Back to Journals » International Journal of Nanomedicine » Volume 12
Antitumor HPV E7-specific CTL activity elicited by in vivo engineered exosomes produced through DNA inoculation
Authors Di Bonito P , Chiozzini C , Arenaccio C, Anticoli S, Manfredi F, Olivetta E, Ferrantelli F , Falcone E , Ruggieri A, Federico M
Received 29 December 2016
Accepted for publication 25 February 2017
Published 23 June 2017 Volume 2017:12 Pages 4579—4591
DOI https://doi.org/10.2147/IJN.S131309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Thomas Webster
Paola Di Bonito,1 Chiara Chiozzini,2 Claudia Arenaccio,2 Simona Anticoli,2 Francesco Manfredi,2 Eleonora Olivetta,2 Flavia Ferrantelli,2 Emiliana Falcone,3 Anna Ruggieri,3 Maurizio Federico2
1Department of Infectious, Parasitic and Immunomediated Diseases, Istituto Superiore di Sanità, Rome, Italy; 2National AIDS Center, Istituto Superiore di Sanità, Rome, Italy; 3Department of Veterinary Public Health and Food Safety, Istituto Superiore di Sanità, Rome, Italy
Abstract: We recently proved that exosomes engineered in vitro to deliver high amounts of HPV E7 upon fusion with the Nefmut exosome-anchoring protein elicit an efficient anti-E7 cytotoxic T lymphocyte immune response. However, in view of a potential clinic application of this finding, our exosome-based immunization strategy was faced with possible technical difficulties including industrial manufacturing, cost of production, and storage. To overcome these hurdles, we designed an as yet unproven exosome-based immunization strategy relying on delivery by intramuscular inoculation of a DNA vector expressing Nefmut fused with HPV E7. In this way, we predicted that the expression of the Nefmut/E7 vector in muscle cells would result in a continuous source of endogenous (ie, produced by the inoculated host) engineered exosomes able to induce an E7-specific immune response. To assess this hypothesis, we first demonstrated that the injection of a Nefmut/green fluorescent protein-expressing vector led to the release of fluorescent exosomes, as detected in plasma of inoculated mice. Then, we observed that mice inoculated intramuscularly with a vector expressing Nefmut/E7 developed a CD8+ T-cell immune response against both Nef and E7. Conversely, no CD8+ T-cell responses were detected upon injection of vectors expressing either the wild-type Nef isoform of E7 alone, most likely a consequence of their inefficient exosome incorporation. The production of immunogenic exosomes in the DNA-injected mice was formally demonstrated by the E7-specific CD8+ T-cell immune response we detected in mice inoculated with exosomes isolated from plasma of mice inoculated with the Nefmut/E7 vector. Finally, we provide evidence that the injection of Nefmut/E7 DNA led to the generation of effective antigen-specific cytotoxic T lymphocytes whose activity was likely part of the potent, therapeutic antitumor effect we observed in mice implanted with TC-1 tumor cells. In summary, we established a novel method to generate immunogenic exosomes in vivo by the intramuscular inoculation of DNA vectors expressing the exosome-anchoring protein Nefmut and its derivatives.
