Back to Journals » OncoTargets and Therapy » Volume 12
Antitumor effects of conditioned media of human fetal dermal mesenchymal stem cells on melanoma cells
Authors Sun B , Wang X, Pan Y, Jiao Y, Qi Y, Gong H, Jiang D
Received 2 February 2019
Accepted for publication 12 April 2019
Published 28 May 2019 Volume 2019:12 Pages 4033—4046
DOI https://doi.org/10.2147/OTT.S203910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Bencheng Sun,1–3,* Xiao Wang,1–2,4,* Yi Pan,1–2,4 Ya Jiao,1,2 Yongjun Qi,1–2,4 Hongmin Gong,1–2,4 Duyin Jiang1–2,4
1Department of Emergency, The Second Hospital of Shandong University, Jinan, Shandong Province, People’s Republic of China; 2Department of Emergency and Department of Burns and Plastic Surgery, The Second Hospital of Shandong University, Jinan, Shandong Province, People’s Republic of China; 3Department of Burn and Plastic Surgery, Linyi People’s Hospital, Linyi, Shandong Province, People’s Republic of China; 4School of Medicine, Shandong University, Jinan, Shandong Province, People’s Republic of China
*These authors contributed equally to this work
Background: Malignant melanoma is the most lethal form of cutaneous tumor and has a high metastatic rate and motility capacity. Owing to the poor prognosis, it is urgent to seek an effective therapeutic regimen. Human mesenchymal stem cells (MSCs) can home to tumor cells and have been shown to play important roles in both promoting and inhibiting tumor development. Fetal dermal MSCs (FDMSCs), derived from fetal skin are a novel source of MSCs. Nevertheless, the antitumor capacity of FDMSCs on malignant melanoma is not clearly understood.
Materials and methods: FDMSCs were extracted from the dorsal skin of fetal tissues. A375 melanoma cells lines were obtained from American Type Culture Collection. The effects of conditioned media from FDMSCs (CM-FDMSC) on A375 melanoma cells were tested in vivo using tumor formation assay and in vitro using cell viability, 5-ethynyl-2ʹ-deoxyuridine incorporation, flow cytometry, TdT-mediated dUTP Nick-End Labeling (TUNEL), wound healing, transwell invasion, and Western blotting.
Results: CM-FDMSC inhibited A375 tumor formation in vivo. In vitro, CM-FDMSC inhibited the tumor-related activities of A375 melanoma cells, as evidenced reductions in viability, migration, and invasion. CM-FDMSC-treated A375 cells showed decreased phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT), and extracellular signal-regulated kinase (ERK) phosphorylation, and up-regulation of Bcl-2-Associated X (BAX) and down-regulation of B-cell lymphoma-2 (BCL-2) expression.
Conclusion: CM-FDMSC can inhibit the tumor-forming behaviors of A375 melanoma cells and inhibit PI3K/AKT and mitogen-activated protein kinase signaling to shift their BCL-2/BAX ratio toward a proapoptotic state. Identification of the bioactive components in CM-FDMSC will be important for translating these findings into novel therapies for malignant melanoma.
Keywords: human fetal dermal mesenchymal stem cells, melanoma, conditioned media, apoptosis, PI3K/AKT signaling pathway, MAPK signaling pathway
Introduction
Malignant melanoma is one of the most aggressive skin tumors derived from malfunctioning of normal melanocytes. The incidence of melanomas continues to increase at a high rate, particularly in western populations.1,2 Although melanoma represents a small proportion of skin cancers, it accounts for 75% of skin cancer deaths in the United States.3,4 The increasing rate of morbidity of melanoma is attributed mainly to its invasive potential and high resistance to many conventional therapies.5,6 Therefore, it is urgent to develop alternative and innovative therapies to improve clinical outcomes.
