Back to Journals » Journal of Inflammation Research » Volume 15
Antioxidant Mitoquinone Alleviates Chronic Pancreatitis via Anti-Fibrotic and Antioxidant Effects
Authors Li M, Yuan Y, Han X, Liu X, Zhang W, Hao J
Received 7 January 2022
Accepted for publication 4 July 2022
Published 3 August 2022 Volume 2022:15 Pages 4409—4420
DOI https://doi.org/10.2147/JIR.S357394
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zili You
Miaomiao Li,1,* Yue Yuan,1,* Xue Han,2 Xinjuan Liu,1 Weizhen Zhang,2 Jianyu Hao1
1Department of Gastroenterology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Physiology and Pathophysiology, Peking University Health Science Center, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianyu Hao, Department of Gastroenterology, Beijing Chao-Yang Hospital, Capital Medical University, No. 8, South Road of Workers Stadium, Chaoyang District, Beijing, 100020, Email [email protected] Weizhen Zhang, Department of Physiology and Pathophysiology, Peking University Health Science Center, No. 38, Xueyuan Road, Haidian District, Beijing, 100191, Email [email protected]
Background: Chronic pancreatitis (CP) is a long-term inflammatory disease of the pancreas that can be caused by various pathogenic factors. Oxidative stress (OS), which is associated with several pancreatic diseases, can induce pancreatic stellate cell (PSC) activation, leading to pancreatic fibrosis. Given the inefficacy of existing treatments for CP, in this study, our objective was to evaluate the therapeutic effect of the antioxidant, mitoquinone (MitoQ).
Methods: First, in vivo, we established a CP mouse model via the repeated injection of cerulein. Mice in the MitoQ group simultaneously received MitoQ daily. After 4 weeks of cerulein injection, pancreatic tissues from mice were evaluated by morphological changes and the expression of fibrosis markers. Further, OS in the collected pancreatic tissue samples was evaluated by determining the level of malondialdehyde (MDA) as well as the expression levels and activities of antioxidants. Furthermore, in vitro, the effect of MitoQ on human PSCs (hPSCs) was evaluated based on PSC activation markers and fibrotic phenotypes, and OS in these treated hPSCs was evaluated by measuring reactive oxygen species (ROS), MDA, and antioxidant levels.
Results: In vivo, MitoQ alleviated pancreatic fibrosis and inhibited OS in the cerulein-induced murine CP model. In vitro, it inhibited PSC activation as well as the subsequent development of the profibrogenic phenotypes by balancing out the levels of free radicals and the intracellular antioxidant system.
Conclusion: MitoQ is a potential candidate for CP treatment.
Keywords: chronic pancreatitis, pancreatic stellate cells, pancreatic fibrosis, oxidative stress, mitochondria-specific antioxidant, MitoQ, superoxide dismutase
Introduction
Chronic pancreatitis (CP), which is characterized by irreversible damage to the pancreas and leads to endocrine and exocrine insufficiency, is a long-term inflammatory disease of the pancreas caused by various pathogenic factors.1 Studies have revealed that patients with CP are at approximately eight-fold higher risk of developing pancreatic cancer than their healthy counterparts.2 Further, current treatments of CP lack efficacy. Reportedly, the main pathological feature of CP is pancreatic fibrosis,3 and pancreatic stellate cells (PSCs) play an indispensable role in its development.4 Normally, PSCs are quiescent, and they are located in the periacinar region, where they regulate the production of extracellular matrix (ECM).5 However, as a response to pancreatic injury, they transform into myofibroblast-like cells as evidenced by an increased expression of alpha-smooth muscle actin (α-SMA) and extracellular matrix components (EMCs).6,7 Recently, PSC activation has drawn increased attention owing to its association with CP,8–10 and given that any agent that can suppress PSC activation might be a potential therapeutic option for patients with CP, it is important to clarify the mechanism of PSC activation.