Keywords: nanovesicles, cytotoxic T lymphocytes, HIV-1 Nef, DNA vectors
Introduction
Exosomes are vesicles ranging 50–150 nm and are released constitutively by all cell types.1 They are generated by inward invagination of endosome membranes. These intraluminal vesicles form the multivesicular bodies (MVBs) which can traffic to the plasma membrane to which they fuse, thereby releasing their vesicular contents in the extracellular milieu. Exosomes are part of the intercellular communication network. They incorporate messenger RNAs, microRNAs, DNA, and proteins which can be functional in target cells. Nanovesicles showing both physical and biochemical features resembling exosomes, but those generated through direct extrusion of plasma membrane have also been described in muscle cells.2–4
The exosome immunogenicity basically relies on the amounts and quality of antigens they incorporate. Exosomes were tested as antitumor immunostimulatory agents, and clinical trials demonstrated that they are well tolerated as cell-free vaccines.5,6 However, their therapeutic efficacy appeared quite limited, posing the need of new methods to increase their immunogenicity. This issue has been addressed through in vitro methods devoted to engineer foreign antigens to increase their display on the exosome membrane.7,8
Exosome biogenesis and HIV budding share the functions of a number of cell proteins including Alix, Tsg101, and several components of the endosomal sorting complex required for transport.9 The convergence of exosome and HIV biogenesis implies the possibility that viral products incorporate in exosomes. Such is the case for HIV-1 Nef, which associates with exosomes through anchoring its N-terminal myristoylation to lipid raft microdomains at the endosome membranes.10 Nef is a 27 kilodalton (kDa) protein lacking enzymatic activities. However, it can act as a scaffold/adaptor element to trigger activation of signal transducing molecules.11
We previously identified a G3C V153L E177G Nef mutant incorporating in exosomes at quite high levels.12 This Nef mutant (referred to as Nefmut) is defective for basically all Nef functions. Its efficiency of incorporation in nanovesicles does not change significantly when fused at its C-terminus with foreign proteins. Manipulating Nefmut allows the incorporation of high amounts of antigens of choice into exosomes, which thus remain protected from external neutralization/degradation factors. We recently reported that the inoculation in mice of exosomes carrying HPV E7 fused with Nefmut induces an effective E7-specific CD8+ T cytotoxic lymphocyte (CTL) response.13 This result demonstrates that, through the Nefmut-based engineering strategy, the already proven poor CTL immunogenicity of exosomes can be overcome. However, this strategy would face possible difficulties in view of potential clinical applications, including in areas of standardization of industrial manufacturing, high cost-effectiveness, and storage of the immunogen. For these reasons, we conceived a still unproven, exosome-based vaccine strategy relying on delivery of DNA vectors expressing Nefmut-based fusion proteins into host animal by intramuscular (IM) inoculation. This strategy relies on the very recent observation that muscle cells also, both proliferating and differentiated, constitutively release exosome-like vesicles. Considering that Nefmut and derivatives thereof associate with exosomes with high efficiency, we predicted that the expression of Nefmut-based vectors in muscle cells would be sufficient to create a continuous source of engineered, immunogenic exosomes.
Here, we demonstrate that IM inoculation of mice with a DNA vector expressing Nefmut/E7 elicits a potent CTL immune response, thereby blocking the growth of already implanted tumor cells. We provide evidence that the production of endogenously engineered exosomes was the basis of the observed antitumor effect.
Materials and methods
Molecular constructs and cell cultures
All molecular constructs were based on IE-CMV-promoted vectors. Vectors expressing Nefmut, Nefmut/green fluorescent protein (GFP),12 NefG2A/GFP,14 wild-type (wt) Nef,15 and HPV E716 have already been described. 293T, murine muscle C2C12 (both obtained from American Type Culture Collection, ATCC), and HPV E7-expressing TC-1 tumor cells (a kind gift from Dr Wu, John Hopkins Medical Institutes, Baltimore, MD, USA) were grown in Dulbecco’s Modified Eagle’s Medium plus 10% heat-inactivated fetal calf serum (FCS). Transfection assays were carried out by the Lipofectamine 2000-based method (Invitrogen, Thero Fisher Scientific, Waltham, MA, USA), which in the case of C2C12 cells was modified by adding liposomes to freshly trypsinized cells. Both EL-4 cells17 (obtained from ATCC) and mouse splenocytes were cultivated in Roswell Park Memorial Institute 1640 (RPMI) medium plus 10% FCS.
Exosome isolation, detection, and characterization
Exosomes were isolated from cell supernatants through differential centrifugations as previously described.18 The recovery of exosomes from the plasma of inoculated mice was carried out in a similar way except that samples were fivefold diluted before starting centrifugations. The amounts of recovered exosomes were evaluated by measuring the activity of acetylcholinesterase (AchE), ie, a classical exosome marker,19 through the use of the Amplex Red kit (Molecular Probes, Thermo Fisher) following the manufacturer’s recommendations. The AchE activity was measured in mU/mL, where 1 mU is defined as the amount of enzyme which hydrolyzes 1 pmole of acetylcholine to choline and acetate per minute at pH 8.0 at 37°C.