Mesenchymal stem cells (MSCs) exist in various tissues, including bone marrow, adipose tissue, synovial membrane, periodontal ligament, and skin.7–10 MSCs are pluripotent progenitor cells and have shown potential in tissue engineering and regenerative medicine.11 Previous studies suggested that MSCs might become a promising treatment strategy for neurological dysfunctions, diabetic, cardiomyopathy, glaucoma, and urological diseases.12–15 Importantly, MSCs can effectively inhibit the development of some types of tumors.16–19 Fetal dermal MSCs (FDMSCs) can be isolated from aborted fetal skin and have the ability to differentiate into multiple cell types, although their full characteristics are still under investigation. Our previous study showed that paracrine factors secreted by FDMSCs could inhibit keloid growth.20
Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway regulates most hallmarks of cancer, including cell survival, metabolism, motility, invasion, and genomic instability.21 Activation of the PI3K pathway can promote tumor development by enhancing cell survival. Mitogen-activated protein kinase (MAPK) signal pathway plays an important role in many biological functions such as cell proliferation, adhesion, survival, and differentiation. It also participates in tumorigenesis and regulates the apoptotic process. MAPK pathway is activated in most melanomas because of the oncogenic serine-threonine protein kinase B-RAF (BRAF) mutations. Inhibiting MAPK pathway shows therapeutic benefit in melanoma treatment.22–24 Thus, we hypothesized that the antitumor effects of FDMSCs were mediated by paracrine manner through PI3K/AKT and MAPK signaling pathways. Our studies revealed that CM-FDMSC inhibits A375 cell behaviors associated with tumor formation in vivo and in vitro. Our data supports a model wherein biologically active factors present in CM-FDMSC inhibit PI3K/AKT and MAPK signaling to promote apoptosis of A375 melanoma cells and suggests that FDMSC-derived paracrine factors could lead to novel therapeutic approaches for melanoma.
Methods and materials
Cell culture and preparation of conditioned media from FDMSCs
Our research was conducted in accordance with the Declaration of Helsinki. All of the patients or their guardians provided written informed consent, and we received the ethical approval of the Ethics Committee of the Second Hospital of Shandong University, Jinan, China, in fetal skin isolation. The ethics certificate was issued on 1st June, 2017 and the certificate number is KYLL-2018(LW)-006. FDMSCs were extracted from the dorsal skin of fetal samples obtained from the Second Hospital of Shandong University and identified as described in our previous study.20 A375 melanoma cells lines were obtained from American Type Culture Collection and were verified by short tandem repeats analysis. FDMSCs and A375 melanoma cells lines were cultured in “complete medium” consisting of DMEM/Ham’s F-12 50/50 (F-12 50/50 Mix) with L-glutamine and 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Corning, USA), containing 10% FBS (Gibco, USA) and 1% 100 U/mLpenicillin-streptomycin (Gibco, USA). Cells were maintained in 100 mm culture dishes (Corning, USA) at 37°C, 5% CO2 in saturated humidity conditions. When FDMSCs reached 80% confluence, the media were changed to 5 mL serum-free DMEM/F-12 (serum-free medium containing 1% 100 U/mL penicillin-streptomycin, SFM), with the media harvested after 48 hrs of incubation and filtered through a 0.45 µm MILLEX-HP syringe filter (Millipore, USA) to generate 1× concentration of FDMSC conditioned medium (CM-FDMSC). To generate 0.5× CM, 1× CM was diluted 1:1 with fresh SFM; to generate 2× CM, 1× CM was concentrated with Amicon® Ultra-15 10K Centrifugal Filter Devices (Millipore, USA) rotated at 2000 (×g) for approximately 4 mins.
Animal assay
Studies were performed in 4–6-week-old female BALB/c nude mice, with a total of 24 mice divided randomly into 4 treatment groups of 6 each. To evaluate the antitumor effects in tumor nude mice model, 1×107 A375 melanoma cells were suspended in 200 μL of different concentrations of CM-FDMSC (control SFM, 0.5× CM, 1× CM, 2× CM) and injected into the right dorsal flank. Tumor volumes were recorded every 3 days with calipers, and tumor volumes (in mm3) were calculated using the formula V=length×width2/2. On the 17th day, mice were euthanized, and tumors were excised, weighed, and collected for histopathological examination by H&E and immunohistochemistry (IHC). In IHC, anti-KI-67 (abcam cat. no. ab15580, 1:500, rabbit), anti-Collagen IV (ZSGB-bio cat. no. ZM-0081, 1:200, mouse), and anti-Fibronectin (ZSGB-bio, cat. no. ZA-0106, 1:200, rabbit) primary antibody were used. Animal experiments were approved by the Ethics Committee of the Second Hospital of Shandong University. Our animal experiments were conducted in accordance with the recommendations of the United Kingdom Co-ordinating Committee on Cancer Research 1998.
