Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Antioxidative and Hypoglycemic Effect of Ta-ermi Extracts on Streptozotocin-Induced Diabetes
Authors Jing S, Zhao Z, Wu J, Yan LJ
Received 14 April 2020
Accepted for publication 28 May 2020
Published 23 June 2020 Volume 2020:13 Pages 2147—2155
DOI https://doi.org/10.2147/DMSO.S258116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Siqun Jing,1 Zhengmei Zhao,2 Jinzi Wu,3 Liang-Jun Yan3
1Yingdong Food College, Shaoguan Unversity, Shaoguan, Guangdong 512005, People’s Republic of China; 2College of Life Sciences and Technology, Xinjiang University, Urumqi, Xinjiang 830046, People’s Republic of China; 3Department of Pharmaceutical Sciences, UNT System College of Pharmacy, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX 76107, USA
Correspondence: Siqun Jing; Liang-Jun Yan Tel/ Fax +86-0751-8120167
; Tel +1 817-735-2386
; Fax +1 817-735-2603
Email [email protected]; [email protected]
Introduction: The purpose of the present study was to reveal the potential positive effect of the Ta-ermi extracts on oxidative stress and streptozotocin (STZ)-diabetic mice and rats treated with Ta-ermi water- and alcohol-extracts.
Methods: The study was carried out using three experimental model: 1) in vitro experiments whereby Ta-ermi extracts were incubated with free radical generators such as 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) and 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) to evaluate Ta-ermi’s antioxidant effects; 2) testing the hypoglycemic effects of Ta-ermi extracts in streptozotocin (STZ)-induced diabetic mice; and 3) testing the beneficial effects of Ta-ermi extracts on mitochondrial complex I function using STZ-diabetic rats.
Results: In vitro antioxidant experiments showed that both of the extracts could scavenge free radicals and exhibited inhibitory effects on glucosidase and aldose reductase with differential effects between water extract and alcohol extract. In the STZ mouse diabetic model, both the water- and alcohol-extracts attenuated body weight decrease, decreased blood glucose levels in a concentration-dependent manner, improved insulin sensitivity, and increased oral glucose tolerance ability. In the STZ-diabetic rat model, both the water- and alcohol-extracts were found to be able to lower blood glucose levels in the diabetic animals with no effects on body weight changes. Moreover, in the STZ-diabetic rats, both the water- and alcohol-extracts of Ta-ermi could inhibit the increase of mitochondrial NADH/ubiquinone oxidoreductase (complex I) activity in the pancreas and enhanced complex I activity in the liver but showed no effect on lung or kidney mitochondrial complex I.
Discussion: The present study points to the potential medicinal value of Ta-ermi’s water and alcohol extracts in lowering blood glucose and decreasing diabetic oxidative stress. One limitation of our study is that the compound or compounds that actually have this beneficial effect in the extracts remain unknown at this time. Therefore, the future studies should be focused on the identification of the components in the extracts that exhibit anti-oxidative and hypoglycemic effects.
Conclusion: Taken together, our studies using different experimental paradigms indicate that Ta-ermi extracts possess antioxidant and anti-diabetic properties and may be employed as functional food ingredients for the remission of diabetes.
Keywords: Ta-ermi extracts, antioxidant activity, anti-diabetic, mitochondrial complex I, streptozotocin
Introduction
Diabetes mellitus (DM) is a group of chronic metabolic diseases characterized by continuously elevated blood glucose levels caused by defects in insulin secretion or insulin resistance. At present, the incidence of diabetes reaches 382 million people worldwide, and is expected to reach 592 million by 2035.1 The chronic feature of diabetes and its complications, together with the economic burden on the patient and their family members, have rendered diabetes the major heath issue on the globe. Type 2 diabetes is an age-associated disorder and a pandemic disease in many countries.2 It is a third leading cause of death following cardiovascular disease and cancer.3 While type I diabetes is due to β cell dysfunction that results in no insulin secretion, type 2 diabetes is an adult-onset disease caused by insulin resistance and dysregulation of glucose metabolism. The pathogenesis of type 2 diabetes is multifactorial, including genetics, environment, lifestyle, and obesity. It has been proven that unhealthy diet is a key risk factor for the formation and development of diabetes. Furthermore, type 2 diabetes can be prevented by diet interventions which can regulate the onset of diabetes and control of blood sugar. It is well known that natural products derived from a variety of organisms and diet regulation can serve an important fundamental measure of therapy for diabetes,4–6 and can effectively control the condition of diabetes and prevent and delay the complications of diabetes.7
Coarse cereals divided into three categories (beans, rice and wheat) are rich in trace elements, vitamins and dietary fiber, especially possess functional properties in terms of promoting intestinal peristalsis and helping diabetic patients to reduce insulin and triglyceride. Hence, there has been growing recognition that coarse cereals would become an ideal raw material for developing diabetic food. Liu et al have investigated this aspect in a diabetic mouse model induced by alloxan and found that a certain amount of quinoa supplemented in the diet can reduce blood glucose.8 Kim et al9 investigated the hypoglycemic effect of whole grain diet on type II diabetes in C57BL/KsJ-db/db mice and pointed out that whole grains can be employed as functional food ingredients to alleviate T2DM by enhancing the PI3K/Akt and AMPK pathways.