Fluorescent exosomes from transfected cell cultures were either directly detected by fluorescence-activated cell sorting (FACS, Gallios, Beckman Coulter, Brea, CA, USA), or, in the case of exosomes isolated from plasma, analyzed upon binding with aldehyde/sulfate latex beads (Molecular Probes, Thermo Fisher). To this end, samples were incubated with 5 μL of beads overnight at room temperature on a rotating plate, washed, and incubated for 1 hour at 4°C with either anti-CD63 mAb MX-49.129.5 from Abcam (Cambridge, UK) or a control isotype. The incubation was then repeated using secondary PE-conjugated Abs. Finally, beads were resuspended in 1× PBS-2% v/v formaldehyde and analyzed with FACS.
Western blot analyses of both cell lysates and exosomes were carried out as described previously.12 Filters were revealed using 1:1,000 diluted sheep anti-Nef antiserum ARP 444 (a generous gift of M Harris, University of Leeds, Leeds, UK), 1:250 diluted anti-β actin AC-74 mAb from Sigma (Milan, Italy), and 1:100 diluted anti-Alix H-270 polyclonal Abs from Santa Cruz (Heidelberg, Germany).
Mice immunization and detection of IFN-γ-producing CD8+ T lymphocytes
All studies with animals described here have been approved by the Ethical Committee of the Istituto Superiore di Sanità, Rome, Italy (protocol number 555/SA/2012) according to Legislative Decree 116/92, which has implemented in Italy the European Directive 86/609/EEC on laboratory animal protection. Animals used in our research have been housed and treated according to the guidelines mentioned in the Legislative Decree. C57 Bl/6 mice were purchased from Charles River Laboratories Italia (Calco, Italy) and inoculated IM two times at 10 day intervals with 50 μg in each quadriceps with plasmid DNA purified with endotoxin-free Qiagen kit (Hilden, Germany). Mice were also inoculated subcutaneously (SC) three times at ten day intervals with exosomes (6 mU AchE equivalents) purified from plasma of DNA injected mice, and sacrificed 10 days after the last immunization. To detect both E7- and Nef-specific CD8+ T-cell immune responses, splenocytes were put in IFN-γ Elispot microwells (Millipore, Billerica, MA) in the presence of 5 μg/mL of either HPV E7 or HIV-1 Nef 8- or 9-mer peptides binding the H-2 Kb complex of C57 Bl/6 mice, ie, DLYCYEQL (aa 21–28) and RAHYNIVTF (aa 49–57) for E7,20 and TAATNADCA (aa 48–56) for Nef.21 H-2 Kb binding HPV E6-specific KLPQLCTEL (aa 18–26) and YDFAFRDL (aa 50–57) peptides20 were used as unrelated peptides. After overnight incubation, IFN-γ Elispot plates were developed (Mabtech AB, Nacka Strand, Sweden) and spot-forming units were counted using an Elispot reader (A.EL.VIS. Elispot reader and Analysis software GmbH, Turin, Italy).
Fluorescence microscope analysis
Slices (7 μm) from quadriceps of inoculated mice were prepared using a cryostat (Leika CM 3050, Wetzlar, Germany) and placed to slides. The slices were then incubated with 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories, Peterborough, UK) together with an antifade mounting medium. Finally, coverslips were mounted on the slides, which were then observed with a Zeiss Axioskop 2 Plus (Oberkochen, Germany) fluorescence microscope.
CTL assay
CD8+ T-cells were isolated from splenocytes by positive immunomagnetic selection (Miltenyi Biotec Gmbh, Teterow, Germany). They were put in coculture for 6 hours in RPMI 10% FCS with EL-4 cells previously labeled with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, Thermo Fisher), following the manufacturer’s recommendation, and treated overnight with either E7 or unrelated peptides. The cocultures were run at different cell ratios (ie, 20:1, 10:1, and 5:1 effector/target cells) in 200 μL of RPMI 20% in U-bottom 96 well plates. Afterward, EL-4 cell mortality was scored by FACS analysis soon after addition of 7-AAD at final concentration of 1 μg/mL.
