Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Antioxidant and Anti-Inflammatory Properties of Melatonin in Patients with Type 2 Diabetes Mellitus with Periodontal Disease Under Non-Surgical Periodontal Therapy: A Double-Blind, Placebo-Controlled Trial
Authors Zare Javid A, Hosseini SA, Gholinezhad H, Moradi L, Haghighi-zadeh MH, Bazyar H
Received 13 December 2019
Accepted for publication 10 March 2020
Published 18 March 2020 Volume 2020:13 Pages 753—761
DOI https://doi.org/10.2147/DMSO.S242208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Ahmad Zare Javid,1,2 Seyed Ahmad Hosseini,1,2 Hassan Gholinezhad,2 Leila Moradi,3 Mohammad Hosein Haghighi-zadeh,4 Hadi Bazyar1,5
1Nutrition and Metabolic Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 2Department of Nutrition, School of Allied Medical Sciences, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 3Health Research Institute, Diabetes Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 4Department of Biostatistics, School of Public Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; 5Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Correspondence: Hadi Bazyar
Nutrition and Metabolic Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Tel +98 9136659629
Email [email protected]
Background and Aim: The imbalance between pro-oxidant and antioxidant systems often leads to further oxidative damage in the pathogenesis of both diabetes and periodontal disease. This study aimed to investigate the antioxidant and anti-inflammatory properties of melatonin in type 2 diabetes mellitus (T2DM) patients with periodontal disease (PD) under non-surgical periodontal therapy (NSPT).
Materials and Methods: In this double-blind clinical trial study, 50 T2DM patients with PD were randomly allocated to intervention and control groups and received 250 mg/day (2 tablets) either melatonin or placebo 1 h before bedtime for 8 weeks. The NSPT was performed for all patients in both groups at the beginning of the study. The serum levels of interleukin-1b (IL-1b), malondialdehyde (MDA), total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured pre- and post-intervention.
Results: Supplementation with melatonin in adjunct to NSPT significantly increased the serum levels of TAC, SOD, CAT, and GPx in the intervention group (P = 0.02, 0.008, 0.004 and 0.004, respectively). The mean changes of SOD, CAT, and GPx were significantly (P = 0.02, 0.04 and 0.04, respectively) greater in the intervention group compared with the control group. Also, after adjusting for confounding factors, the results did not change in terms of significance (P < 0.05). After the intervention, serum levels of MDA and IL-1b were significantly reduced in the intervention group (P < 0.001 and P = 0.008, respectively). The intervention group exhibited lower mean changes of MDA compared with the control group, and these changes were statistically significant (P = 0.008). In addition, after adjusting for confounding factors, the results did not change in terms of significance.
Conclusion: The adjunctive effects of melatonin and NSPT may improve inflammatory and antioxidant parameters in T2DM patients with PD.
Keywords: type 2 diabetes mellitus, periodontal disease, melatonin, inflammatory markers, antioxidant enzymes
Introduction
The prevalence of diabetes mellitus (DM) is rapidly increasing around the world specifically in developing countries.1 The global prevalence of DM is greater than 300 million people.2 In Iran, 11.4% of adults have been estimated to suffer from T2DM.3 T2DM is related to several clinical complications including central obesity, hyperglycemia, dyslipidemia, oxidation of low-density lipoproteins (LDL), inflammation, hypertension, and the prothrombotic state.4 Several studies have shown that obesity, especially abdominal obesity plays a major role in diabetes and metabolic complications associated with it. Also, it is thought that basic inflammation caused by obesity increases the risk of cancer. The adipose tissue secretes various active compounds called adipokines (IL-6, TNF-α, leptin, adiponectin, and other). These compounds produce reactive oxygen species (ROS) and reduce activity of antioxidant enzymes (glutathione peroxidase, superoxide dismutase, and catalase). Therefore, improvement in these adipokines can play a beneficial role in diabetes by decrease oxidative stress.5–7 Infact, T2DM is characterized by chronic inflammation and high levels of oxidative stress. An increase in the amount of ROS and nitrogen species can result in a redox imbalance and plays an important role in the pathogenesis of diabetic complications such as periodontal disease.8
