Back to Journals » Journal of Inflammation Research » Volume 13
Antioxidant, Anti-Inflammatory and Anti-Apoptotic Activities of Nesfatin-1: A Review
Received 22 July 2020
Accepted for publication 29 August 2020
Published 28 September 2020 Volume 2020:13 Pages 607—617
DOI https://doi.org/10.2147/JIR.S273446
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yayun Xu,1– 3 Feihu Chen1– 3
1Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, People’s Republic of China; 2The Key Laboratory of Major Autoimmune Diseases of Anhui Province, Anhui Institute of Innovative Drugs, School of Pharmacy, Anhui Medical University, Hefei, People’s Republic of China; 3The Key Laboratory of Anti-Inflammatory and Immune Medicines, Ministry of Education, Hefei, People’s Republic of China
Correspondence: Feihu Chen
Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, 81 Meishan Road, Hefei 230032, People’s Republic of China
Email [email protected]
Abstract: Nesfatin-1, a newly identified energy-regulating peptide, is widely expressed in the central and peripheral tissues, and has a variety of physiological activities. A large number of recent studies have shown that nesfatin-1 exhibits antioxidant, anti-inflammatory, and anti-apoptotic properties and is involved in the occurrence and progression of various diseases. This review summarizes current data focusing on the therapeutic effects of nesfatin-1 under different pathophysiological conditions and the mechanisms underlying its antioxidant, anti-inflammatory, and anti-apoptotic activities.
Keywords: antioxidant, anti-inflammatory, anti-apoptotic, nesfatin-1, anorexigenic
Introduction
Nesfatin-1 was first discovered in the hypothalamic nuclei and was initially reported to reduce food intake.1 Studies have shown that nesfatin-1 is widely expressed in central and peripheral tissues and has pleiotropic effects.2 More recent evidence indicates that nesfatin-1 exerts antioxidant, anti-inflammatory, and anti-apoptotic effects in different inflammation-related diseases.3,4 In this review, we provide a brief account of the structure, localization, and functions of nesfatin-1, and mainly focus on the experimental evidence for the antioxidant, anti-inflammatory, and anti-apoptotic effects of nesfatin-1 and its possible mechanism.
Structure and Localization of Nesfatin-1
Nesfatin-1, an 82-amino acid peptide discovered by Oh-I in 2006, is derived from the precursor protein nucleobindin-2 (NUCB2).5 The product of the NUCB2 gene is a peptide with 420 amino acid (AA), which is composed of a 396 AA long precursor peptide and a 24 AA long signal peptide (SP). The precursor peptide is cleaved into three different parts by prohormone/proprotein convertase (PC) 1/3 and PC 2: nesfatin-1 (AA 1–82), nesfatin-2 (AA 85–163), and nesfatin-3 (AA 166–396).5 The structure of nesfatin-1 can also be divided into three segments, namely N23, M30, and C29,6 among which M30 is critical for its anorexigenic action.7,8 However, no obvious biological activity has been detected for nesfatin-2 or nesfatin-3.9 Figure 1 shows a schematic representation of nesfatin-1 processing from its precursor, NUCB2.
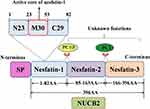 |
Figure 1 Schematic representation of the production of nesfatin-1 from its precursor NUCB2. |
Initially, the paraventricular nucleus (PVN), arcuate nucleus (ARC), supraoptic nucleus (SON), and lateral hypothalamic area (LHA) in the hypothalamus were thought to be the main sites of nesfatin-1 expression.5 Subsequent studies have shown that nesfatin-1 is widely expressed in other regions of the brain also, like brainstem, including dorsal vagal complex (DVC), Edinger-Westphal nucleus, locus coeruleus (LC), lateral parabrachial nucleus, ventrolateral medulla (VLM),10,11 insular cortex, central amygdala, ventrolateral medulla, cerebellar Purkinje cell, pterygopalatine parasympathetic preganglionic neurons, and spinal cord at the lumbar and sacral region.11 In addition to being widely expressed in the central nervous system, nesfatin-1 is also expressed in peripheral tissues, like esophagus,12 stomach, small intestine,13 colon, pancreas,14 liver, adipocytes,15 cardiomyocytes,16 testes,17 ovaries,18 uterus and epididymis.19
Functions of Nesfatin-1
As an energy-regulating peptide, nesfatin-1 is widely expressed in both central and peripheral tissues.20 This, along with the fact that nesfatin-1 can cross the blood-brain barrier,21,22 suggests that nesfatin-1 may have pleiotropic effects. In addition to maintaining the feeding balance, this peptide plays an important role in glucose homeostasis,23 lipid metabolism,24 modulation of gastrointestinal functions,25 cardiovascular,9 and reproductive functions.26 It may also be involved in epilepsy,27 psychological disorders, including stress,28 sleep disorders,29 anxiety,30 and depression.30
Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities of Nesfatin-1 in Multiple Diseases
Besides its anorexigenic and anti-hyperglycemic effects, nesfatin-1 has recently been reported to have potent anti-inflammatory, anti-apoptotic, and anti-oxidative capabilities, which can ameliorate the symptoms of several diseases (Figure 2).