Detection of anti-E7 and anti-Nef antibodies in plasma
Plasma from inoculated mice were pooled, and twofold serial dilutions starting from 1:10 were assayed for the presence of both E7- and Nef-specific Abs as previously described.22 The end-point dilution corresponded to a <0.1 OD absorbance at 450 nm. Each plasma sample was assayed in triplicate, and the mean of the absorbance values was taken as final readout.
Antitumor effects of Nefmut/E7-expressing vector
The antitumor activity was evaluated in mice previously challenged with 2×105 TC-1 cells. DNA inoculations were performed 4 and 11 days after tumor cell challenge following the above reported protocol, and only in mice that developed palpable tumors. Tumor growth was monitored by visual inspection, palpation, and measurement of tumor nodule diameter calculated as (length × width2)/2. At the end of the observation time, tumors were explanted and weighted.
Statistical analysis
When appropriate, data are presented as mean + standard deviation. In some instances, the paired Student’s t-test was used and confirmed using the nonparametric Wilcoxon rank sum test. P<0.05 was considered significant.
Results
Detection of engineered exosomes released by DNA-transfected murine muscle cells
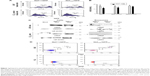
We preliminarily investigated whether, as already assessed in different human cell types, the expression of a Nefmut-expressing DNA vector in murine muscle cells was sufficient to generate engineered exosomes. For the sake of clarity, exosome-like nanovesicles released by murine muscle cells are here defined as exosomes, although their biogenesis somehow differs from that of MVB-generated exosomes.2
Murine muscle C2C12 and, as control, human 293T cells were transfected with vector expressing GFP fused at the C-terminus of either Nefmut or a Nef isotype inefficiently associating with exosomes (ie, NefG2A).23 Transfected cells were monitored for the respective efficiency of transfection (Figure 1A), which in muscle cells appeared to be over 50%. The supernatants were collected, and exosomes isolated through differential centrifugation. Exosome preparations were then titrated in terms of AchE activity. The two cell types produced apparently similar levels of AchE-positive nanovesicles whatever the transfection conditions (Figure 1B). The western blot analysis of 100 μU equivalent of exosomes showed the presence of Nef-derived molecules in exosomes from both 293T and C2C12 cells transfected with Nefmut/GFP but not NefG2A/GFP vectors (Figure 1C). The FACS analysis confirmed the association of fluorescence with the nanovesicles we recovered from C2C12 cells transfected with Nefmut/GFP (Figure 1D).
In sum, we have proven that exosome-like nanovesicles released by murine muscle cells can be engineered by Nefmut-derivatives as previously proven in epithelial-like, human 293T cells.
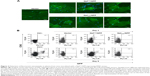
Nefmut-derived products can be detected in exosomes from plasma of DNA-inoculated mice
To assess whether engineered exosomes can be generated in vivo, 50 μg of either Nefmut/GFP, NefG2A/GFP, or empty vector were inoculated in each quadriceps of C57 Bl/6 mice. To assess the expression of injected vectors, 3 days later, slices of muscle tissues obtained from the zones of inoculation were analyzed for the expression of GFP-related products. Consistently with what was already described for wt Nef,24 Nefmut accumulated at the plasma membrane as well as in an intracellular punctate pattern. Differently, the NefG2A mutant localized preferentially in the cytoplasm, as a consequence of the lack of N-terminal myristoylation (Figure 2A). Three and nine days after inoculation, plasma from the inoculated mice was recovered and exosomes were isolated by differential centrifugations. Exosome preparations were titrated in terms of AchE activity, equivalent amounts of them were bound to white aldehyde/sulfate latex beads, and finally labeled for the CD63 exosome marker. By FACS analysis of the exosome–bead complexes, we detected fluorescent exosomes among those isolated only from the plasma of mice injected with Nefmut/GFP vector (Figure 2B).