Cell viability assay
To determine the effect of different concentrations of CM-FDMSC, 2×103 A375 melanoma cells per well were seeded in 96-well plates and cultured in SFM for 24 hrs, after which media were changed to CM-FDMSC (0.5×, 1×, 2×) or control (SFM) media and incubated for an additional 48 hrs. The proportion of viable cells was evaluated using a Cell Counting Kit (CCK)-8 assay (Beyotime; Shanghai, China) according to the manufacturer’s protocol. Briefly, 20 µL CCK-8 solution was added to each well and incubated for 3 hrs at 37°C. The absorbance of each well (containing six auxiliary holes) was measured in 450 nm wavelength using the Victor spectrophotometer (Thermo Fisher Scientific, USA) in three independent experiments.
5-Ethynyl-2ʹ-deoxyuridine (Edu) assay
EdU incorporation was performed using the BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 488 (Beyotime; Shanghai, China) according to the manufacturer’s protocol. CM-FDMSC-treated and control (SFM)-treated cells (1×106/well) were seeded in 6-well plates (Corning, USA) for 48 hrs and then incubated with the same volume of EdU solution for 2 hrs at 37°C. Cells were fixed with 4% paraformaldehyde for 15 mins, permeabilized with 0.3% TritonX-100 for 15 mins, and incubated with 500 μL of click-reactive solution for 30 mins. All nuclei were counterstained with Hoechst 33342. Hoechst-positive nuclei were counted, scored for EdU staining, and the percentage of EdU-positive/Hoechst-positive nuclei CM-FDMSC calculated in at least three independent experiments.
Flow cytometry analysis
In dose-dependent apoptosis assay, A375 melanoma cells were treated with different concentrations of CM-FDMSC or control medium for 48 hrs, harvested, washed twice with PBS, and then treated using Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) flow cytometric assay kit (Invitrogen, USA) following the manufacturer’s instructions. More than 1.0×105 cells were detected. In time-dependent apoptosis assay, cells were treated by 2×CM for 0, 12, 24, 48, and 72 hrs.
TUNEL assay
A375 melanoma cells were treated with different concentrations of CM-FDMSC or control serum-free medium for 48 hrs and then incubated with TUNEL detecting solution (Beyotime; Shanghai, China) for 1 hr at 37°C. Nuclei were counterstained by DAPI. The proportion of apoptotic cell death was evaluated by calculating the percentage of DAPI-positive cells that were TUNEL-positive in five random fields in more than three independent experiments.
Wound healing
For the wound healing assay, A375 melanoma cells were cultured in 60-mm plates until confluent, and then each monolayer was scratched with a 200 μL pipette tip to create a mechanical wound. The cells were cultured in different concentrations of CM-FDMSC for 48 hrs. Bright-field images were captured using an inverted microscope (Nikon, Japan) equipped with Camera and NIS-Elements software (Nikon, Japan).
Transwell invasion assay
The upper chamber of transwells (8 µm pore size polycarbonate membrane, Corning Costar, USA) was coated with 50 µL Matrigel matrix (BD, Franklin Lakes, NJ, USA, 1:3 diluted with serum-free DMEM medium). A375 melanoma cells were suspended (5×105 cells/mL) in different concentrations of CM-FDMSC or control medium, with 200 µL of the suspension was added into the upper transwell chamber and complete medium (800 µL) was added to the lower chamber. After incubation for 14 hrs, cells were fixed with 4% paraformaldehyde, stained with crystal violet (Beyotime; Shanghai, China), and cells on the lower surface of the membrane were counted using a microscope at 200× magnification in different areas in three independent repeated experiments.
Western blotting assay
Western blotting was performed following standard protocols. Briefly, after CM-FDMSC treatment, A375 melanoma cells were lysed with protease and phosphatase inhibitors and total proteins were extracted using RIPA lysis buffer on ice. Proteins were boiled for 3 mins and separated on a 12% polyacrylamide gel. Protein expression and phosphorylation were monitored with specific antibodies and chemiluminescent horseradish peroxidase Substrate (Millipore, USA), and imaged with film (Carestream, China). The images were quantified using Image J. Primary antibodies used in this study were as follows: anti p-PI3K p55/p85 (Cell Signaling Technology, cat. no. 4228, 1:1000, rabbit), anti- PI3K p85 (Cell Signaling Technology, cat. no. 4292, 1:1000, rabbit), anti p-AKT, (Cell Signaling Technology, cat. no. 4060, 1:1000, rabbit), anti-AKT (Cell Signaling Technology, cat. no. 9272, 1:1000, rabbit), anti p-p44/42 MAPK (Erk1/2) (Cell Signaling Technology, cat. no. 4370, 1:1000, rabbit), anti-p44/42 MAPK (Erk1/2) (Cell Signaling Technology, cat. no. 4695, 1:1000, rabbit), anti-BCL-2 (Cell Signaling Technology, cat. no. 2876, 1:1000, mouse), anti-BAX (Cell Signaling Technology, cat. no. 2772, 1:1000, rabbit) and anti-GAPDH (ZSGB-bio, cat. no. TA08, 1:1000, mouse). Secondary antibodies used were, Goat anti-mouse (ZSGB-bio, cat. no. ZB2305, 1:5000) and Goat anti-rabbit (ZSGB-bio, cat. no. ZB2301, 1:5000).