Ta-ermi (Panicum miliaceum L.), belonging to the family of coarse cereals, is a traditional food favored by Kazak people in Xinjiang, China with golden color and full grain whose shape is similar to the commonly known millet. It belongs to a genus of millet. Ta-ermi is mainly distributed in the cities of Yili, Tacheng and other places in Xinjiang with super stress resistance, excellent drought resistance, short growth cycle and high yield. In Ta-ermi, protein content is about 12%- 14%, starch content is about 70%, while sugar content is very low. It has been found that some ethnic minorities often add Ta-ermi to butter tea because it is thought to be a healthy food supplement that can lower blood pressure, blood sugar and blood lipids. Moreover, Ta-ermi is not only consumed by local people in China but also exported to other countries such as Russia and Kazakhstan. Our research group10 extracted Ta-ermi alcohol extract by ethanol reflux extraction with ethyl alcohol density 95%, 70%, 50% and 30%, and found that 30% ethanol extract had the strongest antioxidant activity. Thereafter, we used Ta-ermi to make soda crackers instead of wheat flour, which showed antioxidant effects.10 Our preliminary studies indicate that Ta-ermi contains active ingredients including amino acids, peptides, proteins, polysaccharides, organic acids, saponins, phenols, flavonoids, lactones, coumarins, terpenes, steroids, volatile oils and fats, etc. from its water extract, alcohol extract and petroleum ether extract obtained by systematic experimental methods.
Nonetheless, to the best of our knowledge, there has been no document regarding the anti-diabetic effects of Ta-ermi. Hence, we believe that it is quite necessary and significant to reveal the hypoglycemic effect and mechanism of Ta-ermi extract. The present study was thus aimed to: ⅰ) investigate the capabilities of Ta-ermi water extract and alcohol extract in terms of DPPH· radical scavenging, ABTS+· radical scavenging, inhibitory activity against α-Glucosidase11 and Aldose reductase; ⅱ) evaluate the glucose-lowering ability using streptozotocin (STZ)-induced diabetes in mouse and rat; ⅲ) the effects of Ta-ermi water extract and alcohol extract on mitochondrial complex I activities in the selected tissues isolated from STZ-diabetic rats. We herein report our findings that Ta-ermi extracts exhibit beneficial effects on diabetes. It should be noted that as animal-related experiments were conducted in different laboratories testing different aspects of Ta-ermi’s hypoglycemic effects, either mice or rats were used.
Materials and Methods
Materials
Raw and processed Ta-ermi were kindly provided by Da erhan Farmer’s Professional Cooperative in Tori county (Xinjiang, China) and was stored at 4°C before use. They were washed gently and dried at 50°C in an oven, and then grounded by universal high-speed smashing machines (FW-100, Beijing Yongguangming Medical instrument factory, China), and sieved through a 60 mesh screen. DPPH (1,1-diphenyl-2-trinitrophenylhydrazine) and ABTS (2,2-diazo-bis (3-ethyl-benzothiazole-6-sulfonic acid) diammonium salt were purchased from Shanghai Yuanye Biotechnology Co., Ltd. Alpha-D-glucosidase originated from Aspergillus niger was obtained from Beijing Suo Laibao Technology Co., Ltd., PNPG (4-Nitropheyl-alpha-D-glucopyranoside) was purchased from Shanghai Baoman Biotechnology Co., Ltd. Acarbose (Baitangping) was purchased from Bayer Medical and Health Co., Ltd. (Leverkusen, German). Streptozotocin (STZ) was purchased from Sigma Chemical Inc. (St Louis MO, USA). Insulin (INS) ELISA measurement was purchased from Jiancheng Bioengineering Research Institute (Nanjing, China). All other chemicals were of analytical grade and were obtained from Tianjin Reagent Company (Tianjin, China).