These results indicated that the inoculation in mice of vectors expressing Nefmut derivatives can lead to the generation of engineered exosomes.
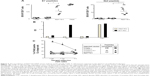
HPV E7-specific CTL response induced by IM inoculation of Nefmut/E7-expressing DNA vector
Next, we evaluated the immunogenicity of DNA vectors expressing Nefmut derivatives. C57 Bl/6 mice (six per group) were inoculated IM in each quadriceps with 50 μg of vectors expressing either Nefmut/E7 or E7 alone or empty vector. Since we previously noticed that E7 associates with exosomes at undetectable levels (not shown), the inclusion of a vector expressing E7 alone was instrumental to evaluate the benefit of the Nefmut fusion in terms of CD8+ T-cell immunogenicity. The inoculations were repeated 10 days later, and after additional 10 days mice were sacrificed. Then, the splenocytes were isolated and cultured overnight in IFN-γ Elispot microwells in the presence of either unrelated, Nef-, or E7-specific nonamers. The CD8+ T-cell activation observed in cultures with unrelated peptides remained at background levels as in the case of splenocytes cultured in the absence of peptides (not shown). On the other hand, peptide-specific cell activation was clearly detectable in splenocytes from mice inoculated with Nefmut/E7-expressing vector (Figure 3A). Conversely, no CD8+ T-cell response was observed in cultures of splenocytes from mice receiving either E7-expressing or empty vector, whatever the peptide used. Notably, anti-E7 antibodies were detected only in plasma from mice inoculated with the vector expressing E7 alone (Figure 3B).
To evaluate whether the CD8+ T-cell response coupled with an E7-specific CTL activity, CD8+ T-cells were isolated from pools of splenocytes and then put in coculture for 6 hours at different cell ratios with CFSE-labeled EL-4 cells pretreated overnight with either unrelated or E7 nonamers. Afterward, the cocultures were labeled with 7-AAD and percentages of dead target cells were scored by FACS analysis. The results reported in Figure 3C show a clear increase of target cell mortality in both 20:1 and 10:1 cocultures comprising CD8+ T lymphocytes from mice inoculated with the Nefmut/E7-expressing vector and EL-4 pretreated with E7-specific nonamers. This result demonstrates the presence of E7-specific CTLs among the CD8+ T lymphocytes isolated from mice inoculated with the Nefmut/E7-expressing vector.
Taken together, these data indicated that IM inoculation of a vector expressing a heterologous antigen fused with Nefmut leads to the induction of a strong antigen-specific CTL response in the absence of antibody production.
Lack of immunogenicity of a DNA vector expressing the wt Nef isoform
To support the idea that high levels of Nefmut incorporation in exosomes were mandatory to elicit the antigen-specific CD8+ T-cell response, we reproduced the immunogenicity experiments by inoculating mice with vectors expressing the wt isoform of Nef, which is known to incorporate in exosomes at much lower extents compared to Nefmut.12 To this end, C57 Bl/6 mice (four per group) were injected IM in each quadriceps with 50 μg of a vector expressing either wt Nef or Nefmut or with the empty vector. The inoculations were repeated 10 days later, and after an additional 10 days the mice were sacrificed. Splenocytes were then isolated and cultured overnight in IFN-γ Elispot microwells in the presence of either unrelated or Nef nonamers. As shown in Figure 4, mice inoculated with the vector expressing wt Nef, differently to those receiving the Nefmut vector, failed to develop a detectable Nef-specific CD8+ T cell response.
This result indicates that the levels of antigen incorporation into exosomes are critical for the immune response, meanwhile suggesting that the functions of wt Nef were not per se involved in the antigen-specific CD8+ T-cell activation.