Oxidative stress (OS) is caused by an imbalance between the production and removal of reactive oxygen species (ROS). Several studies have indicated that OS exists in pancreatic diseases, such as type 2 diabetes mellitus, CP, and pancreatic ductal adenocarcinoma.11–14 With advances in research, it has been demonstrated that OS possibly plays an important role in PSC activation. Based on experiments involving the use of CP animal models, it has been reported that antioxidant supplementation significantly alleviates PSC activation induced by hypoxia11 or hyperglycemia,15 thereby inhibiting pancreatic fibrosis.16,17
Intracellular ROS primarily originate from mitochondria, and their accumulation in this organelle leads to its dysfunction, which ultimately results in cell damage and diseases.18–20 Several advances have been made with respect to mitochondria-targeted antioxidant administration over the years. To date, mitoquinone (MitoQ) is reportedly the best characterized mitochondria-targeted antioxidant.21 Using preclinical models of liver diseases, it has also been demonstrated that MitoQ is an effective therapeutic strategy in various liver diseases.22–24 Therefore, in this study, we aimed to clarify the effect of MitoQ on CP and PSCs activation with the ultimate objective of developing an effective therapeutic strategy for fibrosis-related pancreatic diseases. Our results indicated that CP treatment using MitoQ could lead to the alleviation of pancreatic fibrosis and also attenuate PSC activation by suppressing intracellular OS.
Materials and Methods
Mice
Healthy mice, 6–7-week-old C57BL/6J (20–25 g), were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). The mice were housed under standard laboratory conditions (temperature 18–22°C, relative humidity 40–70%, 12 h/12 h light/dark cycle), the animals were reared for 1 week before the commencement of our animal experiments, which were approved by the Institutional Animal Care and Use Committee of Capital Medical University and performed in accordance with the ARRIVE guidelines.25
CP Model and Treatment
CP was induced via the repeated injection of mice with cerulein (Topscience, Shanghai, China) over a 4-week period (50 µg/kg) at 6-h intervals per day,3 times per week. Cerulein is frequently used to establish murine CP models in this field.26,27 Further, to evaluate the efficacy of MitoQ (MedChemExpress, Shanghai, China), mice in the MitoQ treatment group were administered MitoQ (MedChemExpress) via oral gavage daily (10 mg/kg) during the cerulein injection period. Finally, three days after the last cerulein (Topscience) injection, the mice were sacrificed, and pancreatic tissue samples were collected for observation.
Histology
Pancreas tissue was quickly removed after mice were deeply anesthetized with pentobarbital. After weighing the pancreatic tissue, they were rapidly rinsed thoroughly with phosphate buffered saline for sectioning, pancreas pieces were fixed in 4% paraformaldehyde. The degree of pancreatic fibrosis was evaluated by hematoxylin and eosin (H&E), Masson trichrome and Sirius red staining (performed by Servicebio technology). We quantified the pancreatic fibrosis by calculating the positive area in Sirius red staining.
Cell Culture
Primary human PSCs (hPSCs, ScienCell, San Diego, CA, USA) were cultured in stellate cell medium (ScienCell, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) at 37 °C in 5% CO2 as recommended. After seeding and culturing for at least 24 h, the cells were then treated with 100 nM MitoQ (MedChemExpress) for 48 h or vehicle (DMSO, Invitrogen; Carlsbad, CA, USA).
Cell Proliferation and EdU Incorporation Assays
Cell-counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) and the EdU incorporation assay kit (RiboBio, Guangzhou, China) were used to evaluate cell proliferation. To realize the cck-8 assay, the cells were seeded into 96-well plates at a density of 1000 cells per well. After adding CCK-8 to the culture medium followed by culturing for 2 h, the absorbance corresponding to each well was measured at 450 nm. Cell proliferation and viability were then calculated as previously described.28
Further, for EdU incorporation assay, the cells were seeded into 24-well plates at a density of 8000 cells per well. After treatment with MitoQ or vehicle for 48 h, 25 µM EdU was added followed by incubation for 2 h. Thereafter, the cells were fixed and incubated with Apollo 567 dye that can combine with EdU, and fluorescence intensities were measured using a fluorescence microscope (Leica, Wetzlar, Germany).
Flow Cytometry
A total of 15×104 cells were seeded into each well of a 6-well plate. After treatment with MitoQ or vehicle, the cells were harvested, and apoptosis was examined using an Annexin V-FITC/PI Apoptosis Detection Kit (KeyGEN, Jiangsu, China). Specifically, apoptotic cells were counted by flow cytometry (BD Biosciences, San Jose, CA, USA), and the data obtained were analyzed using FlowJo software version 10 (FlowJo LLC, Ashland, OR, USA).