Preparation of STZ Solution
STZ solution was prepared with a mixed solution of equal volumes of A and B. Briefly, 1000 mg STZ was dissolved in 100 mL mixture solution (A:B=1:1), in which solution A was obtained by adding 2.1 g citric acid into 100 mL sterile water for injection while solution B was prepared by adding 2.94 g sodium citrate into 100 mL sterile water for injection, thus the pH value was 4.2~4.4. STZ solution was kept in dark and used within 30 min of preparation.
Preparation of Ta-ermi Extraction
Ta-ermi water extract was prepared with the water extraction and alcohol precipitation method basically as previously described with modifications.12 Briefly, extraction temperature was at 90°C, ratio of solid to liquid was 1:10. After two extractions, the filtrates were combined and concentrated, and precipitated with threefold volume of 80% ethanol. Finally, the precipitate was collected and freeze-dried. The yield reached 38.6 g extract per kg Ta-ermi raw material. Ta-ermi alcohol extract was obtained with traditional reflux extraction method. Briefly, extraction temperature was at 75°C, ethanol concentration was 70%, ratio of solid to liquid was 1:10. The material was extracted twice, and the filtrate was combined, evaporated and lyophilized using the method as previously described with modifications.13 The yield was up to 47.2 g powder/kg Ta-ermi raw material and the extract was stored at 4°C for further use.
Animals
Diabetes in Mouse Induced by STZ
Six- to eight-week-old Kunming male mice (20±2g) were obtained from Xinjiang Laboratory Medical University Breeding Research Center, and feeding condition was at 22–27°C, humidity of 50–70%, the light and dark alternated for 12 hrs, with 6 mice in each cage. The approved animal protocol number is 2016–0003/SCXK (Xin) and all experiments were carried out according to the guidelines and regulations of Xinjiang Medical University and also were in accordance with NIH guidelines for the Care and Use of Laboratory Animals. For diabetes induction, diabetic mouse was injected (IP injection) with STZ (180 mg/kg BW) while control mouse received a same volume of sodium citrate.14 Three days after SZT injection, blood glucose was determined by a tail-pricking method and mouse having blood glucose value equal or greater than 16.7 mM were considered diabetic. For Ta-ermi treatment of diabetic mice, 2 groups of mice were randomly assigned and Table 1 shows the treatment conditions for each group. All extracts, including distilled water, were administered once per day via gavage for 28 days. Body weight and blood glucose were both measured once per week after treatment was started.
 |
Table 1 Experiments in mouse grouping and treatment conditions |
Oral Glucose Tolerance Test in Mouse
At the end of the treatments, mice were fasted overnight. Then, 0.5% glucose was administered via gavage. Blood glucose was measured at 0.5 h, 1 h, 1.5 h, and 2 h after oral glucose ingestion.