Statistical analysis
For each assay, data for each treatment condition were averaged from three or more independent experiments, and the mean and SD calculated. Student’s t-test was used for single comparisons. Time-course and multi-group analyses were performed using ANOVA. Tukey’s post hoc test was used to detect differences between groups. P-value <0.05 was considered as statistically significant. GraphPad Prism 5 and SPSS 11.5 software were used for graphs and statistical analysis.
Results
CM-FDMSC inhibits melanoma tumor growth in nude mouse
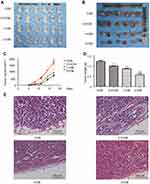
To detect the antitumor activity of CM-FDMSC, we used a melanoma mouse model, in which A375 melanoma cells were injected subcutaneously. The tumor volumes were continuously observed every 3 days and on day 17, tumors were harvested and weighted, respectively (Figure 1A and B). Tumor volumes and weight showed a dose-dependent decrease in CM-treated groups compared to control (Figure 1C and D). H&E and IHC staining of tumors showed histological differences among groups (Figures 1E and 2A–C). Qualitatively, in the control group, there were more tumor cells (containing more blue nuclei) with higher proliferative rate (containing more Ki67 positive cells), less connective tissues (lower collagen IV and fibronectin expression), and the tumor capsules were thin (indicated by the black arrows). With increasing concentrations of CM-FDMSC, the quantity and density of tumor cells decreased, as well as Ki67-positive cells. In addition, the tumor connective tissues became abundant, as shown in the IHC of collagen IV and fibronectin, and the thickness of tumor capsules increased. In 2× CM, a distinct tissue boundary formed (indicated by the black arrows). Collectively, these results demonstrated that CM-FDMSC inhibited tumor growth by A375 cells.
CM-FDMSC inhibits the viability of A375 melanoma cells
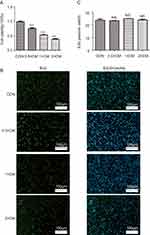
We measured the viability and proliferation of A375 melanoma cells in vitro using CCK-8 kit and EdU incorporation, respectively, to examine if CM-FDMSC can affect tumor cells vitro. There was a dose-dependent decrease in A375 melanoma cell viability with increasing concentrations of CM-FDMSC, as evidenced by changes in A450 absorbance by the formazan dye (Figure 3A). Despite the reduction in cell viability and the visible reduction in the density of Hoechst-positive nuclei in CM-FDMSC-treated A375 melanoma cells (Figure 3B), there were no significant differences in the percent of cells incorporating EdU across treatment groups (Figure 3C). The results demonstrated that the inhibitory effect of CM-FDMSC was attributable to effects on cell viability rather than cell proliferation.
CM-FDMSC induces the apoptosis of A375 melanoma cells
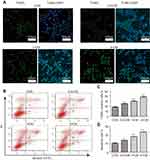
To test whether apoptosis contributed to reduced viability of CM-FDMSC-treated A375 cells, TUNEL and Annexin V-FITC/PI binding assays were performed. The percentage of A375 melanoma cells that were TUNEL/DAPI double positive increased significantly with increasing concentrations of CM-FDMSC (Figure 4A and C). Likewise, Annexin V-FITC/PI binding increased significantly with CM-FDMSC treatment in a dose- and time-dependent manner as measured by flow cytometry (Figures 4B, D, and 5A, B). Thus, CM-FDMSC reduces the viability of A375 melanoma cells by inducing apoptosis, rather than reducing proliferation.