Immunogenicity of exosomes isolated from plasma of mice immunized with Nefmut/E7 DNA
To enforce the hypothesis that the CD8+ T-cell immune response we detected upon inoculation of Nefmut-expressing vectors relied on production of engineered exosomes, we next tested whether exosomes purified from plasma of DNA-inoculated mice were immunogenic in recipient-naïve mice. For this, eight mice were inoculated with vectors expressing E7, Nefmut/E7, or empty vector following the schedule mentioned earlier. Eight days after the last immunization, peripheral blood mononuclear cells were recovered through retro-orbital bleeding and checked for the E7-specific CD8+ T-cell response. As already observed, the injection of the Nefmut/E7-expressing vector, but not that expressing E7 alone, gave rise to a well detectable E7-specific CD8+ T-cell response (Figure 5A). Plasma from the different mouse groups were pooled and exosomes were isolated by differential centrifugation. Afterward, exosomes were titrated in terms of AchE activity, and 6 mU AchE equivalents were injected SC in syngeneic mice three times at 10 day intervals. Finally, mice were sacrificed, and splenocytes tested for the E7-specific CD8+ T-cell responses, which were noticed only in splenocyte cultures from mice inoculated with exosomes purified from mice injected with the Nefmut/E7 DNA vector (Figure 5B).
These results indicate that the IM injection of DNA expressing Nefmut/E7 leads to the production of immunogenic exosomes, hence further supporting the idea that the production of endogenously engineered exosomes was on the basis of the strong E7-specific CD8+ T-cell immune response observed in the DNA-injected mice.
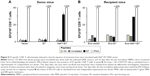
Antitumor therapeutic effect of IM inoculation of Nefmut/E7-expressing DNA vector
Finally, we evaluated the potency of the CD8+ T-cell immune response evoked by injection of Nefmut/E7 expressing vector in terms of antitumor effect. To this end, we set up therapeutic immunization assays on C57 Bl/6 mice inoculated SC with 2×105 TC-1 cells. Mice developing a tumor mass detectable by palpation, ie, of about 2 mm diameter, were then inoculated with 50 μg/quadriceps of vectors expressing either empty vector, Nefmut (four mice per each group) or Nefmut/E7 (six mice) at days 4 and 11 after cell implantation. As control, 4 tumor-implanted mice were injected with the vehicle alone. At day 21, retro-orbital bleeding was carried out on mice injected with Nefmut- or Nefmut/E7-expressing vectors to assess the induction of E7-specific CD8+ T-cell immune response (Figure 6A). The growth of tumors was monitored over 30 days, and thereafter mice were sacrificed, tumors explanted, and weighted. Figure 6B shows that, whereas the injection of control DNA vectors did not influence the growth of implanted tumor cells, their expansion was severely impaired in mice inoculated with Nefmut/E7 vector, with tumor cells apparently being cleared in three mice, as confirmed by tumor weights (Figure 6C).
Hence, the inoculation of Nefmut/E7-expressing DNA vector elicited a CD8+ T-cell immune response also in the presence of tumor cells. Most importantly, this immune response was both strong and rapid enough to inhibit the tumor growth and, in some instances, clear the implanted tumor cells.
Taken together, these results represent a relevant milestone toward possible therapeutic applications of immunization strategies based on Nefmut-based endogenously engineered exosomes.
Discussion
We here describe a simple exosome-mediated strategy to induce unrestricted CTL immunity through IM DNA inoculation. Muscle cells constitutively release exosome-like nanovesicles through extrusion of plasma membrane with a biogenesis at least in part different from that of MVB-generated exosomes. Thus, considering that Nefmut-based in vitro engineered exosomes we previously described were generated in epithelial-like human cells, investigating whether and how efficiently the ectopic expression of Nefmut leads to the release of engineered exosomes in murine cells was mandatory. Data we obtained with C2C12 cells supported the idea that Nefmut accumulates into exosome-like nanovesicles released by murine muscle cells at levels similar to those observed in human cells. Our investigations did not include terminally differentiated muscle cells, ie, the cell type most likely targeted by IM inoculation in mice, since we assumed that the cell differentiation does not significantly impact the mechanisms underlying intracellular vesicle trafficking. Since we expected that the majority of muscle cells targeted by IM DNA inoculation was differentiated, the detection of engineered exosomes in plasma from inoculated mice supported the idea that terminally differentiated muscle cells indeed release engineered exosomes in vivo. We cannot exclude that at least part of injected DNA could target other cell types by means, for instance, of the diffusion of DNA in draining lymph nodes, where it can be captured and internalized by dendritic cells (DCs). However, we believe that DCs contribute limitedly to the overall production of engineered exosomes since SC inoculation of Nefmut/E7-expressing DNA, which is expected to preferentially target DCs, gave rise to a quite weak E7-specific CD8+ T-cell activation (not shown).