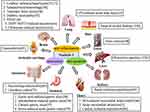 |
Figure 2 Nesfatin-1 exerts antioxidant, anti-inflammatory and anti-apoptotic effects in various diseases. |
Brain-Related Diseases
As cerebral ischemia/reperfusion (I/R) leads to neuroinflammation and neuronal apoptosis,31,32 prevention of these processes is recommended as a treatment to compensate for the brain damage.33 Erfani et al34 showed that nesfatin-1 could significantly improve the memory impairment caused by cerebral I/R by reducing the activity of caspase-3, an apoptosis-associated protein, in the pyramidal cells in CA1 area of the hippocampus, and decreasing the number of Iba-1 positive cells;34 Iba-1 is an immunohistochemical marker for activated microglia.35 Consistent with these results, another study36 demonstrated the usefulness of nesfatin-1 in the treatment of cerebral I/R through inhibition lipid peroxidation, increase in the expression of anti-apoptotic protein Bcl-2, and reduction of the Bax-mediated neuronal apoptosis. The neuroprotective effects of nesfatin-1 through its anti-inflammatory and anti-apoptotic activities has also been reported in two other brain-related diseases; reduction of caspase-3-mediated nerve cell apoptosis and inhibition of release of mediators of inflammation in the brain damage through trauma37 and through subarachnoid hemorrhage.38
Parkinson’s disease (PD), a neurodegenerative disease, is characterized by the loss of dopaminergic neurons in the substantia nigra of the brain.39 Increasing evidence indicate that PD is related to mitochondrial dysfunction through multiple pathways, including free radical generation, inflammation, and apoptosis.40,41 Numerous studies have shown that apoptosis, induced by mitochondrial dysfunction, plays a vital role in the incidence and development of PD,42,43 suggesting that inhibition of apoptosis may be one of the treatment strategies for PD. Recently, rotenone-treated MES23.5 dopaminergic cells, a cellular model of PD, were used to study the neuroprotective effect of nesfatin-1 in PD and to understand the underlying mechanisms. The results indicated that treatment with nesfatin-1 inhibited the production of rotenone-induced reactive oxygen species (ROS), release of mitochondrial cytochrome C, and subsequent activation of caspase-3.44 This suggested that nesfatin-1 exerts a neuroprotective effect in PD through its antioxidant and anti-apoptotic properties. More recently, the neuroprotective effect of nesfatin-1 in PD was further confirmed through in vivo and in vitro experiments, which showed that the anti-apoptotic C-Raf-extracellular signal-regulated protein kinase 1/2 (ERK1/2) signaling pathway mediates the protective effect of nesfatin-1 on dopaminergic neurons against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced neurotoxicity in C57BL/6 mice and 1-methyl-4-phenylpyridillium ion (MPP+)-induced cytotoxicity in MES23.5 cells.45
Diabetic neuropathy (DN), one of the most debilitating outcomes of diabetes mellitus, is thought to be the consequence of oxidative stress,46 inflammation,47 and neural apoptosis.48 More specifically, increased glucose levels damage the mitochondrial membrane and respiratory chain, resulting in the production of large amounts of ROS,49 which accelerate the damage of lipids, proteins, and nucleic acids, and eventually lead to inflammation and neural apoptosis.50 Due to its antioxidant, anti-inflammatory, and anti-apoptotic activities, nesfatin-1 may have an ameliorating effect on DN. It has been reported51 that nesfatin-1 inhibits intracellular ROS overproduction and reduces apoptotic cell death in PC12 cells following high-glucose exposure, a widely used in vitro model for DN, which makes nesfatin-1 a potential drug for the treatment of DN against high glucose-induced cell death.
Gastric Diseases
Expression of nesfatin-1 in the stomach is reported to be 20 times higher than that in the brain tissues in rats.52 Lipopolysaccharide (LPS) administration is known to increase the production and release of gastric NUCB2/nesfatin-1.53 Due to its anti-inflammatory properties, nesfatin-1 may have a protective effect against gastric injury.
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat various ailments. However, serious side effects of these drugs, such as bleeding, acute injury, and gastric ulcers, have been widely reported.54 Recently, potential anti-ulcer and anti-inflammatory effects of nesfatin-1 in a model of gastric ulcer induced by indomethacin, an NSAID with analgesic, antipyretic, and anti-inflammatory effects, were studied.55 The results showed thatnesfatin-1 alleviated the indomethacin-induced gastric injury, by supporting the balance of oxidant and antioxidant systems and inhibiting the production of proinflammatory mediators.55 Gastro-protective effect of nesfatin-1was confirmed using acetic acid-induced gastric ulcer model. Nesfatin-1 promoted the healing of chronic gastric ulcers induced by acetic acid by accelerating the gastric blood flow and mucosal repair, and partially reversing the downregulation of superoxide dismutase (SOD) mRNA.56 Moreover, nesfatin-1 treatment healed the acetic acid-induced gastric injury by inhibiting neutrophil infiltration and proinflammatory cytokine release, and promoting the antioxidant activity.57
Intraperitoneal injection of nesfatin-1 is shown to decrease the gastric lesions induced by water immersion and restraint stress (WRS), probably byinhibitingthe secretion of gastric acid and attenuation of the expression and release of proinflammatory cytokines, including interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α).58
Cardiac Diseases
Detection of nesfatin-1 protein and mRNA in rat cardiac extracts by Western blotting and qRT-PCR59 suggests a physiological role for nesfatin-1 in cardiac myocytes. Myocardial I/R injury often causes oxidative stress and inflammation, which in turn, leads to apoptosis and necrosis of cardiomyocytes.60,61 Nesfatin-1 can prevent this injury by reducing the infarct size and post-ischemic contracture, and by inhibiting the release of lactate dehydrogenase59 through its antioxidant properties. Myocardial infarction (MI) is one of the most common life-threatening diseases, which may lead to other diseases through oxidative stress and inflammation.62 Previous studies have suggested that the plasma level of nesfatin-1 in patients of acute myocardial infarction (AMI) was significantly lower than that in healthy controls.63 Moreover, a negative correlation was reported between the levels of plasma nesfatin-1, and high-sensitivity C-reactive protein and neutrophils in AMI patients,63 indicating that reduced nesfatin-1 levels may abet the pathogenesis of AMI through inflammatory mechanisms. In vivostudiesalso confirmed the cardioprotective effect of nesfatin-1. Isoproterenol (ISO)-induced MI in rats is a commonly used experimental model to evaluate the protective effect of various cardioprotective agents against human MI.64,65 Intraperitoneal administration of nesfatin-1 (10 μg/kg/day) conferred significant cardioprotection against the induced MI in this model by lowering the levels of proinflammatory cytokines and reducing the number of apoptotic and necrotic cells in the myocardium.3