Intracellular and Mitochondrial ROS Assessment
Cells were seeded in 96-well plates at the density of 1000 cells per well and treated with MitoQ or vehicle. DHE kit (Abcam, MA, USA) and MitoSOX Red (Invitrogen, CA, USA) were used according to the manufacturer’s protocols to measure intracellular ROS, respectively.
Measurement of Superoxide Dismutase (SOD) Activity
SOD activity (U/mg protein) in pancreatic tissues and hPSCs (15×104 cells seeded into each well) was measured using an SOD Assay Kit (NJJCBIO, Nanjing, China). In brief, pancreatic tissue and cell samples were homogenized and diluted 10 times using normal saline. After adding the reagents, the mixture was incubated at 37 °C for 20 min. Thereafter, absorbance at 450 nm was measured for each sample. Notably, the amount of enzyme that inhibited 50% of the reduction of WST-1 with the superoxide anion in 20 µL of the reaction system was defined as one unit of SOD activity.
Measurement of Glutathione (GSH) Content
The GSH content in the pancreatic tissues was measured using a GSH assay kit (NJJCBIO, Nanjing, China). First, pancreatic tissue samples were homogenized and diluted 10 times using normal saline. After adding the reagents for each step, the absorbance of each sample at 405 nm was measured. Next, GSH content was evaluated by measuring the amount of the yellow compound produced by the reaction of GSH with dithiodinitrobenzoic acid.
Measurement of MDA Content
The MDA content in serum and hPSCs (15×104 cells seeded into each well) was measured using an MDA Assay Kit via the colorimetric method (NJJCBIO, Nanjing, China). After adding the reagents, the mixture was incubated at 95 °C for 40 min. Next, the MDA content was evaluated by measuring the absorbance at 532 nm. Notably, MDA and thiobarbituric acid condense to form a red-colored mixture with absorbance at 532 nm.
Measurement of Catalase (CAT) Activity in hPSCs
CAT activity was measured using a CAT assay kit (NJJCBIO, Nanjing, China). In brief, 15×104 cells were seeded into each well of a 6-well plate. After treatment with MitoQ or vehicle, the cells were harvested and homogenized. First, the cell homogenate and H2O2 were mixed followed by incubation at 37 °C for 1 min, after which ammonium molybdate was added to the mixture. CAT enzymatic activity was then assessed via absorbance measurements at 405 nm, which is the absorbance corresponding to the amount of the complex composed of the remaining H2O2 and ammonium molybdate.
Immunofluorescence Staining
Immunofluorescence staining for α-SMA expression was performed to identify activated hPSCs. Specifically, 1×104 cells were seeded into each well of a 12-well plate. After treatment with MitoQ or vehicle for 48 h, the cells were fixed with 4% paraformaldehyde, and thereafter, blocked with 5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) solution for 40 min. Next, the cells were incubated with anti α-SMA monoclonal antibody (#7817; Abcam, Cambridge, MA, USA) overnight at 4 °C followed by incubation with the secondary antibody (Alexa Fluor 594-conjugated goat anti-mouse IgG; ZSGB-BIO, Beijing, China) for 2 h. Nuclei were then stained with DAPI (ZSGB-BIO, Beijing, China), and the quantity of α-SMA positive cells was determined by analyzing the images obtained via fluorescence microscopy (Leica, Wetzlar, Germany) using Image J software (National Institute of Health (NIH), Bethesda, MD, USA).
Immunohistochemical Staining
Immunohistochemical staining of α-SMA in pancreatic tissue was performed for evaluating the activation of PSCs after administration of MitoQ. After Paraffin sections dewaxed by xylene and ethanol (100%-70%), the antigens were retrieved and blocked by 5% goat serum. Then slides were incubated with α-SMA antibody (#ab124964, Abcam) at 4°C overnight. Then slides were incubated with horseradish peroxidase conjugated secondary antibodies (ZSGB-BIO) at 37°C for 20 min. The expression of α-SMA in slides were visualized using DAB substrate kit (ZSGB-BIO).