Mouse Tissue Collection and Blood Insulin Measurement
For tissue collection, mouse was sacrificed by decapitation. This was followed by collection of tissues including liver, kidney, spleen, pancreas, and heart. Blood insulin measurement was carried out by an ELISA method and insulin resistance index was expressed as the following: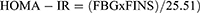
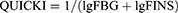
FINS: fasting blood insulin (mIU/L); FBG: fasting blood glucose (mM)
Diabetes in Rat Induced by STZ
For STZ induction of diabetes in rats, Sprague Dawley rats (4–6 weeks old) were purchased from Charles River. The animal protocol number was 2017–0005 and the studies were approved by the Institutional Care and Use Committee of University of North Texas Health Science Center and were in accordance with NIH guidelines for the Care and Use of Laboratory Animals. STZ was freshly prepared in 1 mL of 100 mM citrate buffer (pH 4.5) and injected (IP) to overnight fasted rats at a dosage of 60 mg/kg body weight. Blood glucose levels were determined using blood collected via tail-pricking by Free Style blood glucose test strips made by Abbot (Alameda, CA), and animals with blood levels exceeding 300 mg/dl were considered diabetic.3 Control rats received 1 mL citrate buffer only. For Ta-ermi treatment of STZ-diabetic rats, Ta-ermi was given via gavage once a day for 4 weeks, and both water extract (400 mg/kg) and alcohol extract (400 mg/kg) were administered to diabetic animals.15 Tissue mitochondrial preparation and blue native PAGE (BN-PAGE) analysis of complex I, a routine parameter for assessing mitochondrial dysfunction in our lab, were performed as previously described.16,17 All gel images were documented using an EPSON PERFECTION 1670 scanner. SDS-PAGE and Western blot assays were conducted according to standard protocols as previously reported.18
Assay of DPPH• Radical Scavenging Activity
The DPPH radical scavenging activity was quantified by the method of Pan et al with minor modifications.19 Briefly, 2 mL of 2×10−4 mole/L DPPH was added to 2 mL various concentrations (0.01–0.40 mg/mL) of polysaccharide samples in 70% (v/v) ethanol. The reaction mixture was incubated for 30 min at room temperature in dark. The DPPH radical scavenging activity was determined by measuring the absorbance at 517 nm with ascorbic acid being the positive control.20
Assay of ABTS+• Radical Scavenging Activity
An improved ABTS+•·radical de-colorization assay was carried out involving direct production of the blue/green ABTS+•·chromophore through the reaction between ABTS+•·and potassium persulfate. Addition of antioxidant to the preformed radical cation reduces it to ABTS+•·to an extent on a time scale depending on the antioxidant activity, the concentration of the antioxidant, and the duration of the reaction.21 One milliliter ABTS+·was dissolved in water to make 7 mM concentration of the resultant solution. ABTS+• radical was produced by reacting ABTS+• stock solution with 2.45 mM potassium persulfate. The resultant reaction mixture was allowed to stand in dark at room temperature for 12~16 h before use. The ABTS+• radical solution was diluted with methanol to an absorbance of 0.7 (±0.02) at 734 nm. Then, 100 μL of sample at various concentrations was mixed with 100 μL of diluted ABTS+• radical. Each concentration was analyzed in triplicate and the percentage decrease of absorbance at 734 nm with a microplate reader was calculated for each point and the antioxidant capacity of the tested compounds was expressed as percent inhibition (%).21 The radical scavenging activity of the compounds was measured according to the following equation:
IC50 values were calculated by linear regression of plots, and the average percentage of scavenging capacity was taken from three replicates. Trolox was used as a standard in comparison for the determination of the antioxidant activity.
Determination of α-Glucosidase Inhibitory Activity
The inhibitory effect of the compounds on α-glucosidase was assessed by a conventional method using 4-nitrophenyl-α-D-glucopyranoside (PNPG) as a substrate (0.2 mL 20mmol·L-1).15 Reaction solution contained the following: 2.0 mL 0.1 mol·L−1 potassium phosphate buffer (pH 6.8), 0.1 mL sample solution, 50 μL reduced glutathione (1 mg·mL−1), 0.1 mL α-glucosidase solution (0.57 U·mL−1), which were well mixed. After being pre-incubated for 15 min at 37°C, 0.2 mL of PNPG (20 mmol·L−1) was added and the mixture was further incubated at 37°C for another 15 min.22 The reaction was stopped by the addition of 10 mL of 0.1 mol·L−1 Na2CO3. The amount of 4-nitrophenol was measured spectrophotometrically at 400 nm. The α-glucosidase inhibitory activity was calculated as follows: α-glucosidase inhibitory activity (%) = [Ablank - (Asample - Abackground)]/Ablank × 100, Where Ablank, Asample, Abackground are defined as the absorbance of 100% enzyme activity (only the solvent with the enzyme), test sample with the enzyme and test sample without the enzyme, respectively.23 Acarbose was used as a positive control.