Intestinal Diseases
Necrotizing enterocolitis (NEC), a leading cause of gastrointestinal morbidity in premature infants, is an inflammatory disease with systemic repercussion.66 It is characterized by excessive inflammatory infiltration of intestinal mucosa, resulting in the destruction of the intestinal barrier.67 Therefore, drugs with anti-inflammatory effects are likely to contribute to healing and prevent the development and progression of the disease. Nesfatin-1 ameliorated the survival rate and oxidative damage of NEC-induced neonatal intestine by supporting the balance of the oxidative/antioxidant system, inhibiting the NF-κB-65 pathway, and reversing NEC-induced dysbiosis.68
Ulcerative colitis (UC), an idiopathic inflammatory bowel disease, leads to bloody diarrhea and inflammatory alterations mostly in the large intestine, and affecting the colon and rectum.69 Overproduction of ROS and consequent inflammation of the mucosa are the important causes of tissue injury in UC.70 Recent studies have found that some peptide hormones may have a healing effect on UC. For instance, the anti-inflammatory effect of ghrelin is demonstrated in various types of chronic inflammation, including UC.71 Similarly, intracerebroventricular injection of nesfatin-1can restore the oxidative damage in the model of UC, probably through its anti-inflammatory action, by preventing neutrophil infiltration into the tissue, and its antioxidant activity, by suppressing free radical formation.72
Other Diseases
Testicular torsion is a urological emergency that may cause an ischemia-reperfusion injury to the testes.73 Testicular ischemia leads to over-generation of ROS,74 while the exhaustion of antioxidants, activation of neutrophils, increased production of pro-inflammatory cytokines and adhesion molecules, lead to testicular lipid peroxidation and apoptosis,75,76 eventually damaging germ cells and sperm. Tamer et al77 recently demonstrated the antioxidant, anti-inflammatory, and anti-apoptotic effects of nesfatin-1 on impaired testicular function induced by testis torsion. Treatment with nesfatin-1 reduced the pro-inflammatory cytokine expression, depressed apoptosis, degeneration of seminiferous tubules, and ameliorated the oxidative damage and preserved spermatogenic cells in torsioned rat testes.77 Moreover, nesfatin-1 promotes puberty, spermatogenesis and steroid production in pre-pubertal male mice by directly acting on the testes, accompanied by reduced oxidative stress.78 Similarly, the stimulatory effects of nesfatin-1 on spermatogenesis and steroidogenesis may be related to its antioxidant and anti-apoptotic activities.79 Further, it ameliorates the type-2 diabetes mellitus (T2DM)-associated testicular dysfunction and decreases the production of antioxidative enzymes in mice with T2DM.80 In addition, nesfatin-1 is also expressed in the reproductive organs of female mice and has been reported to be indispensable for the onset of normal puberty in female rats.81 Expression level of nesfatin-1 decreased significantly in pregnant rats, indicating the important role of nesfatin-1 in pregnancy and fetal development.82 Detailed study showed that serum levels of nesfatin-1 were negatively correlated with levels of IL-17A in both normal pregnancy and abortion model mice.83 IL-17A is a landmark cytokine secreted by the helper T cell subset Th17 cells, which plays an important role in the pathogenesis of inflammation, autoimmune diseases, and allogeneic organ rejection. Further studies have confirmed that compared to normal pregnant mice, in abortion model mice serum levels of anti-inflammatory cytokines, such as IL-4, IL-13, and IL-1ra were reduced, and the expression levels of nesfatin-1/NUCB2 in the implantation sites in the uterus were significantly increased.84 These results suggest a close relationship between nesfatin-1 and inflammatory cytokines in female reproductive physiology and pathology, but more studies are needed to support the anti-oxidative, antiapoptotic, and anti-inflammatory roles of nesfatin-1 in female reproductive function.
Osteoarthritis (OA) is a painful joint condition, characterized by the breakdown of the cartilage matrix, progressive degeneration of the articular cartilage, inflammation of the synovial membrane, and osteophyte formation in the joints.85 Increasing evidence suggest that apoptosis of chondrocytes, the cellular component of the cartilage, plays a crucial role in the pathogenesis and development of OA,86–88 making apoptosis a potential target for OA treatment. A previous study has shown that the levels of nesfatin-1 in articular cartilage and serum of patients with OA were significantly higher than in healthy controls.89 Moreover, serum nesfatin-1 levels were positively correlated with high-sensitivity C-reactive protein levels, and synovial nesfatin-1 levels were also positively correlated with IL-18 levels.89 These results indicate a potentially pivotal role of nesfatin-1 in the pathophysiology of OA. More recently, Jiang et al investigated the potential effect of nesfatin-1 on the rat OA model and IL-1β-stimulated chondrocytes, a useful model of OA chondrocytes.90 The results revealed that nesfatin-1 not only inhibits matrix metalloproteinase (MMP) expression and chondrocyte inflammation but also reduces apoptosis in rat chondrocytes,90 indicating a protective effect of nesfatin-1 in OA through its anti-inflammatory and anti-apoptotic activities.
Renal I/R causes severe oxidative damage to tissues and organs, the mechanisms of which may be related to oxidative stress, necrosis, apoptosis, adenosine triphosphate depletion, and calcium dyshomeostasis.91,92 An in vivo study conducted by Jiang et al93 showed that intraperitoneal administration of nesfatin-1 can significantly improve the renal function and mitigate the cellular damage caused by I/R injury in murine model. Moreover, after nesfatin-1 treatment, the malondialdehyde (MDA) level decreased, while SOD and catalase (CAT) activities increased in the experimental rats, compared to untreated rats,93 suggesting that nesfatin-1 ameliorated renal I/R injury by inhibiting oxidative stress. Furthermore, the anti-apoptotic activity ofnesfatin-1 was also thought to be involved in the kidney protection it provides, as indicated by a significant decrease in apoptotic tubular cells, as well as a decrease in caspase-3 activity and an increase in the Bcl-2/Bax ratio.93 Therefore, nesfatin-1 has a therapeutic potential to prevent renal IR injury.