Chronic periodontitis (CP), the most common chronic infection worldwide, and DM are bi-directionally related to each other. DM is considered as a predisposing factor in the etiology of CP. On the other hand, CP can impair metabolic control in patients with DM.9 Patients with T2DM have a greater prevalence, extent, and severity of CP than non-diabetic subjects.10 The accumulation of anaerobic gram-negative bacteria can cause the CP and formation of calculus. CP is a multifactorial, chronic inflammatory disorder and if it is not treated, it may lead to non-reversible damages of oral supportive tissues (periodontal ligament, cementum, and alveolar bone) surrounding the teeth and finally result in tooth loss.11 There are growing evidence suggesting that the pro-inflammatory cytokines produced by gingiva in CP transfer to the systemic circulation and exacerbate DM. Conversely, the elevated levels of pro-inflammatory cytokines in DM can move to the gingiva and aggravate periodontal disease.12 In fact, the chronic inflammation in CP can increase plasma levels of glucose, insulin resistance, and exacerbate the complications of diabetes.13 It seems that oxidative stress is the main link between DM and periodontitis and can activate pro-inflammatory pathways common in both pathologies.14 The increase in the concentrations of protein carbonyl and sulfhydryl contents, MDA and also the reduction in TAC have been proposed as the hallmarks for oxidative stress.15 The other factor involved in the pathogenesis of DM is the disturbance in the antioxidant system.15 The antioxidant defense system consists several complex components such as metal-binding proteins, specific enzymes (SOD, CAT, GPx, and other), and a number of low molecular weight antioxidants such as glutathione, ascorbate, cysteine, and urate.16
There are several dietary components with natural antioxidant properties.17 Melatonin is an active component with antioxidant properties, reported by several studies.18 It is an indoleamine (a derivative of tryptophan), secreted mainly by pinealocytes.19 The main function of melatonin is to regulate the sleep cycle. Studies reported that melatonin is also involved in homeostasis and energy metabolism.20 Melatonin can activate brown adipose tissue and subsequently increase energy expenditure. Moreover, new research found its anti-inflammatory, immunomodulatory as well as antioxidant properties.21 Melatonin can increase the expression of antioxidant enzymes (SOD, CAT, and GPx) and scavenge free radicals.22 It is indicated that NSPT (several sessions of scaling and root planning) alone or in combination with the other therapies for 1- to 3-weeks, can lead to clinical improvement in patients with T2DM.23 The available evidence indicates the beneficial effects of consumption of melatonin supplement as a therapeutic tool in some chronic disorders. The hypothesis of the present study was that the use of melatonin with NSPT is effective in improvement of biomarkers of oxidative stress against the lack of effect. To the best of authors’ knowledge, there are no published reports related to the effects of melatonin supplementation along with NSPT in diabetic patients with PD. The aim of this study was to investigate the antioxidant and anti-inflammatory properties of melatonin supplementation in T2DM patients with PD under NSPT.
Materials and Methods
Study Design and Subjects
This study was a double-blinded, placebo-controlled and single-center trial. In this study, 96 patients with T2DM and PD were recruited from the patients referred to the endocrinology and metabolism clinics of Golestan Hospital of Ahvaz Jundishapur University of Medical Science, Iran from March 2017 to August 2017. Of the 96 patients, 46 patients were excluded (due to lack of inclusion criteria and disapproval to participate in the study) and 50 subjects were selected to participate in the study.
The main inclusion criteria were patients with known T2DM (no more than five years since diagnosis), male and female aged 30–60 years and body mass index of 18.5–30 kg/m2, patients with the severity periodontitis of mild to moderate (PD ≥ 4 mm and CAL = 1–4 mm). The exclusion criteria were as follows: patients with kidney failure, pregnancy, breastfeeding, thyroid disease, traveling more than two weeks, smoking, using any immunosuppressive medications, antioxidants, anti-inflammatory agents, insulin, any mouthwash and antibiotic and noticeable change in consumption of medications, patients with severe periodontitis, and following a specific diet over the past six months. The diagnosis of DM was done based on the American Diabetes Association guidelines:24 those with the fasting plasma sugar (FBS) ≥126 mg/dL and HbA1c ≥6.5% or 2 hr glucose (2 hpp) ≥200 mg/dL.