Effect of Ta-ermi Extracts on Aldose Reductase Activity
The effect of ta-ermi extracts on liver enzyme activity of aldose reductase was determined at 340 nm spectrophotometrically. The consumption of NADPH was measured for activity determination.24 The reaction mix in 1 mL contained D, L-glyceraldehyde (4.7 mM) as a substrate, NADPH (11 mM), phosphate buffer (0.067 M, pH 6.2), the enzyme solution (0.05 mL), and varying concentrations of water- or alcohol-extracts of Ta-ermi. The reference sample contained all the above reagents except the liver tissue sample.25 The molar extinction coefficient is 6.22 M-1 cm-1 at pH 6.2 for NADPH. One enzyme unit was defined as the amount of enzyme that catalyzes the hydrolysis of 1 μmol of substrate at 25°C.26
Statistical Analysis
Microsoft Excel statistical tools were used to analyze the in vitro antioxidant data, and SPSS software Statics 17.0 (SPSS, Chicago, USA) was used for in vivo antioxidant data processing. After completing single-factor variance analysis ANOVA, the least significant difference (LSD) was used to compare the five groups. A value of P < 0.05 was considered significant and a value of P < 0.01 was considered extremely significant.
Results
This study was conducted using three experimental systems with appropriate controls in each system: 1) in vitro effects on free radicals and α-glucosidase; 2) STZ-diabetic mice; 3) STZ-diabetic rats.
In vitro Effect of Ta-ermi Extracts on Free Radicals and α-Glucosidase
We first performed Ta-ermi’s antioxidation ability in vitro, and used ABTS+•· and DPPH· as the free radicals that can react with Ta-ermi extract. For the DPPH radical scavenging assay, vitamin c (Vc) was used as a positive control; while for the ABTS radical scavenging assay, Trolox was used as a positive control. As expected, all the two extracts scavenging ability was weaker than that of pure Vc or Trolox. While both water extract and alcohol extract have free radical scavenging ability, alcohol extract was more effective in both assays (Figure 1A and B). Therefore, the alcohol extract had a greater total antioxidant capacity than the water extract.
Effect of Ta-ermi Extract on α-Glucosidase
We then measured the inhibitory effect of Ta-ermi extracts on α-glucosidase. Results indicated that while both alcohol extract and water extract exhibited a concentration-dependent effect, alcohol extract was more effective than that of the water extract (Figure 1C). In addition, it seems that the effect of the water extract was similar to that of acarbose when the concentration was equal to or greater than 5 mg/mL.
Effects of Ta-ermi Extracts on Diabetes in STZ-Treated Mouse
Effect on Body Weight
We next tested the effects of Ta-ermi on STZ-diabetic mouse. Figure 2A shows Ta-ermi effects on body weight changes. As can be seen, except the control group (group A), the body weight of all diabetic mice exhibited a time-dependent decrease (Group B). For groups E and G, regardless of water or alcohol extracts, the body weight decrease in diabetic mice (shown in Group B) was also reversed by Ta-ermi, with the magnitude varied between water extract and alcohol extract. These results indicate that Ta-ermi extracts have a beneficial effect on diabetes.
Effects of Ta-ermi Effects on Blood Glucose in STZ-Induced Diabetic Mice
As shown in Figure 2B, both the water extract and alcohol extract of Ta-ermi could lower blood glucose levels in STZ-diabetic mice. It does not seem that the decrease in blood glucose is solvent dependent. However, when both water extract and alcohol extract were administered simultaneously, the decrease in glucose in the high dosage group was the most pronounced (data not shown). Such a result indicates that the water extract and the alcohol extract have a synergistic effect in lowering blood glucose in STZ-diabetic mice.
Effects on Insulin Resistance
Figure 2C shows that among all the groups tested, there was a significant (p< 0.05) decrease in insulin resistance in STZ-diabetic mice (Group B). All the Ta-ermi groups showed significant improvement in insulin resistance index, albeit that there was no difference between water extract and alcohol extract.
Effect on OGGT
Figure 2D shows that at 0.5 h after oral glucose ingestion, blood glucose was at its peak value among all the animal groups tested except the diabetic control group that had a peak glucose value at 1 h after oral glucose ingestion. At 2 hr after oral glucose ingestion, the blood glucose levels in water extract and alcohol extract groups were higher than healthy control but lower than in diabetic control. These results indicated that Ta-ermi extracts could improve glucose tolerance in STZ-diabetic mice.