Acute lung injury (ALI), a major cause of morbidity and mortality in both humans and animals, is characterized by strong pulmonary inflammation, resulting in inflammatory cell infiltration, alveolar capillary injury, abnormal release of ROS characterized by excessive oxidative stress, and apoptosis of alveolar epithelial cells.94 Inhibition of these processes is considered to be the key to relieve ALI.95,96 In an animal experiment, recombinant nesfatin-1 significantly ameliorated the symptoms of ALI and reduced the level of inflammation and oxidative stress in lung tissue of mice treated with LPS.4 Similartherapeutic effects of nesfatin-1 were observed in the human alveolar epithelial cell line BEAS-2B incubated with LPS, an ALI cell model.4
Hyperglycemia affects and delays wound healing, but the specific underlying mechanism is still unclear.97 It has been suggested that prolonged inflammation, delayed re-epithelialization, and consistent oxidative stress may correlate the high blood glucose levels with impaired wound healing.98 Treatment for three consecutive days with nesfatin-1 (2 μg/kg/day) inhibited apoptosis and oxidative stress in the skin, decreased the plasma levels of inflammatory factors IL-1β and interleukin-6 (IL-6), and improved surgical wound healing in both normoglycemic and hyperglycemic rats.99 This indicates that nesfatin-1 may play a potent role in promoting wound healing through its antioxidant, anti-inflammatory, and anti-apoptotic potential.
Obstructive jaundice is a medical condition caused by blockage of the body’s biliary system.100 The main causes of the obstructive jaundice and the consequent liver damage are persistent inflammation and oxidative stress.101 Because of this, various antioxidants have been tested in experimental obstructive jaundice models.102–104 Solmaz et al105 found that nesfatin-1 reduced the oxidative damage of the liver through its anti-inflammatory and antioxidant effects,105 and may become a potential drug for the treatment of obstructive jaundice.
Mechanisms and Pathways That Mediate the Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects of Nesfatin-1
Increasing evidence indicate the role of ROS as secondary messengers in the initiation and amplification of cell signaling, which have key roles in cell proliferation, apoptosis, oxidative damage of cells, and inflammation.106,107 More specifically, imbalances in ROS homeostasis may lead to oxidation-anti-oxidation imbalance, increase oxidative stress, and result in oxidative damage to biological macromolecules such as lipids, DNA, and proteins.108 ROS have also been reported as key mediators of inflammation.109 By activating the NF-κB pathway and enhancing the sensitivity of IL-1β, TNF-α, and IL-6, ROS can induce cellular inflammation.110 Promotion of apoptosis is another crucial feature of ROS.111 ROS promote apoptosis by increasing mitochondrial membrane permeability, mediating cytochrome C release, and enhancing caspase 9 activation and formation of apoptosis complex.112 The intracellular processes of ROS-induced oxidative stress, inflammation, and apoptosis are summarized in Figure 3.
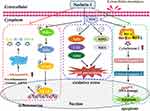 |
Figure 3 Mechanisms and intracellular processes through which nesfatin-1 exerts its antioxidant, anti-inflammatory and anti-apoptotic effects. |
Some therapeutic agents can target an interconnected network of signaling pathways to treat diseases. For example, selenium nanoparticles contribute to neuronal survival by targeting different cellular signaling pathways that regulate cellular metabolism, oxidative defense system, inflammatory reactions, autophagy, and apoptotic cell death.113 Nesfatin-1 plays a protective role in a variety of diseases by regulating ROS-induced oxidative stress, inflammation, and apoptosis (Figure 3). Nesfatin-1 treatment exerts its antioxidant effect by inhibiting intracellular ROS overproduction and maintaining the balance of oxidant/antioxidant systems, by decreasing the levels of lactate dehydrogenase, MPA, and MDA, and increasing the levels of SOD, CAT, and glutathione (GSH), as well as suppressing the free radical formation. Nesfatin-1 exerts its anti-inflammatory effects through several mechanisms. (1) Regulation of inflammatory cells. Nesfatin-1 inhibits inflammation by inhibiting neutrophil infiltration, decreasing the number of Iba-1-positive cells, and inhibiting astrocyte activation. (2) Decreasing the subsequent release of inflammatory mediators. (3) Modulation of various inflammation-related signaling pathways, including the NF-κB pathway, Akt/GSK-3β pathway, and CREB signaling pathway.
The anti-apoptotic effect of nesfatin-1 is relevant to the inhibition of mitochondria-associated apoptotic signaling via caspase-dependent pathways by decreasing the loss of mitochondrial membrane potential, increasing the Bcl-2/Bax ratio, inhibiting the release of cytochrome C from mitochondria, and decreasing caspase-3 activity. Additionally, activation of the C-Raf-ERK1/2 pathway is reported to be involved in the protective effects of nesfatin-1 against apoptosis.Table 1 shows the cellular, molecular, and biochemical mechanisms of nesfatin-1 exerting antioxidant, anti-inflammatory, and anti-apoptotic effects in multiple diseases.
 |
Table 1 Mechanisms of Nesfatin-1 Exerting Antioxidant, Anti-Inflammatory and Anti-Apoptotic Effects |
Conclusion
The reviewed data suggest that nesfatin-1 plays an ameliorative role in different pathophysiological conditions through its antioxidant, anti-inflammatory, and anti-apoptotic properties. Because the receptor of nesfatin-1 has not yet been identified, the exact mechanisms involved in the biological effects of nesfatin-1 are still unclear. Detailed understanding of the biological processes underlying the antioxidant, anti-inflammatory, and anti-apoptotic effects of nesfatin-1 may pave the way for the design of new promising drugs.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81873986).
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Oh-I S, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712.
2. Schalla M, Unniappan S, Lambrecht N, Mori M, Taché Y, Stengel A. Nucb2/nesfatin-1 - inhibitory effects on food intake, body weight and metabolism. Peptides. 2020;128:170308.
3. Tasatargil A, Kuscu N, Dalaklioglu S, et al. Cardioprotective effect of nesfatin-1 against isoproterenol-induced myocardial infarction in rats: role of the akt/gsk-3β pathway. Peptides. 2017;95:1–9. doi:10.1016/j.peptides.2017.07.003
4. Wang Z, Chen S, Zou X, Tian L, Sui S, Liu N. Nesfatin-1 alleviates acute lung injury through reducing inflammation and oxidative stress via the regulation of hmgb1. Eur Rev Med Pharmacol Sci. 2020;24:5071–5081.
5. Oh IS, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi:10.1038/nature05162
6. Shimizu H, Ohsaki A, Oh IS, Okada S, Mori M. A new anorexigenic protein, nesfatin-1. Peptides. 2009;30:995–998.
7. Shimizu H, Oh IS, Hashimoto K, et al. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi:10.1210/en.2008-0598
8. Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642–1647. doi:10.1152/ajpregu.00804.2009
9. Angelone T, Rocca C, Pasqua T. Nesfatin-1 in cardiovascular orchestration: from bench to bedside. Pharmacol Res. 2020;156:104766.
10. Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mrna/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–579. doi:10.1016/j.neuroscience.2008.07.054
11. Goebel-Stengel M, Wang L, Stengel A, Tache Y. Localization of nesfatin-1 neurons in the mouse brain and functional implication. Brain Res. 2011;1396:20–34. doi:10.1016/j.brainres.2011.04.031
12. Jiang S, Zhou W, Zhang X, et al. Developmental expression and distribution of nesfatin-1/nucb2 in the canine digestive system. Acta Histochem. 2016;118:90–96. doi:10.1016/j.acthis.2015.11.010
13. ZB T, RJ D, GR S, LZ W, XJ K, XL D. Expression of gastrointestinal nesfatin-1 and gastric emptying in ventromedial hypothalamic nucleus- and ventrolateral hypothalamic nucleus-lesioned rats. World j Gastroenterol. 2014;20:6897–6905. doi:10.3748/wjg.v20.i22.6897
14. Yang Y, Zhang B, Nakata M, Nakae J, Mori M, Yada T. Islet beta-cell-produced nucb2/nesfatin-1 maintains insulin secretion and glycemia along with suppressing ucp-2 in beta-cells. JPS. 2019;69:733–739. doi:10.1007/s12576-019-00689-2
15. Ramanjaneya M, Chen J, Brown JE, et al. Identification of nesfatin-1 in human and murine adipose tissue: A novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151:3169–3180. doi:10.1210/en.2009-1358
16. Feijoo-Bandin S, Rodriguez-Penas D, Garcia-Rua V, et al. Nesfatin-1 in human and murine cardiomyocytes: synthesis, secretion, and mobilization of glut-4. Endocrinology. 2013;154:4757–4767. doi:10.1210/en.2013-1497
17. Garcia-Galiano D, Pineda R, Ilhan T, et al. Cellular distribution, regulated expression, and functional role of the anorexigenic peptide, nucb2/nesfatin-1, in the testis. Endocrinology. 2012;153:1959–1971. doi:10.1210/en.2011-2032
18. Gonzalez R, Shepperd E, Thiruppugazh V, et al. Nesfatin-1 regulates the hypothalamo-pituitary-ovarian axis of fish. Biol Reprod. 2012;87:84. doi:10.1095/biolreprod.112.099630
19. Kim J, Chung Y, Kim H, Im E, Lee H, Yang H. The tissue distribution of nesfatin-1/nucb2 in mouse. Develop Reproduction. 2014;18:301–309. doi:10.12717/DR.2014.18.4.301
20. Schalla MA, Stengel A. Current understanding of the role of nesfatin-1. J Endocrine Society. 2018;2:1188–1206. doi:10.1210/js.2018-00246
21. Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372–2381. doi:10.1016/j.peptides.2007.10.008
22. Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223–2228. doi:10.1016/j.peptides.2007.09.005
23. Li Z, Gao L, Tang H, et al. Peripheral effects of nesfatin-1 on glucose homeostasis. PLoS One. 2013;8:e71513. doi:10.1371/journal.pone.0071513
24. Blanco A, Velasco C, Bertucci J, Soengas J, Unniappan S. Nesfatin-1 regulates feeding, glucosensing and lipid metabolism in rainbow trout. Front Endocrinol. 2018;9:484. doi:10.3389/fendo.2018.00484
25. Goebel-Stengel M, Stengel A. Role of brain nucb2/nesfatin-1 in the stress-induced modulation of gastrointestinal functions. Curr Neuropharmacol. 2016;14:882–891. doi:10.2174/1570159X14666160601153202
26. García-Galiano D, Tena-Sempere M. Emerging roles of nucb2/nesfatin-1 in the metabolic control of reproduction. Curr Pharm Des. 2013;19:6966–6972. doi:10.2174/138161281939131127142531
27. Aydin S, Dag E, Ozkan Y, et al. Time-dependent changes in the serum levels of prolactin, nesfatin-1 and ghrelin as a marker of epileptic attacks young male patients. Peptides. 2011;32:1276–1280. doi:10.1016/j.peptides.2011.04.021
28. Wei Y, Li J, Wang H, Wang G. Nucb2/nesfatin-1: expression and functions in the regulation of emotion and stress. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:221–227. doi:10.1016/j.pnpbp.2017.09.024
29. Vas S, Ádori C, Könczöl K, et al. Nesfatin-1/nucb2 as a potential new element of sleep regulation in rats. PLoS One. 2013;8:e59809. doi:10.1371/journal.pone.0059809
30. Weibert E, Hofmann T, Stengel A. Role of nesfatin-1 in anxiety, depression and the response to stress. Psychoneuroendocrinology. 2019;100:58–66. doi:10.1016/j.psyneuen.2018.09.037
31. Zhang E, Chen Q, Wang J, Li D, Wan Z, Ju X. Protective role of microRNA-27a upregulation and hsp90 silencing against cerebral ischemia-reperfusion injury in rats by activating pi3k/akt/mTOR signaling pathway. Int Immunopharmacol. 2020;86:106635. doi:10.1016/j.intimp.2020.106635
32. DC M, NN Z, YN Z, HS C. Kv1.3 channel blockade alleviates cerebral ischemia/reperfusion injury by reshaping m1/m2 phenotypes and compromising the activation of nlrp3 inflammasome in microglia. Exp Neurol. 2020;113399.
33. Chu K, Yin B, Wang J, et al. Inhibition of p2x7 receptor ameliorates transient global cerebral ischemia/reperfusion injury via modulating inflammatory responses in the rat hippocampus. J Neuroinflammation. 2012;9:69.
34. Erfani S, Moghimi A, Aboutaleb N, Khaksari M. Nesfatin-1 improve spatial memory impairment following transient global cerebral ischemia/reperfusion via inhibiting microglial and caspase-3 activation. J Mol Neurosci. 2018;65:377–384. doi:10.1007/s12031-018-1105-3
35. Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi:10.1016/S0169-328X(98)00040-0
36. Erfani S, Moghimi A, Aboutaleb N, Khaksari M. Protective effects of nucleobinding-2 after cerebral ischemia via modulating bcl-2/bax ratio and reducing glial fibrillary acid protein expression. Basic Clin Neurosci. 2019;10:451–459.