The immune response detected in DNA-inoculated mice appeared much stronger than what we previously observed in mice injected with in vitro produced engineered exosomes.13 A direct comparative experiments cannot be run in view of the different nature of the two immunogens. However, we noticed that the DNA inoculation elicited an E7-specific CD8+ T-cell activation strong enough to be clearly detected without the in vitro, peptide-directed stimulation/expansion of specific CD8+ T lymphocytes which, on the contrary, was required to detect the immune response in mice inoculated with in vitro produced exosomes.13 This apparently stronger immunogenicity was likely a consequence of the continuous release from DNA-recipient muscle cells of immunogenic exosomes ready to be internalized by both local and distal antigen-presenting cells. Differently, it is known that both in vitro and ex vivo produced exosomes have a quite short half-life upon injection, ie, about 2 minutes, with a prompt accumulation in liver and spleen, and only residual amounts detectable after 4 hours.25–28 The detection of fluorescent exosomes in plasma of mice inoculated with Nefmut/GFP-expressing vector represented a key finding supporting the idea that engineered exosomes can indeed be produced upon DNA injection. The lack of Nef-specific CD8+ T-immune response in mice injected with wt Nef highlighted the importance for the immune response of the efficient association with exosomes, meanwhile excluding the involvement of other Nef functions. Similarly, no E7-specific CD8+ T-cell immune response was detectable in mice injected with vector expressing E7 alone, which per se associates with exosomes poorly, in the presence, however, of a well detectable anti-E7 antibody production. This result further supports the idea that high levels of antigen incorporation into exosomes are mandatory to elicit a strong antigen-specific CTL activity.
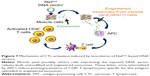
Most important, the key role of endogenously engineered exosomes in the antigen-specific immune response was demonstrated by the evidence that exosomes isolated from plasma of DNA-injected mice were immunogenic when inoculated in naïve recipient mice. On the other hand, the DNA-induced immune response appeared fast and strong enough to efficiently counteract the growth of syngeneic tumor cells implanted before the immunizations. The mechanism underlying the CD8+ T-cell activation induced by inoculation of Nefmut-based DNA vectors are depicted in Figure 7. Muscle cells (and, possibly, other cell types) expressing the injected DNA vector release engineered exosomes. These are expected to be internalized by antigen-presenting cells. The internalization of Nefmut-based exosomes leads to cross-presentation of the associated antigens and, by consequence, priming of antigen-specific CD8+ T-cells. Boosting of the immune response can be generated, besides the second round of DNA injection, by the continuous release of immunogenic exosomes from DNA recipient cells.
Conclusion
The data reported here allow us to propose a novel strategy to induce CTL immunity based on endogenously engineered exosomes. The major advantages of our vaccine platform are the cost-effectiveness of immunogens, ease of storage, potent immunogenicity, and significant intrinsic flexibility in terms of choice of the immunogen.