The subjects were randomly (by a random permuted block procedure, block design, based on the combined analysis) allocated to two groups of intervention (n = 25) and placebo (n = 25). The intervention group received 250 mg melatonin (as 2 tablets; purchased from Nature Made, USA with the ingredients of sodium starch glycolate, magnesium stearate, and 3 mg net melatonin) and the control group received 250 mg placebo (as 2 tablets made by the Faculty of Pharmacy in Ahvaz Jundishapur University of Medical Sciences with the ingredients containing cellulose, silicon dioxide, magnesium stearate, starch and a few taste of peppermint oil matching with the melatonin tablets for shape, color, size, and taste) for 8 weeks. The melatonin and placebo tablets were recommended to use one hour before sleeping at night. Furthermore, both groups underwent the NSPT along with melatonin supplementation at baseline. The assessment method of periodontal disease has been previously reported in our published paper.17 Also, some instructions for dental hygiene (how to brush and use dental floss correctly) were explained to patients. The patients were asked to avoid use mouthwash.
In this study both the researchers and patients were blinded. To ensure consuming supplements or placebo by the patients, they were reminded through phone calls or text messages and were asked to return any untaken tablets. The subjects who did not consume more than 10% of the tablets were excluded from the study.
Assessment of Anthropometric Parameters
Anthropometric measurements were done by a trained researcher at baseline and the end of the 8-week intervention. Height was measured without shoes to the nearest 0.5 cm, and weight was measured without shoes and with light clothing using Seca scale (Seca, Hamburg, Germany). BMI was also calculated as the body weight in kg by height in m2. Waist circumference (WC) was measured by a tape measure, while the subjects were at the end of breathing out and the midpoint of lower rib and iliac crest.
Outcomes
In the present study, the primary outcome was the malondialdehyde (MDA) and the secondary outcomes were inflammatory and other oxidative stress biomarkers.
Assessment of Biochemical Parameters
After an overnight fasting, the venous blood samples were collected at baseline and the end of the study and were immediately centrifuged (3000×g, 10 min, 4ºC). The serum supernatant was separated and stored at −70°C until the analysis of interleukin-1b (IL-1b), malondialdehyde (MDA), total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Serum levels of MDA, TAC, SOD, CAT, and GPx were measured by reliable spectrophotometric methods using Zell Bio GmbH kit (Germany). Serum levels of IL-1b were measured by enzyme-linked immunosorbent assay (ELISA) kit according to the kit instructions (Human IL-1β Elisa kit [IBL, Germany]).
Dietary Intake and Physical Activity Assessment
A three-day food record was used to assess the dietary intake at baseline and end of the study. The data extracted from dietary intakes were analyzed by Nutritionist 4 software. The national food composition tables were used as a reference to analyze food intake. A classified physical activity questionnaire based on physical activity questionnaire (IPAQ) was used to measure the physical activity of the subjects. The validity and reliability of this questionnaire were previously confirmed.25
Ethics Approval
This study was approved by the Ethics Committee of the Ahvaz Jundishapur University of Medical Science (Ref No. IR.AJUMS.REC.1396.157) and was registered in the Iranian Registry of Clinical Trials website (IRCT2017030831993N4). Written informed consent was obtained from all subjects.
Statistical Analysis
Considering MDA as the main variable and the Alamdari NM and coworkers’ study,26 the sample size was calculated with a 95% confidence interval according to the following formula.
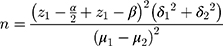
The calculated sample size was 23 subjects in each group, however, considering with a 10% probable withdraw, 25 subjects were involved in each group.
The Kolmogorov–Smirnov test was used to assess the normality distribution of variables. The continuous and categorical data are reported as means ± standard deviations and frequency (%), respectively. The Paired sample t-test was also used to compare the results within groups post-intervention. The Chi-square test was used to compare the qualitative variables. Also, the Independent sample t-test was used to compare the results between two groups. The Analysis of covariance (ANCOVA) was applied for assessment any differences between two groups at the end of study after adjusting for covariates (age, sex, energy, BMI, physical activity, disease duration, and drugs). P < 0.05 were considered as significant. Statistical analysis was performed using the SPSS version 19.