Western Blot Analysis
For protein extraction from hPSCs, a total of 15×104 hPSCs were seeded into each well of a 6-well plate. After treatment with MitoQ or vehicle for 48 h, proteins were extracted using radioimmunoprecipitation assay (RIPA) buffer (Solarbio, Beijing, China). For pancreatic tissue protein, the pancreatic tissue samples were diluted 10 times using RIPA and homogenized. Next, the samples were centrifuged at 12,000 g or 15 min at 4 °C and the concentrations of various proteins in the supernatant solution were measured using the BCA protein assay kit (Beyotime, Shanghai, China). To separate the proteins, 10% SDS–polyacrylamide gels (Beyotime) were used. Next, the proteins were transferred onto polyvinylidene difluoride (PVDF, Millipore, Billerica, MA, USA) membranes, and after blocking with 5% skimmed milk, the membranes were incubated overnight with primary antibodies at 4 °C (the list of primary antibodies used is provided in Supplementary Table 3. Thereafter, the membranes were incubated with a type of near-infrared fluorescent dye-conjugated secondary antibody (LI-COR, Lincoln, NE, USA) at room temperature for 1 h, and finally, scanned using an Odyssey infrared fluorescence imaging system (LI-COR). The images obtained were further analyzed using Image J software (NIH).
Real-Time Quantitative Polymerase Chain Reaction (RT-PCR)
Total RNA of hPSCs and pancreatic tissues were prepared by Trizol (Invitrogen). Reverse transcription of each sample was performed with PrimeScrip RT Master Mix (Taraka, Dalian, China). RT-PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems, Foster city, CA, USA). Primers (Invitrogen) were shown in Supplementary Tables 1–2.
Statistical Analysis
All the results are shown as Mean±SEM (Prism 8; GraphPad Software). The data was calculated by unpaired t-test by SPSS software (SPSS 19.0, Chicago, USA). There are at least three independent experiments in each group. p<0.05 was considered a significant difference.
Results
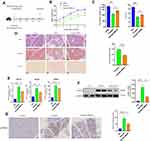
MitoQ Alleviated Cerulein-Induced Pancreatic Fibrosis in the CP Mouse Model in vivo
CP was induced in mice via the repeated administration of cerulein (50 µg/kg) at 6-h intervals each day three times a week over a period of 4 weeks as previously described.26,27 Further, mice in the MitoQ treatment group received MitoQ (10 mg/kg) daily via oral gavage during the 4-week period of cerulein injection (Figure 1A). Our results indicated that during the 4-week-long cerulein treatment period, the body weights of mice in the cerulein-only treatment group exhibited a downward trend, indicating a nutrient absorption disorder. The body weights of mice in the cerulein+MitoQ group also exhibited a decreasing trend; however, the degree of weight loss was much lower (Figure 1B). Further, the cerulein-only treated mice showed significant decreases in pancreas weight and the pancreas to body weight (p/w) ratio (Figure 1C), as well as obvious histological changes in the pancreatic tissue, including glandular atrophy, fibrosis, and inflammatory cell infiltration (Figure 1D). Further, Masson-trichrome and Sirius Red staining of the pancreatic tissue showed the deposition of collagen fibers in the pancreas of the CP mice (Figure 1D). These results indicated that the CP model was successfully established. Conversely, MitoQ treatment brought about increases in pancreas weight and the p/w ratio (Figure 1C). This treatment also decreased collagen deposition in the pancreas (Figure 1D) compared with that in the pancreatic tissue from cerulein-only treated mice.
In addition to the morphological changes observed in their pancreatic tissue, mice in the CP group also showed higher levels of fibrosis-related genes, such as collagen type 1 alpha (Col1a), collagen type III (Col III), and TIMP metalloprotease inhibitor 1 (Timp1), relative to the control mice. Interestingly, the expression levels of all these genes were downregulated following MitoQ treatment (Figure 1E). Further, α-SMA was identified as a key indicator for evaluating PSC activation. As shown in Figure 1F and G, Western blot analysis and immunohistochemistry indicated an increase in α-SMA level in the pancreas of mice in the cerulein-only treatment group, while in the cerulein+MitoQ treatment group, α-SMA expression was downregulated, suggesting that MitoQ possibly affects PSC activation.
Taken together, these findings suggested that MitoQ treatment can alleviate cerulein-induced pancreatic fibrosis in vivo, and its inhibitory effect may be due to the inhibition of PSC activation.