CM-FDMSC inhibits migration and invasion of A375 melanoma cells
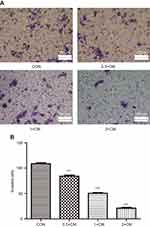
Another aspect of tumorigenicity is the ability of cells to migrate and metastasize. Therefore, monolayer wound healing assays were performed to examine whether CM-FDMSC could affect the migration capacity of A375 melanoma cells (Figure 6A). After 48 hrs of CM-FDMSC treatment, A375 melanoma cell migration was markedly reduced in a dose-dependent manner (Figure 6A and B). We next examined the effect of CM-FDMSC on the invasive capabilities of A375 melanoma cells using transwell invasion assays (Figure 7A). We found that pretreatment with CM-FDMSC significantly inhibited the invasive capacity of the A375 melanoma cells in a dose-dependent manner as evidenced by the reduction in the number of cells present on the lower surface of the membranes compared to controls (Figure 7B). Taken together, our results suggested that CM-FDMSC inhibited both the migration and invasion capacity of A375 melanoma cells.
The PI3K/AKT pathway, MAPK pathway, and mitochondria-mediated internal apoptosis pathway are regulated in A375 melanoma cells
The PI3K/AKT signaling is considered an important pathway in tumorigenesis, and the MAPK pathway is activated in most melanomas. To further find the underlying mechanism of apoptosis-promoting effect and determine whether the antitumor effect of CM-FDMSC is associated with altered PI3K/AKT and MAPK signaling, the expression of BAX, BCL-2, PI3K, AKT, and ERK and the phosphorylation of PI3K, AKT, ERK (Figure 8A–C) were observed by Western blot. CM-FDMSC induced significant increases in expression of the proapoptotic protein BAX and significant decreases in expression of the anti-apoptotic protein BCL-2 (Figure 8D and E), consistent with activation of the mitochondria-mediated internal apoptosis pathway. It is well known that the ratio of BCL-2/BAX is a significant indication of cell apoptosis.25,26 Thus, the dose-dependent decrease in the BCL-2/BAX ratio and increase in both TUNEL and Annexin staining of A375 melanoma cells with increasing concentrations of CM-FDMSC are consistent with induction of apoptosis. In addition, the phosphorylation of PI3K, AKT, and ERK decreased with increasing concentrations of CM-FDMSC (Figure 8F–H), although total PI3K, AKT, and ERK levels were not significantly different between groups. Thus, the decrease in the p-PI3K/PI3K, p-AKT/AKT, and p-ERK/ERK ratios with increasing concentrations of CM-FDMSC indicates inhibition of PI3K/AKT and MAPK signaling.
Discussion
Stem cell therapy has emerged as a promising strategy to prolong survival time and improve the treatment efficiency of patients with cancer compared to some traditional therapies. Previously, our team demonstrated that FDMSCs could accelerate apoptosis of keloid fibroblasts through regulating BCL-2 and BAX expression levels, providing a novel treatment therapy for keloid.20 Furthermore, another report revealed that human fetal bone marrow-derived MSCs could inhibit cell proliferation on hepatocellular carcinoma cells through reduced activation of insulin-like growth factor (IGF)-1R/PI3K/AKT signaling.19 Interestingly, skin MSCs were eventually shown to be derived from melanocyte precursors.27 Thus, we hypothesized that human FDMSCs would also exert antitumor effects on A375 melanoma cells, because of their lineage relationships with adult MSCs. Our previous studies confirmed that FDMSCs could accelerate the apoptosis of keloid fibroblasts through in a paracrine way, without direct cell contact.18,28 The results of the current study support our hypothesis and show that CM-FDMSC inhibits the bioactivity of A375 melanoma cells and has antitumor effects in vivo and in vitro.
CM-FDMSC contains factors that reduce various aspects of tumorigenicity of A375 melanoma cells. We confirmed that CM-FDMSC inhibits A375 melanoma cell expansion by inducing apoptosis of A375 melanoma cells, rather than inhibiting the proliferation, consistent with previous reports.29 In addition, migration and invasion abilities of A375 melanoma cells were suppressed by CM-FDMSC in both the monolayer wound healing and transwell invasion assays and again were consistent with previous reports.30 In contrast, conditioned media from bone marrow MSCs significantly promoted the progression of head and neck cancer progression by enhancing cell proliferation and migration, and activation of the PI3K/AKT/mTOR signaling pathway.31 Thus, the antitumoror pro-tumor effects of MSCs derived factors in CM-FDMSC are likely to depend on many factors, such as the cellular origin of the MSCs, the dose of MSC exposure, the tumor cell characteristics, and the specific microenvironment.