Results
Baseline Variables
Table 1 shows the baseline data. All data in this study were normally distributed. Forty-four subjects (22 subjects in both groups) completed the study (Figure 1). The mean age of the subjects in the intervention and control group was 53.72 ± 6.68 and 51.45 ± 5.03 years old, respectively. There were no significant differences in age, sex, weight, BMI, duration of suffering from DM, and medications (data not shown) between two groups at baseline (P ≥ 0.05). Also, no significant differences were observed in physical activity and dietary data such as intakes of energy, macronutrients, and micronutrients including antioxidant vitamins C, E, A, beta-carotene, α-tocopherol and selenium between two groups at baseline and after the intervention (P ≥ 0.05). The data of dietary intakes of the subjects in this study were previously published.17
 |
Table 1 Baseline Characteristics of the Subjects |
The Effects of Melatonin on IL-1β and MDA
There were no significant differences in the mean serum levels of IL-1β and MDA between two groups (P ≥ 0.05) at baseline. Melatonin supplementation along with NSPT significantly decreased the mean serum levels of IL-1β and MDA in the intervention group compared with the baseline (2.41 ± 0.55 vs 2.06 ± 0.48 pg/mL, respectively; P = 0.008) and MDA (17.2 ± 1.82 vs 16.13 ± 1.76 µM, respectively; P < 0.001). Whereas, the decrease in these parameters were not significant in the control group (Table 2). Moreover, the mean changes of serum levels of MDA were significantly lower in the intervention group compared with the control group (−1.07 ± 0.92 vs −0.31 ± 0.88 µM, respectively; P = 0.008). Also, after adjusting for confounding factors, the results did not change in terms of significance (Table 3).
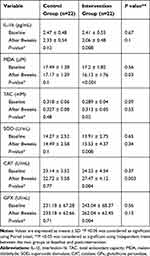 |
Table 2 The Mean ± SD of Inflammatory and Antioxidant Markers at Baseline and Post-Intervention |
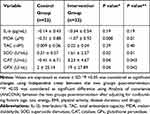 |
Table 3 The Mean Changes of Inflammatory and Antioxidant Markers at Post-Intervention |
The Effects of Melatonin on SOD, CAT, GPx, and TAC
No significant differences were observed in the mean serum levels of SOD, CAT, GPx and, TAC between two groups at baseline (P ≥ 0.05). The mean serum levels of SOD, GPx, CAT, and TAC were significantly increased in the intervention group compared with the baseline after the intervention (13.91 ± 2.75 vs 15.53 ± 4.37 U/mL, respectively; P = 0.008), (243.04 ± 68.37 vs 262.04 ± 62.45 U/mL, respectively; P = 0.004), (24.23 ± 4.54 vs 27.47 ± 4.12 mM, respectively; P = 0.004) and (0.289 ± 0.04 vs 0.313 ± 0.05 mM, respectively; P = 0.02) (Table 2). In addition, the mean changes of serum levels of SOD, GPx, and CAT were significantly greater in the intervention group compared with the control group (P = 0.02, P = 0.04 and P = 0.04; respectively). Also, after adjusting for confounding factors, the results did not change in terms of significance (Table 3).
Melatonin and Periodontal Status (PD, CAL, BOP, and Plaque)
The effects of intervention on periodontal indices have been reported in our previous article.17
Discussion
Increased oxidative stress is a widely accepted agent in the development and progression of DM and its complications such as PD. Melatonin is proposed to have a potent antioxidant capability and protective properties against oxidative stress.27 This study was the only clinical trial investigating the effects of melatonin supplementation along with the NSPT in T2DM patients with PD. The results of the present study showed that the 8 weeks consumption of melatonin supplementation in adjunct to the NSPT significantly increased the serum levels of TAC, SOD, CAT, and GPx. Also, serum levels of MDA and IL-1b were significantly decreased in the intervention group.