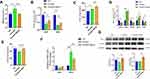
MitoQ Inhibited OS in Pancreatic Tissue from CP Mice
Reportedly, OS is involved in the progression of CP.1 Therefore, in this study, we examined the effect of MitoQ on the level of MDA, an indicator of OS, in serum. As showed in Figure 2A, the cerulein-only treatment group showed a higher MDA level, while MitoQ administration resulted in a decrease in MDA levels. This observation indicated that MitoQ exerts antioxidant effects. Reportedly, cells have antioxidant systems, comprising both enzymatic and non-enzymatic components that protect them against damage induced by oxygen free radicals.19 Therefore, to confirm the antioxidant capacity of MitoQ in CP, we detected the levels of both enzymatic and non-enzymatic antioxidants in pancreatic tissue samples from cerulein-only and cerulein+MitoQ-treated mice. SOD is an antioxidant enzyme that accelerates the conversion of superoxide anions to hydrogen peroxide,29 and in mammals, its exists in three subtypes, namely, SOD1, SOD2, and SOD3; specifically, SOD1 and SOD2 are widely expressed in cells.18 As shown in Figure 2B, the expression levels of genes encoding SOD1 (Sod1) and SOD2 (Sod2) were downregulated in pancreatic tissue from the CP mice. However, MitoQ treatment significantly enhanced the transcription of these genes (p < 0.05). Additionally, with respect to SOD enzyme activity, we observed that compared with the enzyme activity corresponding to mice in the control group, CP mice showed a higher level of SOD activity as a defense against OS. Additionally, the MitoQ treatment showed a protective effect in pancreatic tissue against ROS by further enhancing SOD activity (p < 0.05; Figure 2C). This treatment improved the transcription and expression of the genes encoding the glutathione, peroxidases 8 (GPX8), and CAT (p < 0.05; Figure 2D and G). Further, MitoQ enhanced the expression of GSH (Figure 2E), which belongs to the non-enzymatic antioxidant system. Reportedly, NADPH oxidase (NOX) is a major source of intracellular ROS;30,31 in this study, we observed that the MitoQ treatment showed the ability to attenuate NOX2 transcription (p < 0.05; Figure 2F).
Therefore, MitoQ could improve the defense capacity of the antioxidant system in pancreatic tissue during CP development.
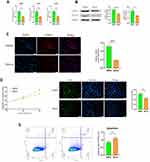
MitoQ Inhibited hPSC Activation as Well as the Development of Profibrogenic Phenotype in vitro
PSC activation plays an important role in CP development.32 In this study, we observed that activated PSCs resulted in increased α-SMA expression and the excessive secretion of ECM components, including COLI and COLIII. However, as illustrated in Figure 3A, MitoQ treatment for 48 h reduced α-SMA, COL1a, and COLIII mRNA levels (p < 0.05) as compared with their levels in the control group. Moreover, α-SMA and COL1a protein levels decreased significantly following the MitoQ treatment (p < 0.05; Figure 3B). To further evaluate α-SMA expression, we performed immunofluorescence staining on MitoQ-treated cells. As shown in Figure 3C, the fluorescence intensity of α-SMA observed for the MitoQ-treated cells was lower than that observed for the control cells (p < 0.05).
It has also been demonstrated that a high degree of hPSC cell proliferation will promote pancreatic fibrosis. Hence, we also evaluated the effect of MitoQ on the proliferation of hPSCs via cell-counting assays and EdU incorporation assays. As shown in Figure 3D, compared with the cell proliferation in the control group, the MitoQ treatment inhibited hPSC proliferation after 48 h of treatment. Moreover, flow cytometry showed that the apoptosis rate in the MitoQ-treated group was significantly higher than that in the control group (p < 0.05; Figure 3E).
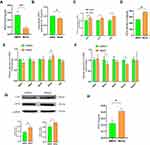
MitoQ Suppressed OS in hPSCs
OS promotes PSC activation.11,33 To verify whether MitoQ inhibited hPSC activation by suppressing OS, we examined its effects on intracellular MDA and OS. As shown in Figure 4A and B, MitoQ reduced intracellular MDA level and ROS generation, relative to the control. Moreover, it enhanced the defense system of the cell against ROS by upregulating the transcription of Sod1, Sod2, and Sod3 (Figure 4C). It also enabled the cells to overcome OS by increasing the SOD activity, as compared to that in the control group (Figure 4D). GPXs and CAT also play an important role in the antioxidant system. As shown in Figure 4E, G and H, MitoQ treatment improved the expression of GPX8 and CAT proteins, and also enhanced CAT activity compared with the control. NOX and dual oxidase (DUOX) enzymes, which are two main enzymes that promote ROS production, belong to the NADPH oxidase family;34 we observed that MitoQ treatment downregulated the mRNA expression levels of both Nox4 and Duox1 (Figure 4F).