FDMSCs can express and secrete a set of biologically functional chemokines and tumor-suppressing agents, and a large body of studies focused on the specific components in CM-FDMSC that actually active in tumor inhibition. The candidate molecules are interferon-beta, tumor necrosis factor alpha, TGF-β1, and other immunomodulatory factors altering the tumor microenvironment and immune state.32–34 Studies also identified that Dickkopf-1 (DKK-1) secreted by MSCs can inhibit cancer cell growth and proliferation by negatively regulating Wingless/Integrated (WNT) signaling.35,36 In a recent report, the results showed that the anti-proliferative factor in human fetal MSCs to inhibit liver cancer growth may be a direct effect of proteolysis of IGF-binding protein-3.19 Therefore, the antitumor effect of CM-FDMSC may be caused by a combination effect of different molecular targeting different pathways. The precise mode of these candidate molecules exerting the inhibitor effect will require further investigations.
In our study, we also investigated the mechanism of CM-FDMSC-induced apoptosis of A375 melanoma cells at the protein level. BAX and BCL-2 are important markers for the mitochondria-mediate apoptotic pathway. Meanwhile, BAX and BCL-2 are downstream molecules of PI3K/AKT signaling pathway. In A375 melanoma cells, expression of BAX was up-regulated and expression of BCL-2 was down-regulated after treatment with CM-FDMSC. The down-regulation of PI3K, AKT, and ERK activity may lead to cell cycle arrest and cell apoptosis. We further showed that phosphorylation of PI3K, AKT, and ERK was decreased in CM-FDMSC-treated A375 melanoma cells, compared with the control groups. Our findings are in agreement with a previous study showing that relevant MSCs inhibit Kaposi’s sarcoma by a neutralizing antibody against E-cadherin through down-regulation of PI3K-AKT signaling.37 In the research by Han, umbilical cord tissue-derived MSCs were shown to inhibit tumor growth in co-culture condition, via activation of JNK and downregulation of PI3K/Akt and ERK signaling.38 Thus, our results can explain the effects of CM-FDMSC in A375 melanoma cells, such as apoptosis, migration, and invasion.
Fetal MSCs are considered as the new source of MSCs with great potential, and the advantages of fetal MSCs have been well documented that they are lower immunogenicity, easier expansion in vitro, and higher proliferation potential and differentiation capability compared to adult MSCs derived from adipose.19 Human umbilical cord blood is also a typical source of MSCs, but the successful isolating rate was much lower than bone marrow, umbilical cord, and adipose tissue.39–42 FDMSCs are relatively new players in cancer research and are showing potential for developing safe and effective clinical applications.43 Furthermore, FDMSCs cannot be recognized as targets by T lymphocytes in cell transplantation, and they can maintain the differentiation potential up to passage 25.44 Moreover, owing to the histological origin, FDMSCs may have some special advantages in wound healing and skin-origin disease, and FDMSCs are considered as the main effector cells in fetal skin scarless wound healing.45,46 Therefore, FDMSCs may be an ideal source for cell therapy. Conditioned media are a combination of cell paracrine factors. FDMSCs are high secretive capacity cells, and their functional roles are mostly depending on the paracrine factors in CM. The secretion profile in CM of FDMSCs is different from various tissues and reflects the unique advantages, cell biological characteristics, and intrinsic properties listed above. This study demonstrated the critical role of CM-FDMSC in A375 melanoma cells. Although we found the inhibition of PI3K/AKT and MAPK signaling may explain, at least in part, the inhibitory effects of FDMSCs on A375 melanoma cells, previous studies showed that a variety of signaling pathways were involved in A375 tumorigenicity, including Wnt/β-catenin signaling pathway, and nuclear factor-kappa B signaling pathway.47,48 In addition, the components in conditioned media are extremely complex, including growth factors, cytokines, extracellular vesicles, and other soluble molecules. Further investigations are required to explore the interactions between MSCs and cancer microenvironment. Also, the differences between CM of varied cells are in need of further investigation. Hence, the underlying mechanisms of CM-FDMSC antitumor effect require further investigation to isolate and characterize the active components secreted by FDMSCs and to extend our in vivo and in vitro findings to other melanoma cell lines, such as SK-Mel-28, WM-115, and MV3, and to primary melanomas. Ultimately, this research offers an exciting research direction with potential applications in developing novel clinical applications for treating melanoma.
Conclusion
In conclusion, our studies showed that CM-FDMSC can inhibit the tumor activity of A375 melanoma cells by suppressing the tumor formation in vivo, cell viability, migration, and invasion, and induces apoptosis of A375 melanoma cells in vitro in a dose- and time-dependent manner. The results showed that phosphorylation of PI3K, AKT, and ERK was decreased in A375 melanoma cells after different concentrations of CM-FDMSC treatment suggesting that the antitumor effects of CM-FDMSC on A375 melanoma cells may act, at least in part, through regulating PI3K/AKT and MAPK signaling pathways. Our studies highlight a promising treatment strategy for malignant melanoma.