37. Tang CH, Fu XJ, Xu XL, Wei XJ, Pan HS. The anti-inflammatory and anti-apoptotic effects of nesfatin-1 in the traumatic rat brain. Peptides. 2012;36:39–45. doi:10.1016/j.peptides.2012.04.014
38. Ozsavci D, Ersahin M, Sener A, et al. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage-induced oxidative brain damage in rats. Neurosurgery. 2011;68:
39. Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi:10.1016/S0896-6273(03)00568-3
40. PR A. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: finding ways for prevention. Med Res Rev. 2020.
41. SR S, MF C. Mitochondrial dysfunction and oxidative stress in parkinson’s disease. Prog Neurobiol. 2013;17–32.
42. Yamaguchi A, Ishikawa K-I, Inoshita T. Identifying therapeutic agents for amelioration of mitochondrial clearance disorder in neurons of familial parkinson disease. Stem Cell Rep. 2020;14:1060–1075. doi:10.1016/j.stemcr.2020.04.011
43. MH C, WJ Q, et al. Mitochondrial dysfunction, oxidative stress, and apoptosis revealed by proteomic and transcriptomic analyses of the striata in two mouse models of parkinson’s disease. J Proteome Res. 2008;7:666–677.
44. Tan Z, Xu H, Shen X, Jiang H. Nesfatin-1 antagonized rotenone-induced neurotoxicity in mes23.5. Dopaminergic Cells Peptides. 2015;69:109–114. doi:10.1016/j.peptides.2015.04.019
45. Shen XL, Song N, Du XX, Li Y, Xie JX, Jiang H. Nesfatin-1 protects dopaminergic neurons against mpp(+)/mptp-induced neurotoxicity through the c-raf-erk1/2-dependent anti-apoptotic pathway. Sci Rep. 2017;7:40961. doi:10.1038/srep40961
46. Chen J, Liu W, Yi H, Hu X, Peng L, Yang F. The natural rotenoid deguelin ameliorates diabetic neuropathy by decreasing oxidative stress and plasma glucose levels in rats via the nrf2 signalling pathway. Cell Physiol Biochem. 2018;48:1164–1176. doi:10.1159/000491983
47. Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL. Inflammation as a therapeutic target for diabetic neuropathies. Curr Diab Rep. 2016;16:29. doi:10.1007/s11892-016-0727-5
48. Pan H, Ding Y, Yan N, Nie Y, Li M, Tong L. Trehalose prevents sciatic nerve damage to and apoptosis of schwann cells of streptozotocin-induced diabetic c57bl/6j mice. Biomed Pharmacother. 2018;105:907–914. doi:10.1016/j.biopha.2018.06.069
49. Zhang Y, Xi X, Mei Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ros/pink1/parkin signal pathway. Biomed Pharmacother. 2019;111:1315–1325. doi:10.1016/j.biopha.2019.01.034
50. You L, Chen J, Liu W. Enterovirus 71 induces neural cell apoptosis and autophagy through promoting acox1 downregulation and ros generation. Virulence. 2020;11:537–553. doi:10.1080/21505594.2020.1766790
51. Nazarnezhad S, Rahmati M, Shayannia A, Abbasi Z, Salehi M, Khaksari M. Nesfatin-1 protects pc12 cells against high glucose-induced cytotoxicity via inhibiting oxidative stress, autophagy and apoptosis. Neurotoxicology. 2019;74:196–202. doi:10.1016/j.neuro.2019.07.001
52. Stengel A, Goebel M, Yakubov I, et al. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–238. doi:10.1210/en.2008-0747
53. Stengel A, Goebel-Stengel M, Jawien J, Kobelt P, Taché Y, Lambrecht N. Lipopolysaccharide increases gastric and circulating nucb2/nesfatin-1 concentrations in rats. Peptides. 2011;32:1942–1947.
54. Kwiecień S, Magierowska K, Śliwowski Z, Wójcik D, Magierowski M, Brzozowski T. New insight into the mechanisms of gastroduodenal injury induced by nonsteroidal anti-inflammatory drugs: practical implications. Pol Arch Med Wewn. 2015;125:191–198.
55. Kolgazi M, Cantali-Ozturk C, Deniz R, et al. Nesfatin-1 alleviates gastric damage via direct antioxidant mechanisms. J Surg Res. 2015;193(1):111–118. doi:10.1016/j.jss.2014.06.057
56. Szlachcic A, Majka J, Strzalka M, et al. Experimental healing of preexisting gastric ulcers induced by hormones controlling food intake ghrelin, orexin-a and nesfatin-1 is impaired under diabetic conditions. A key to understanding the diabetic gastropathy? J Physiol Pharmacol. 2013;64:625–637.
57. Kolgazi M, Ozdemir-Kumral Z, Cantali-Ozturk C, et al. Anti-inflammatory effects of nesfatin-1 on acetic acid-induced gastric ulcer in rats: involvement of cyclo-oxygenase pathway. J Physiol Pharmacol. 2017;68:765–777.