Thus, MitoQ possibly exerted its inhibitory effect on hPSC activation by balancing the free radicals and the antioxidant system, thereby suppressing OS.
Discussion
CP is a long-term, irreversible, inflammatory disease of the pancreas that is accompanied by increased OS in pancreatic tissue.35 Specifically, when pancreatic tissue is affected by pathogenic factors, acinar cells are damaged by OS, resulting in increased neutrophil infiltration, and hence pancreatic inflammation36 as well as increased selective macrophage activation against the early pro-inflammatory effects of neutrophils. Further, under such conditions, macrophage activation initiates tissue repair, leading to the activation of PSCs.37,38 In recent years, several clinical studies have been conducted to evaluate the efficacy of antioxidant drugs as treatment for CP.39–42 In most of these clinical studies, the drug combination strategy was adopted; however, the effect of the antioxidant drugs on CP differed among the various clinical trials. Therefore, exploring new, powerful antioxidant drugs for CP treatment is necessary.
Mitochondria are the main source of ROS in cells;20 antioxidant drugs that target mitochondria are effective in reducing OS,19 which triggers PSC activation by activating the MAPK signaling pathway, resulting in α-SMA overexpression as well as enhanced cell proliferative and migratory abilities.43,44 Further, pro-oxidants promote the expression of fibromodulin via MAPK/AP-1 signaling, ie, the upregulation of fibromodulin favors PSC activation, while its knockdown inhibits PSC activation.33 Our observations in this study indicated that MitoQ can alleviate cerulein-induced pancreatic fibrosis in CP mice by reducing intracellular OS in pancreatic tissue, thereby suppressing the activation of hPSCs. However, in a previous study, it was reported that the administration of MitoQ seems to exacerbate acinar cell damage in cerulein-induced acute pancreatitis mice.45 In this previous study, MitoQ was administered via intraperitoneal injection twice before the first three injections of cerulein at 10 mg/kg and 25 mg/kg body weight. It was observed that inhibition of ROS suppressed protective apoptosis and enhances pancreatic acinar cell necrosis. In our study, the dose of MitoQ, which was administered via oral gavage, was 10 mg/kg body weight per day. Thus, our MitoQ dose was much lower than that used in the abovementioned previous study. This implies that the beneficial effects of an appropriate level of ROS were maintained at this dosage. However, further studies are needed to clarify the relationship between ROS level and pancreatic cell damage.
MitoQ has been used in clinical trials involving patients with hepatitis C. It has been observed that MitoQ administration decreases serum alanine transaminase (ALT) and aspartate aminotransferase (AST) levels by inhibiting mitochondrial oxidative damage.23 The results of another clinical trial indicated that MitoQ is beneficial to vascular endothelial protection.46 Further, the chronic administration of MitoQ has been known to exert protective effects on mitochondrial and nuclear genomes in human muscle tissues.47
Free radicals originate from the mitochondria during oxygen metabolism, and usually, O2 is reduced to the superoxide ion (O2•−), which in turn, can be reduced to H2O2 under the catalytic action of SOD or converted to other harmful oxygen radicals in the absence of SOD. Subsequently, H2O2 can be decomposed to form water (H2O) by catalase (CAT) or the GSH/GPx system, thereby converting the harmful free radicals into harmless non-free radicals.20,34 Conversely, loss of SOD activity increases OS-induced damage to DNA, lipids, and proteins.48,49 Previous studies have shown that the phenotype of Sod1−/− mice is characterized by extensive OS-induced damage to organs,18 accelerated cellular senescence,50,51 and prolonged tissue repair time.52 In this study, we observed that MitoQ could improve mRNA expression level and activity of SOD enzyme in the pancreatic tissue of CP mice as well as in hPSCs. Moreover, it could induce the increased expression of the GSH/GPx system and CAT. Therefore, it may exert its antioxidant effect by enhancing the conversion of free oxygen radicals to water.