Acknowledgments
This work was supported by The National Natural Science Foundation of China (grant no. 81873934), Science and Technology Development Projects of Shandong province (grant no. 2015GSF118041), Youth Fund of the 2nd Hospital of Shandong University (grant no. 2018YT14). We thank members of the central laboratory of the second hospital of Shandong University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Miller AJ, Mihm MC
2. Uzarska M, Czajkowski R, Schwartz RA, Bajek A, Zegarska B, Drewa T. Chemoprevention of skin melanoma: facts and myths. Melanoma Res. 2013;23(6):426–433. doi:10.1097/CMR.0000000000000016
3. Akabane H, Sullivan RJ. the future of molecular analysis in melanoma: diagnostics to direct molecularly targeted therapy. Am J Clin Dermatol. 2016;17(1):1–10. doi:10.1007/s40257-015-0159-z
4. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi:10.3322/caac.21220
5. Tuong W, Cheng LS, Armstrong AW. Melanoma: epidemiology, diagnosis, treatment, and outcomes. Dermatol Clin. 2012;30(1):113–124, ix. doi:10.1016/j.det.2011.08.006
6. Trunzer K, Pavlick AC, Schuchter L, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol. 2013;31(14):1767–1774. doi:10.1200/JCO.2012.44.7888
7. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi:10.1089/107632701300062859
8. De Bari C, Dell‘Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi:10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P
9. Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi:10.1016/S0140-6736(04)16627-0
10. Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778–784. doi:10.1038/ncb0901-778
11. Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi:10.1016/j.stem.2012.05.015
12. Saito F, Nakatani T, Iwase M, et al. Spinal cord injury treatment with intrathecal autologous bone marrow stromal cell transplantation: the first clinical trial case report. J Trauma. 2008;64(1):53–59. doi:10.1097/TA.0b013e31815b847d
13. Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29(1):5–10. doi:10.1002/stem.556
14. Manuguerra-Gagne R, Boulos PR, Ammar A, et al. Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells. 2013;31(6):1136–1148. doi:10.1002/stem.1364
15. Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. Jama. 2012;307(11):1169–1177. doi:10.1001/jama.2012.316
16. Qiao L, Xu Z, Zhao T, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18(4):500–507. doi:10.1038/cr.2008.40
17. Zhang L, Su XS, Ye JS, Wang YY, Guan Z, Yin YF. Bone marrow mesenchymal stem cells suppress metastatic tumor development in mouse by modulating immune system. Stem Cell Res Ther. 2015;6:45. doi:10.1186/s13287-015-0039-8
18. Zhang J, Hou L, Zhao D, et al. Inhibitory effect and mechanism of mesenchymal stem cells on melanoma cells. Clin Transl Oncol. 2017;19(11):1358–1374. doi:10.1007/s12094-017-1677-3
19. Yulyana Y, Ho IA, Sia KC, et al. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Mol Ther. 2015;23(4):746–756. doi:10.1038/mt.2015.13
20. Jiao Y, Wang X, Zhang J, Qi Y, Gong H, Jiang D. Inhibiting function of human fetal dermal mesenchymal stem cells on bioactivities of keloid fibroblasts. Stem Cell Res Ther. 2017;8(1):170. doi:10.1186/s13287-017-0624-0
21. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
22. Konieczkowski DJ, Johannessen CM, Abudayyeh O, et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 2014;4(7):816–827. doi:10.1158/2159-8290.CD-13-0424
23. Brighton HE, Angus SP, Bo T, et al. New mechanisms of resistance to MEK inhibitors in melanoma revealed by intravital imaging. Cancer Res. 2018;78(2):542–557. doi:10.1158/0008-5472.CAN-17-1653
24. Romano G, Chen PL, Song P, et al. A pre-existing rare PIK3CAE545K subpopulation confers clinical resistance to MEK plus CDK4/6 inhibition in NRAS melanoma and is dependent on S6K1 signaling. Cancer Discov. 2018. doi:10.1158/2159-8290.CD-17-0745
25. Mei JM, Niu CS. Effects of CDNF on 6-OHDA-induced apoptosis in PC12 cells via modulation of Bcl-2/Bax and caspase-3 activation. Neurol Sci. 2014;35(8):1275–1280. doi:10.1007/s10072-014-1700-1