58. Szlachcic A, Sliwowski Z, Krzysiek-Maczka G, et al. New satiety hormone nesfatin-1 protects gastric mucosa against stress-induced injury: mechanistic roles of prostaglandins, nitric oxide, sensory nerves and vanilloid receptors. Peptides. 2013;49:9–20. doi:10.1016/j.peptides.2013.07.017
59. Angelone T, Filice E, Pasqua T, et al. Nesfatin-1 as a novel cardiac peptide: identification, functional characterization, and protection against ischemia/reperfusion injury. CMLS. 2013;70:495–509. doi:10.1007/s00018-012-1138-7
60. Chen Y, Lin H, Wang Q, Hou J, Mao Z, Li Y. Protective role of silibinin against myocardial ischemia/reperfusion injury-induced cardiac dysfunction. Int J Biol Sci. 2020;16:1972–1988. doi:10.7150/ijbs.39259
61. Huang Z, Xu W, Wu J, Lu X, Chen X. MicroRNA-374a protects against myocardial ischemia-reperfusion injury in mice by targeting the mapk6 pathway. Life Sci. 2019;232:116619. doi:10.1016/j.lfs.2019.116619
62. Ghartavol M, Gholizadeh-Ghaleh Aziz S, Babaei G, Hossein Farjah G, Hassan Khadem AM. The protective impact of betaine on the tissue structure and renal function in isoproterenol-induced myocardial infarction in rat. Mol Gene Genomic Med. 2019;7:e00579. doi:10.1002/mgg3.579
63. Dai H, Li X, He T, et al. Decreased plasma nesfatin-1 levels in patients with acute myocardial infarction. Peptides. 2013;46:167–171. doi:10.1016/j.peptides.2013.06.006
64. Meeran M, Laham F, Al-Taee H, Azimullah S, Ojha S. Protective effects of α-bisabolol on altered hemodynamics, lipid peroxidation, and nonenzymatic antioxidants in isoproterenol-induced myocardial infarction: in vivo and in vitro evidences. J Biochem Mol Toxicol. 2018;32:e22200. doi:10.1002/jbt.22200
65. Nagoor Meeran M, Laham F, Azimullah S, Tariq S, Ojha S. Α-bisabolol abrogates isoproterenol-induced myocardial infarction by inhibiting mitochondrial dysfunction and intrinsic pathway of apoptosis in rats. Mol Cell Biochem. 2019;453:89–102.
66. Eaton S, Rees C, Hall N. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology. 2017;111:423–430. doi:10.1159/000458462
67. Seo S, Miyake H, Alganabi M, et al. Vasoactive intestinal peptide decreases inflammation and tight junction disruption in experimental necrotizing enterocolitis. J Pediatr Surg. 2019;54:2520–2523. doi:10.1016/j.jpedsurg.2019.08.038
68. Karadeniz Cerit K, Koyuncuoglu T, Yagmur D, et al. Nesfatin-1 ameliorates oxidative bowel injury in rats with necrotizing enterocolitis: the role of the microbiota composition and claudin-3 expression. J Pediatr Surg. 2020. doi:10.1016/j.jpedsurg.2020.02.025
69. Neurath M, Leppkes M. Resolution of ulcerative colitis. Semin Immunopathol. 2019;41:747–756. doi:10.1007/s00281-019-00751-6
70. Yao D, Dong M, Dai C, Wu S. Inflammation and inflammatory cytokine contribute to the initiation and development of ulcerative colitis and its associated cancer. Inflamm Bowel Dis. 2019;25:1595–1602. doi:10.1093/ibd/izz149
71. Ceranowicz P, Warzecha Z, Cieszkowski J, et al. Essential role of growth hormone and igf-1 in therapeutic effect of ghrelin in the course of acetic acid-induced colitis. Int J Mol Sci. 2017;18.
72. Ozturk C, Oktay S, Yuksel M, Akakin D, Yarat A, Kasimay Cakir O. Anti-inflammatory effects of nesfatin-1 in rats with acetic acid - induced colitis and underlying mechanisms. J Physiol Pharmacol. 2015;66:741–750.
73. BK S, Watson G, Townell N. Testicular torsion. BMJ. 2010;341:c3213. doi:10.1136/bmj.c3213
74. SM W, ZZ Y, Zhou J. Curcumin attenuates ischemia-reperfusion injury in rat testis. Fertil Steril. 2009;91:271–277. doi:10.1016/j.fertnstert.2007.10.082
75. TE Ş, Yüksel M, Özyılmaz-Yay N. Apocynin attenuates testicular ischemia-reperfusion injury in rats. J Pediatr Surg. 2015;50:1382–1387. doi:10.1016/j.jpedsurg.2014.11.033
76. Taati M, Moghadasi M, Dezfoulian O, Asadian P, Zendehdel M. Effects of ghrelin on germ cell apoptosis and proinflammatory cytokines production in ischemia-reperfusion of the rat testis. Iranian J Reproductive Med. 2015;13:85–92.
77. Arabaci Tamer S, Yildirim A, Koroglu MK, Cevik O, Ercan F, Yegen BC. Nesfatin-1 ameliorates testicular injury and supports gonadal function in rats induced with testis torsion. Peptides. 2018;107:1–9. doi:10.1016/j.peptides.2018.07.005
78. Ranjan A, Choubey M, Yada T, Krishna A. Immunohistochemical localization and possible functions of nesfatin-1 in the testis of mice during pubertal development and sexual maturation. J Mol Histol. 2019;50:533–549. doi:10.1007/s10735-019-09846-8
79. Ranjan A, Choubey M, Yada T, Krishna A. Direct effects of neuropeptide nesfatin-1 on testicular spermatogenesis and steroidogenesis of the adult mice. Gen Comp Endocrinol. 2019;271:49–60. doi:10.1016/j.ygcen.2018.10.022
80. Ranjan A, Choubey M, Yada T, Krishna A. Nesfatin-1 ameliorates type-2 diabetes-associated reproductive dysfunction in male mice. J Endocrinol Invest. 2020;43:515–528. doi:10.1007/s40618-019-01136-0
81. García-Galiano D, Navarro V, Roa J, et al. The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci. 2010;30:7783–7792. doi:10.1523/JNEUROSCI.5828-09.2010
82. Garcés M, Poveda N, Sanchez E, et al. Regulation of nucb2/nesfatin-1 throughout rat pregnancy. Physiol Behav. 2014;133:216–222. doi:10.1016/j.physbeh.2014.05.042
83. Chung Y, Kim H, Im E, Kim P, Yang H. Th 17 cells and nesfatin-1 are associated with spontaneous abortion in the cba/j × dba/2 mouse model. Develop Reproduction. 2015;19:243–252. doi:10.12717/DR.2015.19.4.243