Acknowledgments
This work had Istituto Superiore di Sanità (ISS) review board approval and was supported by the grant of “Ricerca Finalizzata” project RF-2010-2308334 from the Ministry of Health, Italy. We thank Massimo Sanchez, ISS for help with the FACS analysis of free exosomes; Andrea Giovannelli, Claudia d’Agostino, Paolo Frassanito, and Carlo Giovannelli, ISS, for their support in animal housing and experimentation; and Pietro Arciero and Stefania de Menna, ISS, for technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16:24–43. | ||
Forterre A, Jalabert A, Berger E, et al. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? PLoS One. 2014;9:e84153. | ||
Guescini M, Guidolin G, Vallorani L, et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;16:1977–1984. | ||
Romancino DP, Paterniti G, Campos Y, et al. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 2013;587:1379–1384. | ||
Dai S, Wei D, Wu Z, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. | ||
Escudier B, Dorval T, Chaput N, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. | ||
Hartman ZC, Wei J, Glass OK, et al. Increasing vaccine potency through exosome antigen targeting. Vaccine. 2011;29:9361–9367. | ||
Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med. 2007;85:511–521. | ||
Usami Y, Popov S, Popova E, Inoue M, Weissenhorn W, Göttlinger HG. The ESCRT pathway and HIV-1 budding. Biochem Soc Trans. 2009;37:181–184. | ||
Lenassi M, Cagney G, Liao M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. | ||
Foster JL, Denial SJ, Temple BR, Garcia JV. Mechanisms of HIV-1 Nef function and intracellular signaling. J Neuroimmune Pharmacol. 2011;6:230–246. | ||
Lattanzi L, Federico M. A strategy of antigen incorporation into exosomes: comparing cross-presentation levels of antigens delivered by engineered exosomes and by lentiviral virus-like particles. Vaccine. 2012;30:7229–7237. | ||
Di Bonito P, Ridolfi B, Columba-Cabezas S, et al. HPV-E7 delivered by engineered exosomes elicits a protective CD8+ T cell-mediated immune response. Viruses. 2015;7:1079–1099. | ||
Keppler OT, Allespach I, Schüller L, Fenard D, Greene WC, Fackler OT. Rodent cells support key functions of the human immunodeficiency virus type 1 pathogenicity factor Nef. J Virol. 2005;79:1655–1665. | ||
D’Aloja P, Olivetta E, Bona R, et al. Gag, vif, and nef genes contribute to the homologous viral interference induced by a nonproducer human immunodeficiency virus type 1 (HIV-1) variant: identification of novel HIV 1-inhibiting viral protein mutants. J Virol. 1988;72:4308–4319. | ||
Massa S, Simeone P, Muller A, Benvenuto E, Venuti A, Franconi R. Antitumor activity of DNA vaccines based on the human papillomavirus-16 E7 protein genetically fused to a plant virus coat protein. Hum Gene Ther. 2008;19:354–364. | ||
Gorer PA. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950;4:372–379. | ||
Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Prot Cell Biol. 2006;3. | ||
Rieu S, Géminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000;267:583–590. | ||
Bauer S, Heeg K, Wagner H, Lipford GB. Identification of H-2Kb binding and immunogenic peptides from human papilloma virus tumour antigens E6 and E7. Scand J Immunol. 1995;42:317–323. | ||
Liang X, Fu TM, Xie X, Emini EA, Shiver JW. Development of HIV-1 Nef vaccine components: immunogenicity study of Nef mutants lacking myristoylation and dileucine motif in mice. Vaccine. 2002;20:413–421. | ||
Di Bonito P, Grasso F, Mochi S, et al. Serum antibody response to Human papillomavirus (HPV) infections detected by a novel ELISA technique based on denatured recombinant HPV16 L1, L2, E4, E6 and E7 proteins. Infect Agent Cancer. 2006;1:6. | ||
Arenaccio C, Chiozzini C, Columba-Cabezas S, Manfredi F, Federico M. Cell activation and HIV-1 replication in unstimulated CD4+ T lymphocytes ingesting exosomes from cells expressing defective HIV-1. Retrovirology. 2014;11:46. | ||
Greenberg ME, Bronson S, Lock M, Neumannn M, Pavlakis GN, Skowronski J. Co-localization of HIV-1 Nef with AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1998;16:6764–6776. | ||
Imai T, Takahashi Y, Nishikawa M, et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238. | ||
Morishita M, Takahashi Y, Nishikawa M, et al. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J Pharm Sci. 2015;104:705–713. | ||
Takahashi Y, Nishikawa M, Shinotsuka H, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84. | ||
Yamashita T, Takahashi Y, Nishikawa M, Takakura Y. Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood circulation. Eur J Pharm Biopharm. 2016;98:1–8. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.