In conclusion, MitoQ, a mitochondria-targeted antioxidant, has the potential to alleviate pancreatic fibrosis by decreasing OS in pancreatic tissue and inhibiting PSCs activation; possibly, it reduced intracellular OS by promoting SOD expression and activity and subsequently accelerating the scavenging of oxygen free radicals. One limitation of this study is that the mechanism by which MitoQ enhanced the expression and activity of antioxidants was not explored. Thus, further studies are necessary to determine how MitoQ upregulates the expression and activity of SOD, GPX8, and CAT, so that new and specific treatment targets can be developed for patients with CP.
Ethics Approval and Consent to Participate
Animal experiments were approved by the Institutional Animal Care and Use Committee of Capital Medical University.
Funding
This research was funded by the National Natural Science Foundation of China (Grant no. 82070656 and 81900587) and Golden Seed Research Fund (Grant no. CYJZ202144).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396(10249):499–512.
2. Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: a Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112(9):1366–1372.
3. Singh VK, Yadav D, Garg PK. Diagnosis and Management of Chronic Pancreatitis: a Review. JAMA. 2019;322(24):2422–2434.
4. Ferdek PE, Jakubowska MA. Biology of pancreatic stellate cells-more than just pancreatic cancer. Eur j Physiol. 2017;469(9):1039–1050.
5. Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43(1):128–133.
6. Xue R, Jia K, Wang J, et al. A Rising Star in Pancreatic Diseases: pancreatic Stellate Cells. Front Physiol. 2018;9:754.
7. Bynigeri RR, Jakkampudi A, Jangala R, et al. Pancreatic stellate cell: pandora’s box for pancreatic disease biology. World j Gastroenterol. 2017;23(3):382–405.
8. Ramakrishnan P, Loh WM, Gopinath SCB, et al. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm Sin B. 2020;10(3):399–413.
9. Zhao Q, Manohar M, Wei Y, Pandol SJ, Habtezion A. STING signalling protects against chronic pancreatitis by modulating Th17 response. Gut. 2019;68(10):1827–1837.
10. Li L, Wang G, Hu JS, et al. RB1CC1-enhanced autophagy facilitates PSCs activation and pancreatic fibrogenesis in chronic pancreatitis. Cell Death Dis. 2018;9(10):952.
11. Kim JJ, Lee E, Ryu GR, Ko SH, Ahn YB, Song KH. Hypoxia Increases β-Cell Death by Activating Pancreatic Stellate Cells within the Islet. Diabetes Metab J. 2020;44(6):919–927.
12. Ren Y, Zhang J, Wang M, et al. Identification of irisin as a therapeutic agent that inhibits oxidative stress and fibrosis in a murine model of chronic pancreatitis. Biomed Pharmacother. 2020;126:110101.
13. Tandon RK, Garg PK. Oxidative stress in chronic pancreatitis: pathophysiological relevance and management. Antioxid Redox Signal. 2011;15(10):2757–2766.
14. Daher B, Parks SK, Durivault J, et al. Genetic Ablation of the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. Cancer Res. 2019;79(15):3877–3890.
15. Kim JW, Park SY, You YH, et al. Suppression of ROS Production by Exendin-4 in PSC Attenuates the High Glucose-Induced Islet Fibrosis. PLoS One. 2016;11(12):e0163187.
16. Tasci I, Deveci S, Isik AT, et al. Allopurinol in rat chronic pancreatitis: effects on pancreatic stellate cell activation. Pancreas. 2007;35(4):366–371.
17. Xue R, Wang J, Yang L, et al. Coenzyme Q10 Ameliorates Pancreatic Fibrosis via the ROS-Triggered mTOR Signaling Pathway. Oxid Med Cell Longev. 2019;2019:8039694.
18. Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217(6):1915–1928.
19. Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1066–1077.
20. Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol Sci. 2017;38(7):592–607.
21. Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103.
22. Mukhopadhyay P, Horváth B, Zsengellėr Z, et al. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic Biol Med. 2012;53(5):1123–1138.
23. Gane EJ, Weilert F, Orr DW, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a Phase II study of hepatitis C patients. Liver Int. 2010;30(7):1019–1026.
24. Vilaseca M, García-Calderó H, Lafoz E, et al. Mitochondria-targeted antioxidant mitoquinone deactivates human and rat hepatic stellate cells and reduces portal hypertension in cirrhotic rats. Liver Int. 2017;37(7):1002–1012.
25. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412.