Studies in STZ-Diabetic Rats
Effect of Ta-ermi on Aldose Reductase
It is well known that aldose reductase is activated in diabetes. We wondered whether Ta-ermi extract would have any effect on aldose reductase. To investigate this, diabetes in rats was induced by STZ injection. Four weeks after STZ injection, liver was collected and homogenized. The homogenate was added with Ta-ermi extract, and aldose reductase activity in homogenate was then measured spectrophotometrically. Results indicate that both water extract and alcohol extract exhibited inhibitory effects on the enzyme when expressed as a percentage of STZ (no extracts) (Figure 3A). Our result also indicates that the effect of water extract on aldose reductase activity was similar than that of the alcohol extract.
 |
Figure 3 Studies carried out using STZ-diabetic Sprague Dawley rats and corresponding healthy controls. Shown are effects on aldose reductase activity (A), blood glucose levels (B), body weight (C), and effects on mitochondrial complex I activities for each indicated tissues (D), whereby complex I activity was analyzed by BN-PAGE as previously described16,17 In (B), * indicates p<0.05 when compared with STZ only. |
Effects of Ta-ermi on Blood Glucose and Body Weight Changes of STZ Diabetes Rats
We STZ rats were treated with either water extract or alcohol extract for 4 weeks after STZ injection, we measured blood glucose levels and body weight of the treated rats. Results indicate that Ta-ermi had glucose-lowering ability (Figure 3B) but did not show any significant effect on body weight change (Figure 3C).
Effect on Mitochondrial Complex I
We have previously reported that pancreatic mitochondrial complex I activity is elevated in diabetes.27 To investigate whether Ta-ermi has any effect on complex I activity, we measured complex I activity in several tissues by BN-PAGE. While there was no significant effect observed in the lung and the kidney, both extracts inhibited an increase in complex I activity in the pancreas and enhanced complex I activity in the liver (Figure 3D). These results indicate that there might be cooperative effects between Ta-ermi and complex I in combating diabetic hyperglycemia in the pancreas and the liver.
Discussion
It is known that Ta-ermi has medical values. In the present investigation, we attempted to reveal the antioxidant and anti-diabetic potential of Ta-ermi by three experimental systems: in vitro free radical scavenging activities and effects on α-glucosidase; in STZ-diabetic mice and in STZ-diabetic rats. Firstly, our in vitro studies using water and alcohol extracts of Ta-ermi preparations have found that in vitro both extracts exhibit free radical scavenging capacity and the alcohol extract was more effective than the water extract. Interestingly, the alcohol extracts inhibited α-glycosidase activity more effectively than did the water extracts. While the reason for this discrepancy is unknown at this time, it is possible that the components in the alcohol extracts may bind to the enzyme more tightly.
Secondly, by in vivo studies using STZ mice, we found that both the water extract and the alcohol extract could decrease blood glucose, improve insulin sensitivity and glucose tolerance.
Thirdly, by using STZ-diabetic rats, we determined the effect of both water and alcohol extracts on body weight changes and blood glucose levels, while we did not observe any effects on body weight changes for STZ/water and STZ/alcohol groups, we did observe that both water and alcohol extracts could significantly lower blood glucose levels in the diabetic rats. While the magnitude of decrease was not huge, our results demonstrate that the extracts of Ta-ermi could be promising as anti-diabetic supplements.
In STZ rat study, we also determined the effect of the extracts on diabetes-related aldose reductase, an enzyme known to be activated by diabetes hyperglycemia in the poly pathway.2,28 In this regard, we determined the effects of both water and alcohol extracts. Our results indicate that certain components in both extracts exhibited an inhibitory effect on aldose reductase. Although the identity of these components remains unknown for the time being, our study points to the potential medicinal value of Ta-ermi extracts in lowering diabetic oxidative stress that can be caused by elevated aldose reductase activities.29
While we did not perform studies on the effects of either water or alcohol extract on overall mitochondrial function, we picked one enzyme, ie, complex I, for the evaluation of the extracts. Our results indicate that while the extracts did not show any detectable effects on mitochondrial complex I isolated from lung and kidney, the extracts exhibited an inhibitory effect on complex I activity increase in the pancreas and pronounced enhancing effect on liver mitochondrial complex I, indicating that the extracts of both water and alcohol preparation could fortify pancreatic or liver mitochondrial function in diabetes. It would be interesting to investigate the mechanism of liver mitochondrial complex I upregulation by these extracts in future studies. Also, it should be noted that in the pancreas mitochondria, our findings that pancreatic mitochondrial complex I was upregulated by STZ treatment further confirm our previous report that pancreatic mitochondrial complex I shows hyperactivity in diabetic pancreas due to persistent hyperglycemia. And this complex I upregulation in the pancreas could be mitigated by the Ta-ermi extracts (Figure 3D).