Similar to the present study, a clinical trial showed that consumption of melatonin either with the dosage of 5 mg or with the dosage of 2 mg for 30 days significantly increased SOD-1 activity in two groups of T2DM patients and healthy controls. Whereas, only the 2 mg dosage significantly increased CAT activity in two groups and 5 mg did not have a significant effect. Also, only the 5 mg dosage significantly increased GPx-1 activity in diabetic patients and the 2 mg did not have a significant effect. Both dosages significantly decreased MDA (Lipid peroxidation marker).28 The results of this study suggest that both dosages of melatonin may have similar therapeutic effects on antioxidant defense system. Therefore, it is suggested that melatonin can exert its antioxidant properties at low dosages. However, in our study, the 6 mg dosages of melatonin significantly increased all three antioxidant enzymes (SOD, CAT, and GPx). It is suggested that the design of our study, greater dosage of melatonin, using melatonin supplementation along with NSPT, and the longer period of the intervention in this study may be some reasons behind obtaining significant results in our study. In another study, Özdem et al found that melatonin administration reduced MDA and increased GSH-Px levels in heart tissue in rats with periodontitis, but in contrast to the results of this study, there was no significant increase in serum levels of SOD.29 The difference in the target subjects, the method of research, the supplement dosage, and the duration of the intervention are suggested to be possible reasons lead to the diversity in the results for SOD. In agreement with the findings of this study, in a clinical trial the consumption of melatonin supplementation (10 mg for 12 weeks) in diabetic patients with coronary heart disease resulted in significant increases in plasma GSH and nitric oxide (NO) and significant decreases in MDA, protein carbonyl (PCO) and serum high sensitivity C-reactive protein (hs-CRP) levels.30 Moreover, the treatment with melatonin (10 mg/kg) in type 2 diabetic rats for 3 weeks caused a significant decrease in reactive oxygen species (ROS), oxido-nitrosative stress markers, including thiobarbituric acid reactive substances (TBARS), nitrite, and depleted glutathione (GSH) levels in the hippocampus of melatonin-treated group.31 It is suggested that melatonin is one of the most functional scavengers of free radicals. Melatonin acts as a direct scavenger and neutralizes several free radicals such as singlet oxygen, superoxide anion radical, hydroxyl radical, hydroperoxide, lipid peroxide radical, and peroxynitrite. Melatonin can also protect against the oxidative stress by improving the mitochondrial function, stimulating the expressions and activating the antioxidant enzymes including CAT, SOD, and GPx.32 It is indicated that the potent anti-oxidative effect of melatonin is partially related to its lipophilic and hydrophilic properties, allowing it to easily transfer through all bio-barriers where it is highly available in subcellular organelles such as cell membrane and mitochondria. Regarding that the mitochondria is considered as the main site of ROS production, melatonin can strongly protect these organelles against the oxidative damage. Also, there are decreasing in the oxidative stress and increasing in the antioxidant capacity reported in the literature after the NSPT.33 Brock et al reported that NSPT with some improvements in clinical parameters can also improve the antioxidant defense in gingival crevicular fluid (GCF) and serum in CP patients.34 In another study, it was found that there was a significant increase in GPx and total antioxidative status (TAS) of saliva and decrease (but not significant) in SOD activity in CP patients after the NSPT.35 In our study, the oxidative and inflammatory status of the control group (received only NSPT) did not significantly change. Perhaps, the low level of systemic inflammation in the gum and a small amount of bleeding on probing caused that patients do not respond well to NSPT in the control group. The study limitation in our study is the inadequate number of study groups. It is suggested that further studies are needed to be conducted with four study groups in future (group1; Diabetes + no periodontal treatment + placebo, group2; Diabetes + no periodontal treatment + melatonin, group3; Diabetes + NSPT + placebo, group4; Diabetes + NSPT + melatonin).
Conclusion
It is suggested that supplementation with melatonin along with the NSPT may be effective in the improvement of the oxidative and inflammatory status in T2DM patients with PD. Therefore, the consumption of melatonin supplement in adjunct to NSPT may be recommended as a part of the therapeutic approach in controlling DM and CP.
Abbreviations
CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione S-transferase; HC, hip circumference; IL-1b, interleukin-1b; MDA, malondialdehyde; NSPT, non-surgical periodontal therapy; PD, periodontal disease; SOD, superoxide dismutase; TAC, total antioxidant capacity; T2DM, type 2 diabetes mellitus; WC, waist circumference; WHR, waist to hip ratio.