26. Klauss S, Schorn S, Teller S, et al. Genetically induced vs. classical animal models of chronic pancreatitis: a critical comparison. FASEB j. 2018;1:fj201800241RR.
27. Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144(6):1180–1193.
28. Li Z, Zhang X, Jin T, Hao J. Nicotine promotes activation of human pancreatic stellate cells through inducing autophagy via α7nAChR-mediated JAK2/STAT3 signaling pathway. Life Sci. 2020;243:117301.
29. Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem. 1997;272(30):18515–18517.
30. Martinez-Useros J, Li W, Cabeza-Morales M, Garcia-Foncillas J. Oxidative Stress: a New Target for Pancreatic Cancer Prognosis and Treatment. J Clin Med. 2017;6:3.
31. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313.
32. Sherman MH. Stellate Cells in Tissue Repair, Inflammation, and Cancer. Annu Rev Cell Dev Biol. 2018;34:333–355.
33. An W, Zhu JW, Jiang F, et al. Fibromodulin is upregulated by oxidative stress through the MAPK/AP-1 pathway to promote pancreatic stellate cell activation. Pancreatology. 2020;20(2):278–287.
34. Aviello G, Knaus UG. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 2018;11(4):1011–1023.
35. Swentek L, Chung D, Ichii H. Antioxidant Therapy in Pancreatitis. Antioxidants. 2021;10:5.
36. Gu H, Werner J, Bergmann F, Whitcomb DC, Büchler MW, Fortunato F. Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death Dis. 2013;4(10):e816.
37. Sendler M, van den Brandt C, Glaubitz J, et al. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis. Gastroenterology. 2020;158(1):253–69.e14.
38. Xue J, Sharma V, Hsieh MH, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158.
39. Singh N, Ahuja V, Sachdev V, et al. Antioxidants for Pancreatic Functions in Chronic Pancreatitis: a Double-blind Randomized Placebo-controlled Pilot Study. J Clin Gastroenterol. 2020;54(3):284–293.
40. Bhardwaj P, Garg PK, Maulik SK, Saraya A, Tandon RK, Acharya SK. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology. 2009;136(1):149–59.e2.
41. Siriwardena AK, Mason JM, Sheen AJ, Makin AJ, Shah NS. Antioxidant therapy does not reduce pain in patients with chronic pancreatitis: the ANTICIPATE study. Gastroenterology. 2012;143(3):655–63.e1.
42. Rustagi T, Njei B. Antioxidant therapy for pain reduction in patients with chronic pancreatitis: a systematic review and meta-analysis. Pancreas. 2015;44(5):812–818.
43. Masamune A, Kikuta K, Satoh M, Satoh A, Shimosegawa T. Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J Pharmacol Exp Ther. 2002;302(1):36–42.
44. Kikuta K, Masamune A, Satoh M, Suzuki N, Satoh K, Shimosegawa T. Hydrogen peroxide activates activator protein-1 and mitogen-activated protein kinases in pancreatic stellate cells. Mol Cell Biochem. 2006;291(1–2):11–20.
45. Huang W, Cash N, Wen L, et al. Effects of the mitochondria-targeted antioxidant mitoquinone in murine acute pancreatitis. Mediators Inflamm. 2015;2015:901780.
46. Rossman MJ, Santos-Parker JR, Steward CAC, et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension. 2018;71(6):1056–1063.
47. Williamson J, Hughes CM, Cobley JN, Davison GW. The mitochondria-targeted antioxidant MitoQ, attenuates exercise-induced mitochondrial DNA damage. Redox Biol. 2020;36:101673.
48. Van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16(1):29–37.
49. Elchuri S, Oberley TD, Qi W, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24(3):367–380.
50. Zhang Y, Unnikrishnan A, Deepa SS, et al. A new role for oxidative stress in aging: the accelerated aging phenotype in Sod1(-/)(-) mice is correlated to increased cellular senescence. Redox Biol. 2017;11:30–37.
51. Zhang Y, Ikeno Y, Bokov A, et al. Dietary restriction attenuates the accelerated aging phenotype of Sod1(-/-) mice. Free Radic Biol Med. 2013;60:300–306.
52. Iuchi Y, Roy D, Okada F, et al. Spontaneous skin damage and delayed wound healing in SOD1-deficient mice. Mol Cell Biochem. 2010;341(1–2):181–194.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.