In summary, the present study demonstrates that Ta-ermi extracts could scavenge reactive oxygen species, inhibit both the enzyme activities of glucosidase and aldose reductase. Furthermore, the present study also demonstrates that Ta-ermi extracts could lower blood glucose levels and improve insulin sensitivity and glucose tolerance in STZ-diabetic rodents. Moreover, our study further indicates that in STZ-diabetic rats, Ta-ermi extracts had no effects on lung and kidney mitochondrial complex I activity, but inhibited complex I activity increase in the pancreas and enhanced complex I activity in the liver. Taken together, the present study indicates that Ta-ermi may have promising potential in fighting diabetes. Our findings are in line with those of others that herbal products or plant extracts including polyphenols, phytoconstituents present in water and alcohol extracts have therapeutic values.30–32
Acknowledgments
This work was supported by National Science Foundation of China (No. 31770385) and by Professor Research Start-up Fund of Shaoguan University (No. 433-99000611).
Disclosure
The authors report no conflict of interest in this work.
References
1. Lee EH, van der Bijl J, Shortridge-Baggett LM, Han SJ, Moon SH. Psychometric properties of the diabetes management self-efficacy scale in Korean patients with type 2 diabetes. Int J Endocrinol. 2015;2015:780701. doi:10.1155/2015/780701
2. Luo X, Li R, Yan LJ. Roles of pyruvate, NADH, and mitochondrial complex i in redox balance and imbalance in β cell function and dysfunction. J Diabetes Res. 2015;2015:1–12. doi:10.1155/2015/512618
3. Wu J, Jin Z, Yan LJ. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017;11:51–59. doi:10.1016/j.redox.2016.11.003
4. Ramachandran S, Rajasekaran A, Manisenthilkumar KT. Investigation of hypoglycemic, hypolipidemic and antioxidant activities of aqueous extract of Terminalia paniculata bark in diabetic rats. Asian Pac J Trop Biomed. 2012;2(4):262–268. doi:10.1016/S2221-1691(12)60020-3
5. Wen W, Lin Y, Ti Z. Antidiabetic, antihyperlipidemic, antioxidant, anti-inflammatory activities of ethanolic seed extract of annona reticulata l. In streptozotocin induced diabetic rats. Front Endocrinol (Lausanne). 2019;10:716. doi:10.3389/fendo.2019.00716
6. Strugala P, D’zydzan O, Brodyak I, et al. Antidiabetic and antioxidative potential of the blue congo variety of purple potato extract in streptozotocin-induced diabetic rats. Molecules. 2019;24(17):3126. doi:10.3390/molecules24173126
7. Miller CK, Edwards L, Kissling G, Sanville L. Nutrition education improves metabolic outcomes among older adults with diabetes mellitus: results from a randomized controlled trial. Prev Med. 2002;34(2):252–259. doi:10.1006/pmed.2001.0985
8. Liu -Y-Y, Cai Y-X, Wang S-E, Zhao C-F. Effects of quinoa on blood glucose levels in diabetic mice. Acta Nutrimenta Sinica. 2019;41(3):261–264.
9. Kim C, Lee J, Kim MB, Hwang JK. Hypoglycemic effect of whole grain diet in C57BL/KsJ-db/db mice by activating PI3K/Akt and AMPK pathways. Food Sci Biotechnol. 2019;28(3):895–905. doi:10.1007/s10068-018-0533-8
10. Zhao Z-M, Lasheng Z, Jing S-Q, et al. Antioxidant effect of Ta-er-mi and its application in soda biscuit. Food Ferment Ind. 2017;43(9):166–170.
11. Cakar U, Grozdanic N, Pejin B, et al. Impact of vinification procedure on fruit wine inhibitory activity against α-glucosidase. Food Biosci. 2018;25:1–7. doi:10.1016/j.fbio.2018.06.009
12. Mehta P, Lohidasan S, Mahadik KR. Pharmacokinetic behaviour of clinically important TCM prescriptions. Orient Pharm Exp Med. 2017;17(3):171–188. doi:10.1007/s13596-017-0281-y
13. Mehta P, Dhapte VV. Propulsive PAT paradigm: optimization of Freeze Drying Process. Int J Pharm Sci Rev Res. 2014;28(2):240–246.