Ethics and Consent Statement
Study design was done according to the guidelines of the Helsinki Declaration and all procedures involving human patients were approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Ethical Code. IR.AJUMS.REC.1396.157). In this study, written informed consent was obtained from all patients before initiating the study.
Data Sharing Statement
The datasets are not publicly available because of lack of agreement for disclosing individual raw data in public but are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the Nutrition and Metabolic Disorders Research Center, and Research Center of Diabetes, Endocrinology and Metabolism clinic employees of Ahvaz University Golestan Hospital and Dental Clinic of Ahvaz Jundishapur University of Medical Sciences.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by Vice-Chancellor for Research Affairs of Ahvaz Jundishapur University of Medical Sciences (NRC-9605).
Disclosure
The authors have declared that they have no conflicts of interest in this work.
References
1. Yan L, Hui GJ. Subcutaneous implanted system for the treatment of type 2 diabetes. CAMS. 2011;33(4):473–477. doi:10.3881/j.issn.1000-503X.2011.04.025
2. Neumann A, Schwarz P, Lindholm L. Estimating the cost-effectiveness of lifestyle intervention programmes to prevent diabetes based on an example from Germany: Markov modelling. CERA. 2011;9(1):17. doi:10.1186/1478-7547-9-17
3. Esteghamati A, Larijani B, Aghajani MH, et al. Diabetes in Iran: prospective analysis from first nationwide diabetes report of National Program for Prevention and Control of Diabetes (NPPCD-2016). Sci Rep. 2017;7(1):13461. doi:10.1038/s41598-017-13379-z
4. Barre D, Mizier-Barre K, Stelmach E, et al. Flaxseed lignan complex administration in older human type 2 diabetics manages central obesity and prothrombosis—an invitation to further investigation into polypharmacy reduction. J Nutr Metab. 2012;2012:7. doi:10.1155/2012/585170
5. Epingeac ME, Gaman MA, Diaconu CC, Gad M, Gaman AM. The evaluation of oxidative stress levels in obesity. Rev Chim (Bucharest). 2019;70:2241–2244. doi:10.37358/RC.19.6.7314
6. Mamdouh M, Shaban S, Ibrahim Abushouk A, Zaki MMM, Ahmed OM, Abdel-Daim MM. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J Diabetes Res. 2017;2017:11. doi:10.1155/2017/8095926
7. Elisia I, Lam V, Cho B, et al. Exploratory examination of inflammation state, immune response and blood cell composition in a human obese cohort to identify potential markers predicting cancer risk. PLoS One. 2020;15(2):e0228633. doi:10.1371/journal.pone.0228633
8. Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14(1):453. doi:10.1007/s11892-013-0453-1
9. Khumaedi AI, Purnamasari D, Wijaya IP, Soeroso YJD. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes Metab Syndr. 2019;13(2):1675–1678. doi:10.1016/j.dsx.2019.03.023
10. Quintero AJ, Chaparro A, Quirynen M, et al. Effect of two periodontal treatment modalities in patients with uncontrolled type 2 diabetes mellitus: a randomized clinical trial. J Clin Periodontol. 2018;45(9):1098–1106. doi:10.1111/jcpe.12991
11. Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;11(2):72.