26. Zhao L, Zhu L, Guo X. Valproic acid attenuates Abeta25-35-induced neurotoxicity in PC12 cells through suppression of mitochondria-mediated apoptotic pathway. Biomed Pharmacother. 2018;106:77–82. doi:10.1016/j.biopha.2018.06.080
27. Wong CE, Paratore C, Dours-Zimmermann MT, et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175(6):1005–1015. doi:10.1083/jcb.200606062
28. Lacerda L, Debeb BG, Smith D, et al. Mesenchymal stem cells mediate the clinical phenotype of inflammatory breast cancer in a preclinical model. Breast Cancer Res. 2015;17:42. doi:10.1186/s13058-015-0549-4
29. Yan F, Li X, Li N, et al. Immunoproapoptotic molecule scFv-Fdt-tBid modified mesenchymal stem cells for prostate cancer dual-targeted therapy. Cancer Lett. 2017;402:32–42. doi:10.1016/j.canlet.2017.05.003
30. Xie H, Liao N, Lan F, Cai Z, Liu X, Liu J. 3D-cultured adipose tissue-derived stem cells inhibit liver cancer cell migration and invasion through suppressing epithelial-mesenchymal transition. Int J Mol Med. 2018;41(3):1385–1396. doi:10.3892/ijmm.2017.3336
31. Liu C, Feng X, Wang B, et al. Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci. 2018;109(3):688–698. doi:10.1111/cas.13479
32. Shahrokhi S, Daneshmandi S, Menaa F. Tumor necrosis factor-alpha/CD40 ligand-engineered mesenchymal stem cells greatly enhanced the antitumor immune response and lifespan in mice. Hum Gene Ther. 2014;25(3):240–253. doi:10.1089/hum.2013.193
33. Kidd S, Caldwell L, Dietrich M, et al. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12(5):615–625. doi:10.3109/14653241003631815
34. Takahara K, Ii M, Inamoto T, et al. Adipose-derived stromal cells inhibit prostate cancer cell proliferation inducing apoptosis. Biochem Biophys Res Commun. 2014;446(4):1102–1107. doi:10.1016/j.bbrc.2014.03.080
35. Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269(1):67–77. doi:10.1016/j.canlet.2008.04.032
36. Zhu Y, Sun Z, Han Q, et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23(5):925–933. doi:10.1038/leu.2008.384
37. Khakoo AY, Pati S, Anderson SA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi‘s sarcoma. J Exp Med. 2006;203(5):1235–1247. doi:10.1084/jem.20051921
38. Han I, Yun M, Kim EO, Kim B, Jung MH, Kim SH. Umbilical cord tissue-derived mesenchymal stem cells induce apoptosis in PC-3 prostate cancer cells through activation of JNK and downregulation of PI3K/AKT signaling. Stem Cell Res Ther. 2014;5(2):54. doi:10.1186/scrt443
39. Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–18001. doi:10.3390/ijms140917986
40. Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi:10.1634/stemcells.2005-0342
41. Lv F, Lu M, Cheung KM, Leung VY, Zhou G. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr Stem Cell Res Ther. 2012;7(6):389–399.
42. Zeddou M, Briquet A, Relic B, et al. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 2010;34(7):693–701. doi:10.1042/CBI20090414
43. Hohlfeld J, de Buys Roessingh A, Hirt-Burri N, et al. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366(9488):840–842. doi:10.1016/S0140-6736(05)67107-3
44. Chinnici CM, Amico G, Monti M, et al. Isolation and characterization of multipotent cells from human fetal dermis. Cell Transplant. 2014;23(10):1169–1185. doi:10.3727/096368913X668618
45. Wegmeyer H, Broske AM, Leddin M, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013;22(19):2606–2618. doi:10.1089/scd.2013.0016
46. Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126(4):1172–1180. doi:10.1097/PRS.0b013e3181eae781
47. Pearlman RL, Montes de Oca MK, Pal HC, Afaq F. Potential therapeutic targets of epithelial-mesenchymal transition in melanoma. Cancer Lett. 2017;391:125–140. doi:10.1016/j.canlet.2017.01.029
48. Park WY, Hong BJ, Lee J, Choi C, Kim MY. H3K27 demethylase JMJD3 employs the NF-kappaB and BMP signaling pathways to modulate the tumor microenvironment and promote melanoma progression and metastasis. Cancer Res. 2016;76(1):161–170. doi:10.1158/0008-5472.CAN-15-0536
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.