14. Bhakkiyalakshmi E, Sireesh D, Sakthivadivel M, Sivasubramanian S, Gunasekaran P, Ramkumar KM. Anti-hyperlipidemic and anti-peroxidative role of pterostilbene via Nrf2 signaling in experimental diabetes. Eur J Pharmacol. 2016;777:9–16. doi:10.1016/j.ejphar.2016.02.054
15. Zhang Y, Ren C, Lu G, et al. Anti-diabetic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int Immunopharmacol. 2014;22(1):248–257. doi:10.1016/j.intimp.2014.06.039
16. Luo X, Wu J, Jin Z, Yan LJ. Non-gradient blue native polyacrylamide gel electrophoresis. Curr Protoc Protein Sci. 2017;87(1):1929 11–19 29 12. doi:10.1002/cpps.21
17. Yan LJ, Forster MJ. Resolving mitochondrial protein complexes using nongradient blue native polyacrylamide gel electrophoresis. Anal Biochem. 2009;389(2):143–149. doi:10.1016/j.ab.2009.03.043
18. Yan LJ, Sohal RS. Gel electrophoretic quantitation of protein carbonyls derivatized with tritiated sodium borohydride. Anal Biochem. 1998;265(1):176–182. doi:10.1006/abio.1998.2868
19. Pan YM, Wang K, Huang S, et al. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel. Food Chem. 2008;106(3):1264–1270. doi:10.1016/j.foodchem.2007.07.033
20. Jing S, Zhang X, Yan LJ. Antioxidant activity, antitumor effect, and antiaging property of proanthocyanidins extracted from Kunlun Chrysanthemum flowers. Oxid Med Cell Longev. 2015;2015:983484. doi:10.1155/2015/983484
21. Guo G, Yue L, Fan S, Jing S, Yan LJ. Antioxidant and antiproliferative activities of purslane seed oil. J Hypertens. 2016;5(02):2. doi:10.4172/2167-1095.1000218
22. Montori-Grau M, Tarrats N, Osorio-Conles O, et al. Glucose dependence of glycogen synthase activity regulation by GSK3 and MEK/ERK inhibitors and angiotensin-(1-7) action on these pathways in cultured human myotubes. Cell Signal. 2013;25(5):1318–1327. doi:10.1016/j.cellsig.2013.02.014
23. Habicht SD, Ludwig C, Yang RY, Krawinkel MB. Momordica charantia and type 2 diabetes: from in vitro to human studies. Curr Diabetes Rev. 2014;10(1):48–60. doi:10.2174/1573399809666131126152044
24. Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep. 2007;7(5):373–380. doi:10.1007/s11910-007-0058-7
25. Ren C, Zhang Y, Cui W, et al. A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. Int J Biol Macromol. 2015;72:951–959. doi:10.1016/j.ijbiomac.2014.09.060
26. Bezborodkina NN, Chestnova AY, Okovity SV, Kudryavtsev BN. Activity of glycogen synthase and glycogen phosphorylase in normal and cirrhotic rat liver during glycogen synthesis from glucose or fructose. Exp Toxicol Pathol. 2014;66(2–3):147–154. doi:10.1016/j.etp.2013.12.001
27. Wu J, Luo X, Thangthaeng N, et al. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem Biophys Rep. 2017;11:119–129. doi:10.1016/j.bbrep.2017.07.007
28. Yan LJ. Redox imbalance stress in diabetes mellitus: role of the polyol pathway. Animal Model Exp Med. 2018;1(1):7–13. doi:10.1002/ame2.12001
29. Wu J, Jin Z, Zheng H, Yan LJ. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab Syndr Obes. 2016;9:145–153. doi:10.2147/DMSO.S106087
30. Mehta P, Dhapte V. A comprehensive review on pharmacokinetic profile of some traditional Chinese medicines. N J Sci. 2016;2016:7830367. doi:10.1155/2016/7830367
31. Mehta P, Shah R, Lohidasan S, Mahadik KR. Pharmacokinetic profile of phytoconstituent(s) isolated from medicinal plants – a comprehensive review. J Tradit Complement Med. 2015;5(4):207–227. doi:10.1016/j.jtcme.2014.11.041
32. Tesanovic K, Pejin B, Sibul F, et al. A comparative overview of antioxidative properties and phenolic profiles of different fungal origins: fruiting bodies and submerged cultures of Coprinus comatus and Coprinellus truncorum. J Food Sci Technol. 2017;54:430–438. doi:10.1007/s13197-016-2479-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