12. Gurav AN. Periodontitis and insulin resistance: casual or causal relationship? Diabetes Metab J. 2012;36(6):404–411. doi:10.4093/dmj.2012.36.6.404
13. Calle M, Fernandez MJD. Inflammation and type 2 diabetes. Arch Dermatol Res. 2012;38(3):183–191. doi:10.1007/s00403-017-1775-7
14. Patil VS, Patil VP, Gokhale N, Acharya A, Kangokar PJ. Chronic periodontitis in type 2 diabetes mellitus: oxidative stress as a common factor in periodontal tissue injury. J Clin Diagn Res. 2016;10(4):BC12. doi:10.7860/JCDR/2016/17350.7542
15. Tiwari BK, Pandey KB, Abidi A, Rizvi SI. Markers of oxidative stress during diabetes mellitus. J Biomark. 2013;2013:1–8. doi:10.1155/2013/378790
16. Gawlik K, Naskalski J, Fedak D, et al. Markers of antioxidant defense in patients with type 2 diabetes. Oxid Med Cell Longev. 2016;2016:1–6. doi:10.1155/2016/2352361
17. Bazyar H, Gholinezhad H, Moradi L, et al. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: a double-blind, placebo-controlled trial. Inflammopharmacology. 2019;27(1):67–76. doi:10.1007/s10787-018-0539-0
18. Reiter RJ, TAN DX. Melatonin: an antioxidant in edible plants. Ann N Y Acad Sci. 2002;957(1):341–344. doi:10.1111/j.1749-6632.2002.tb02938.x
19. Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiol. 2014;29(5):325–333. doi:10.1152/physiol.00011.2014
20. Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52(2):139–166. doi:10.1111/j.1600-079X.2011.00934.x
21. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin LJ. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61(3):253–278. doi:10.1111/jpi.12360
22. Rodriguez C, Mayo JC, Sainz RM, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9. doi:10.1046/j.1600-079X.2003.00092.x
23. Santos VR, Lima JA, De Mendonça AC, Braz Maximo MB, Faveri M, Duarte PM. Effectiveness of full‐mouth and partial‐mouth scaling and root planing in treating chronic periodontitis in subjects with type 2 diabetes. J Periodontol. 2009;80(8):1237–1245. doi:10.1902/jop.2009.090030
24. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi:10.2337/dc14-S081.
25. Gh FM. Evaluation of the reliability and validity of Azad-Fesharaki’s physical activity questionnaire (AFPAQ). AMUJ. 2011;14(56):36–44.
26. Alamdari NM, Mahdavi R, Roshanravan N, Yaghin NL, Ostadrahimi A, Faramarzi E. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm Metab Res. 2015;47(7):504–508. doi:10.1055/s-0034-1384587
27. Alqasim AA, Noureldin EEM, Hammadi SH, Esheba GE. Effect of melatonin versus vitamin D as antioxidant and Hepatoprotective agents in STZ-induced diabetic rats. J Diabetes Metab Disord. 2017;16(1):41. doi:10.1186/s40200-017-0322-6
28. Rybka J, Kędziora-Kornatowska K, Kupczyk D, Muszalik M, Kornatowski M, Kędziora J. Antioxidant effect of immediate-versus sustained-release melatonin in type 2 diabetes mellitus and healthy controls. Drug Deliv. 2016;23(3):804–807. doi:10.3109/10717544.2014.917343
29. Özdem M, Kırzıoğlu FY, Yılmaz HR, et al. Antioxidant effects of melatonin in heart tissue after induction of experimental periodontitis in rats. J Oral Sci. 2017;59(1):23–29. doi:10.2334/josnusd.16-0034
30. Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;38(1):191–196. doi:10.1016/j.clnu.2017.12.004
31. Xuyan Z, Ping YJ, Zhongjing W, et al. Melatonin reverses type 2 diabetes-induced cognitive deficits via attenuation of oxidative/nitrosative stress and NF-κB-mediated neuroinflammation in rat hippocampus. Trop J Pharm Res. 2017;16(12):2865–2875. doi:10.4314/tjpr.v16i12.10
32. Prado NJ, Ferder L, Manucha W, Diez ER. Anti-inflammatory effects of melatonin in obesity and hypertension. Curr Hypertens Rep. 2018;20(5):45. doi:10.1007/s11906-018-0842-6
33. Akpinar A, Toker H, Ozdemir H, Bostanci V, Aydin HJ. The effects of non-surgical periodontal therapy on oxidant and anti-oxidant status in smokers with chronic periodontitis. Arch Oral Biol. 2013;58(6):717–723. doi:10.1016/j.archoralbio.2012.11.009
34. Brock G, Butterworth C, Matthews J, Chapple IJ. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31(7):515–521. doi:10.1111/j.1600-051X.2004.00509.x
35. Novaković N, Cakić S, Todorović T, et al. Antioxidative status of saliva before and after non-surgical periodontal treatment. Srp Arh Celok Lek. 2013;141(3–4):163–168. doi:10.2298/SARH1304163N
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

