant human ovarian cancer cells. Ultrasound Obstet Gynecol. 2009;33(3):355–359. doi:10.1002/uog.6258
17. Wood AK, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41(4):905–928. doi:10.1016/j.ultrasmedbio.2014.11.019
18. Lu DY, Chang CS, Yeh WL, et al. The novel phloroglucinol derivative BFP induces apoptosis of glioma cancer through reactive oxygen species and endoplasmic reticulum stress pathways. Phytomedicine. 2012;19(12):1093–1100. doi:10.1016/j.phymed.2012.06.010
19. Shi JM, Bai LL, Zhang DM, et al. Saxifragifolin D induces the interplay between apoptosis and autophagy in breast cancer cells through ROS-dependent endoplasmic reticulum stress. Biochem Pharmacol. 2013;85(7):913–926. doi:10.1016/j.bcp.2013.01.009
20. Giuntini F, Foglietta F, Marucco AM, et al. Insight into ultrasound-mediated reactive oxygen species generation by various metal-porphyrin complexes. Free Radic Biol Med. 2018;121:190–201. doi:10.1016/j.freeradbiomed.2018.05.002
21. Yokouchi M, Hiramatsu N, Hayakawa K, et al. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem. 2008;283(7):4252–4260. doi:10.1074/jbc.M705951200
22. Hu LB, Wang Y, Chen I, et al. Hsp90 inhibitor SNX-2112 enhances TRAIL-induced apoptosis of human cervical cancer cells via the ROS-mediated JNK-p53-autophagy-DR5 pathway. Oxid Med Cell Longev. 2019;2019:9675450. doi:10.1155/2019/9675450
23. Jhaveri K, Taldone T, Modi S, et al. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823(3):742–755. doi:10.1016/j.bbamcr.2011.10.008
24. Yu T, Wang Z, Jiang S. Potentiation of cytotoxicity of adriamycin on human ovarian carcinoma cell line 3AO by low-level ultrasound. Ultrasonics. 2001;39(4):307–309. doi:10.1016/S0041-624X(01)00051-8
25. Harrison GH, Balcer-Kubiczek EK, Gutierrez PL. In vitro mechanisms of chemopotentiation by tone-burst ultrasound. Ultrasound Med Biol. 1996;22(3):355–362. doi:10.1016/0301-5629(95)02053-5
26. Escoffre JM, Zeghimi A, Novell A, Bouakaz A. In-vivo gene delivery by sonoporation: recent progress and prospects. Curr Gene Ther. 2013;13(1):2–14. doi:10.2174/156652313804806606
27. Trendowski M. The promise of sonodynamic therapy. Cancer Metastasis Rev. 2014;33(1):143–160. doi:10.1007/s10555-013-9461-5
28. Wang H, Liu Q, Zhang K, et al. Comparison between sonodynamic and photodynamic effect on MDA-MB-231 cells. J Photochem Photobiol B. 2013;127:182–191. doi:10.1016/j.jphotobiol.2013.08.015
29. Pan XT, Wang HY, Wang SH, et al. Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci China Life Sci. 2018;61(4):415–426. doi:10.1007/s11427-017-9262-x
30. Zeghimi A, Escoffre JM, Bouakaz A. Role of endocytosis in sonoporation-mediated membrane permeabilization and uptake of small molecules: a electron microscopy study. Phys Biol. 2015;12(6):066007. doi:10.1088/1478-3975/12/6/066007
31. Hao DN, Song YB, Che Z, et al. Calcium overload and in vitro apoptosis of the C6 glioma cells mediated by sonodynamic therapy (hematoporphyrin monomethyl ether and ultrasound). Cell Biochem Biophys. 2014;70(2):1445–1452. doi:10.1007/s12013-014-0081-7
32. Li ZY, Yang Y, Ming M, et al. Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem Biophys Res Commun. 2011;414(1):5–8. doi:10.1016/j.bbrc.2011.09.046
33. Healy SJ, Gorman AM, Mousavi-Shafaei P, et al. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625(1–3):234–246. doi:10.1016/j.ejphar.2009.06.064
34. Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3(11):597–605. doi:10.1158/1541-7786.MCR-05-0221
35. Chen Y, Brandizzi F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013;23(11):547–555. doi:10.1016/j.tcb.2013.06.005
36. Szegezdi E, Logue SE, Gorman AM, et al. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi:10.1038/sj.embor.7400779
37. Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5(7):723–728. doi:10.4161/cbt.5.7.2967
38. Saha S, Bhanja P, Partanen A, et al. Low intensity focused ultrasound (LOFU) modulates unfolded protein response and sensitizes prostate cancer to 17AAG. Oncoscience. 2014;1(6):434–445. doi:10.18632/oncoscience.48
39. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi:10.1146/annurev.arplant.55.031903.141701
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.