84. Chung Y, Kim H, Seon S, Yang H. Serum cytokine levels are related to nesfatin-1/nucb2 expression in the implantation sites of spontaneous abortion model of cba/j × dba/2 mice. Develop Reproduction. 2017;21:35–46. doi:10.12717/DR.2017.21.1.035
85. Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71-72:40–50. doi:10.1016/j.matbio.2018.05.008
86. BW W, Jiang Y, Yao Z-L, Chen P-S, Yu B, Wang S-N. Aucubin protects chondrocytes against il-1β-induced apoptosis in vitro and inhibits osteoarthritis in mice model. Drug Des Devel Ther. 2019;13:3529–3538. doi:10.2147/DDDT.S210220
87. Liu Q, Wang J, Sun Y, Han S. Chondroitin sulfate from sturgeon bone protects chondrocytes via inhibiting apoptosis in osteoarthritis. Int J Biol Macromol. 2019;134:1113–1119. doi:10.1016/j.ijbiomac.2019.05.110
88. Sun J, Wei X, Lu Y. Glutaredoxin 1 (grx1) inhibits oxidative stress and apoptosis of chondrocytes by regulating creb/ho-1 in osteoarthritis. Mol Immunol. 2017;90:211–218. doi:10.1016/j.molimm.2017.08.006
89. Jiang L, Bao J, Zhou X, Xiong Y, Wu L. Increased serum levels and chondrocyte expression of nesfatin-1 in patients with osteoarthritis and its relation with bmi, hscrp, and il-18. Mediators Inflamm. 2013;2013:631251. doi:10.1155/2013/631251
90. Jiang L, Xu K, Li J, et al. Nesfatin-1 suppresses interleukin-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging. 2020;12:1760–1777. doi:10.18632/aging.102711
91. Awad A, El-Sharif A. Curcumin immune-mediated and anti-apoptotic mechanisms protect against renal ischemia/reperfusion and distant organ induced injuries. Int Immunopharmacol. 2011;11:992–996. doi:10.1016/j.intimp.2011.02.015
92. Thurman J. Triggers of inflammation after renal ischemia/reperfusion. Clin immunol. 2007;123:7–13. doi:10.1016/j.clim.2006.09.008
93. Jiang G, Wang M, Wang L, et al. The protective effect of nesfatin-1 against renal ischemia-reperfusion injury in rats. Ren Fail. 2015;37:882–889. doi:10.3109/0886022X.2015.1015426
94. Li T, Zou C. The role of deubiquitinating enzymes in acute lung injury and acute respiratory distress syndrome. Int J Mol Sci. 2020;21.
95. Yanling Q, Xiaoning C, Fei B, Liyun F, Huizhong H, Daqing S. Inhibition of nlrp9b attenuates acute lung injury through suppressing inflammation, apoptosis and oxidative stress in murine and cell models. Biochem Biophys Res Commun. 2018;503:436–443. doi:10.1016/j.bbrc.2018.04.079
96. Lin W, Chen C, Huang Y, et al. Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis. Sci Rep. 2015;5:12463. doi:10.1038/srep12463
97. Shakya S, Wang Y, Mack J, Maytin E. Hyperglycemia-induced changes in hyaluronan contribute to impaired skin wound healing in diabetes: review and perspective. Int J Cell Biol. 2015;2015:701738. doi:10.1155/2015/701738
98. Li M, Yu H, Pan H, et al. Nrf2 suppression delays diabetic wound healing through sustained oxidative stress and inflammation. Front Pharmacol. 2019;10:1099. doi:10.3389/fphar.2019.01099
99. Solmaz A, Bahadir E, Gulcicek OB, et al. Nesfatin-1 improves oxidative skin injury in normoglycemic or hyperglycemic rats. Peptides. 2016;78:1–10. doi:10.1016/j.peptides.2015.12.006
100. Pavlidis E, Pavlidis T. Pathophysiological consequences of obstructive jaundice and perioperative management. HBPD INT. 2018;17:17–21. doi:10.1016/j.hbpd.2018.01.008
101. Unal Y, Tuncal S, Kosmaz K, et al. The effect of calcium dobesilate on liver damage in experimental obstructive jaundice. J Investigative Surgery. 2019;32:238–244. doi:10.1080/08941939.2018.1451936
102. Soylu A, Aydogdu N, Basaran U, et al. Antioxidants vitamin e and c attenuate hepatic fibrosis in biliary-obstructed rats. World J Gastroenterol. 2006;12:6835–6841. doi:10.3748/wjg.v12.i42.6835
103. Yilmaz E, Arikanoğlu Z, Turkoğlu A, Kiliç E, Yüksel H, Gümüş M. The protective effects of pomegranate on liver and remote organs caused by experimental obstructive jaundice model. Eur Rev Med Pharmacol Sci. 2016;20:767–772.
104. Fu Y, Hua C, Zhou J, Cheng B, Zhang J. Protective effects of ginseng total saponins against hepatic ischemia/reperfusion injury in experimental obstructive jaundice rats. Pharm Biol. 2013;51:1545–1551. doi:10.3109/13880209.2013.802352
105. Solmaz A, Gülçiçek O, Erçetin C, et al. Nesfatin-1 alleviates extrahepatic cholestatic damage of liver in rats. Bosnian J Basic Med Sci. 2016;16:247–253.
106. Zhou DR, Eid R, Miller KA, Boucher E, Mandato CA, Greenwood MT. Intracellular second messengers mediate stress inducible hormesis and programmed cell death: A review. Biochimica Et Biophysica Acta Molecular Cell Res. 2019;1866:773–792. doi:10.1016/j.bbamcr.2019.01.016
107. Zhang H, Wang Z, Liu R, et al. Reactive oxygen species stimulated pulmonary epithelial cells mediate the alveolar recruitment of fasl(+) killer b cells in lps-induced acute lung injuries. J Leukoc Biol. 2018;104:1187–1198. doi:10.1002/JLB.3A0218-075R
108. Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PI
109. Sho T, Xu J. Role and mechanism of ros scavengers in alleviating nlrp3-mediated inflammation. Biotechnol Appl Biochem. 2019;66:4–13. doi:10.1002/bab.1700
110. Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and nf-kappab signaling. Cell Res. 2011;21:103–115. doi:10.1038/cr.2010.178
111. Su LJ, Zhang JH, Gomez H, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi:10.1155/2019/5080843
112. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863:2977–2992. doi:10.1016/j.bbamcr.2016.09.012
113. Amani H, Habibey R, Shokri F, et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019;9:6044.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
